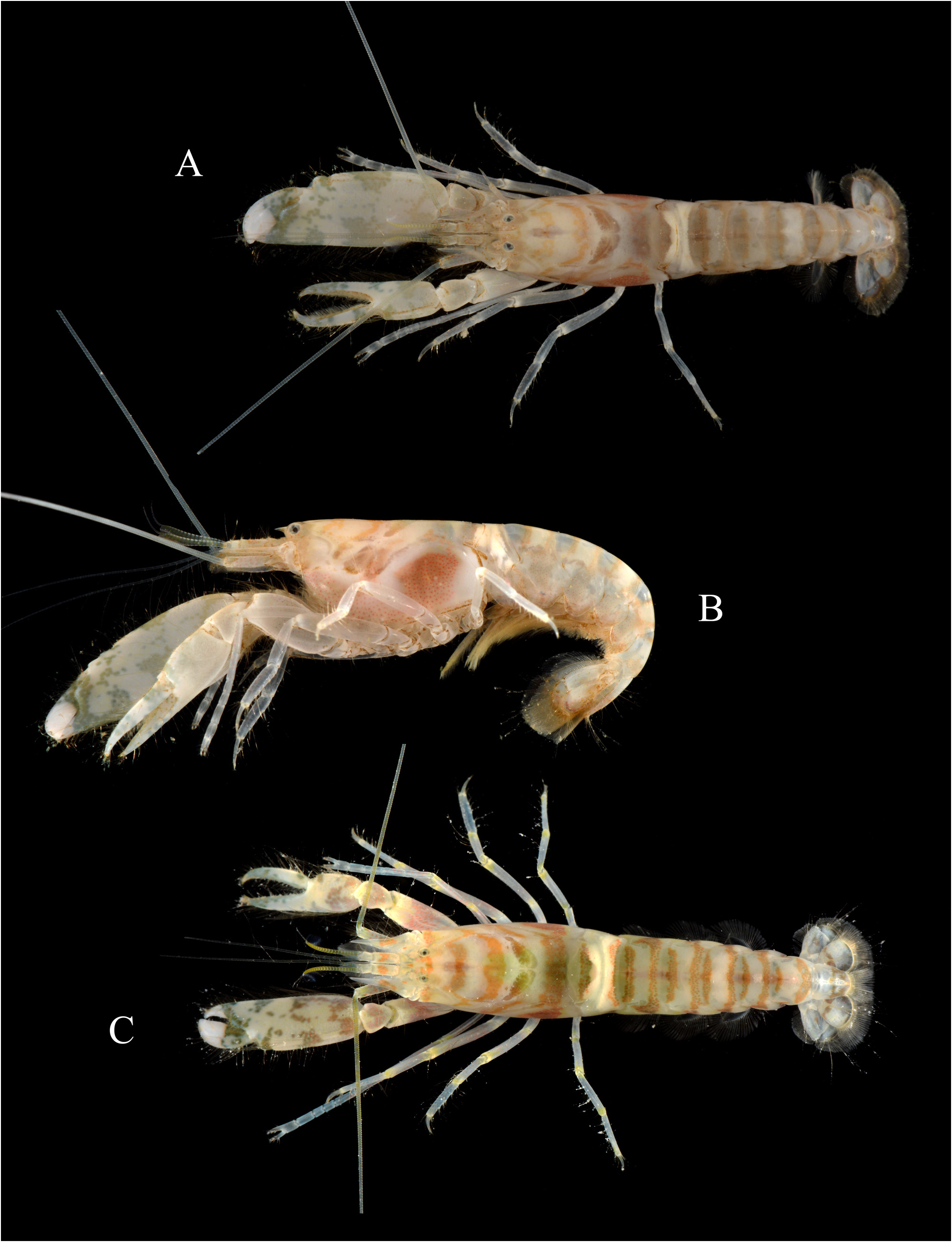
Alpheus sciolii, Anker, 2022
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5092.3.2 |
|
publication LSID |
lsid:zoobank.org:pub:B41C48B3-CD91-47EF-82E4-57884BDC4FB9 |
|
DOI |
https://doi.org/10.5281/zenodo.5888954 |
|
persistent identifier |
https://treatment.plazi.org/id/03BF87EB-0513-0510-B4B1-8CFFFCF31956 |
|
treatment provided by |
Plazi |
|
scientific name |
Alpheus sciolii |
| status |
sp. nov. |
Alpheus sciolii sp. nov.
( Figs. 4–7 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 , 8C View FIGURE 8 )
Alpheus cf. bellulus View in CoL — Marin 2008: 382, fig. 9b (as A. bellulus View in CoL in the figure legend) [not A. bellulus Miya & Miyake, 1969 View in CoL ]. Alpheus sp. aff. bellulus — Anker & De Grave 2019: 148, fig. 4C.
Alpheus aff. djiboutensis View in CoL — Hurt et al. 2021: 2, fig. 1D.
Alpheus sp. — Debelius 2001: 153, colour photograph.
Alpheus sp. 2 — Minemizu 2013: 101, colour photograph.
Alpheus sp. 6 — Kuiter & Debelius 2009: 152, 4 colour photographs.
Type material. Holotype: male (cl 8.6 mm), MNHN-IU-2018-5667, Solomon Islands, New Georgia, Munda, Hopei Island, shallow sand flat, suction (yabby) pump, depth 0.5–1 m, leg. A. Anker, 14.09.2016 [fcn SOL-106] . Paratypes: 1 male (cl 9.3 mm), MNHN-IU-2018-5669, Solomon Islands, New Georgia, Munda, Sosuhite Island, shallow sand flat with coral rubble, depth 0.5–2 m, in burrow under large piece of coral rubble, leg. A. Anker, 19.09.2016 [fcn SOL-047]; 1 ov. female (cl 10.7 mm), MNHN-IU-2018-5668, Australia, Queensland, Heron Island , southern side, shallow reef flat, coarse sand, suction (yabby) pump, 0.5–1 m, leg. A. Anker, 28.09.2016 [fcn HE-011] ; 1 male (cl 6.6 mm), 1 female (cl 8.4 mm), MNHN-IU-2018-5670, Australia, Queensland, Heron Island , southern side, shallow reef flat, coarse sand, suction (yabby) pump, 0–0.5 m, leg. A. Anker, 24.09.2016 [fcn HE-120] ; 1 male (cl 16.0 mm, missing both chelipeds), MNHN-IU-2018-5525, Australia, Queensland, Heron Island , southern side, shallow reef flat, coarse sand, suction (yabby) pump, 0–0.5 m, leg. A. Anker, 25.09.2016 [fcn HE-026] .
Additional material. 1 male (cl 14.5 mm), MNHN-IU-2018-5671, Vietnam, Nha Trang Bay , Tre Island, Tre Bay, intertidal area near mangrove, suction (yabby) pump, with goby, leg. I.N. Marin, 12.07.2006 .
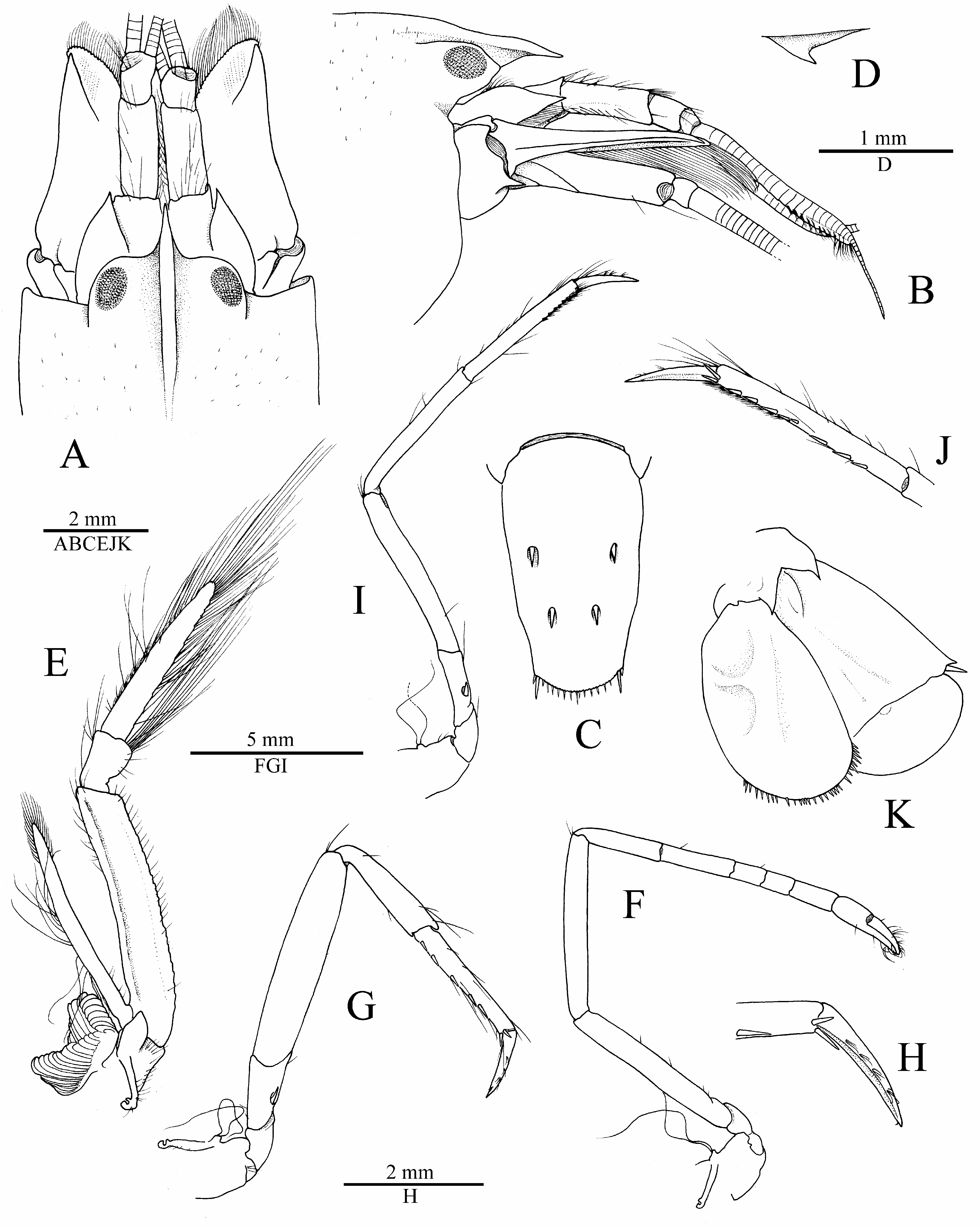
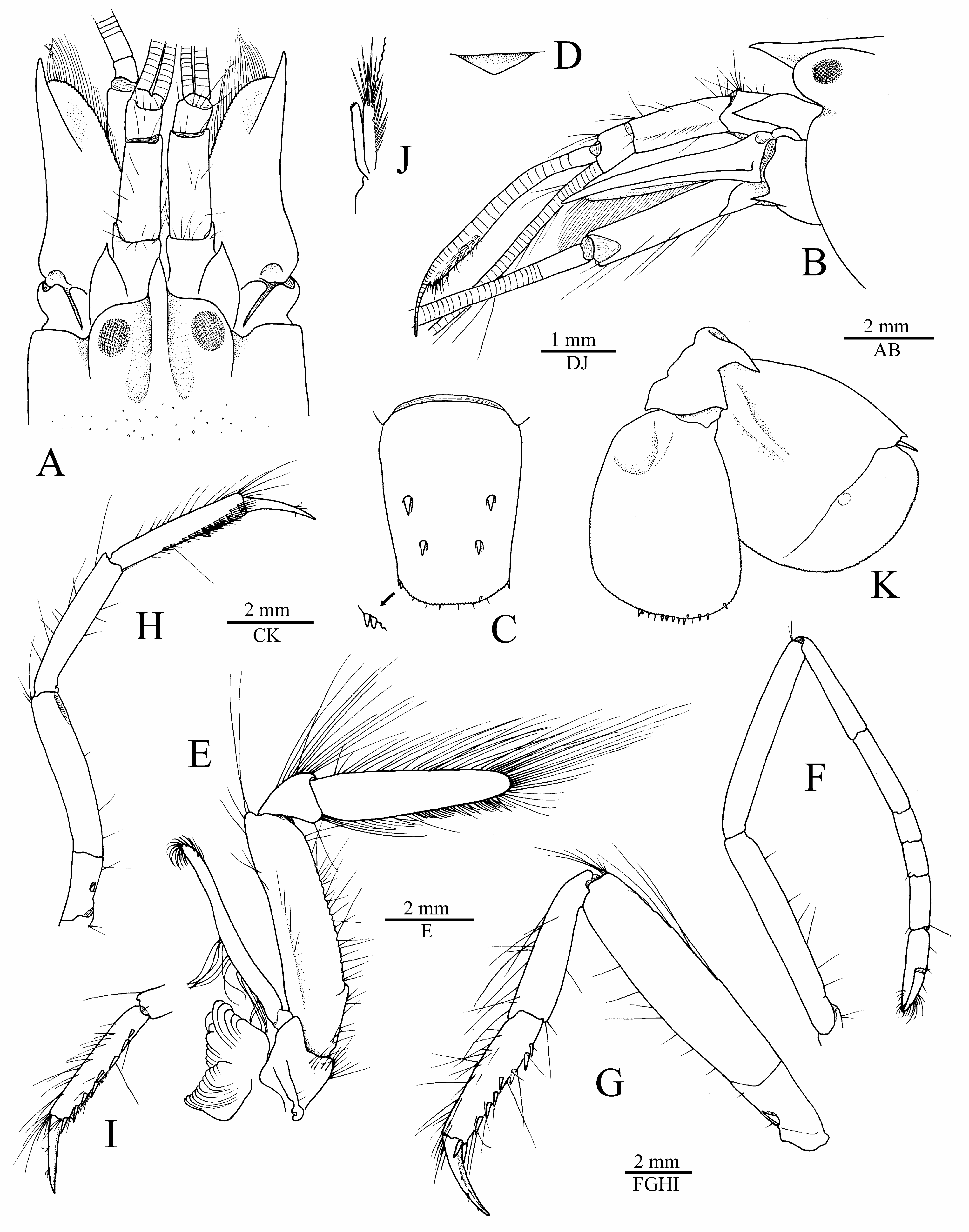
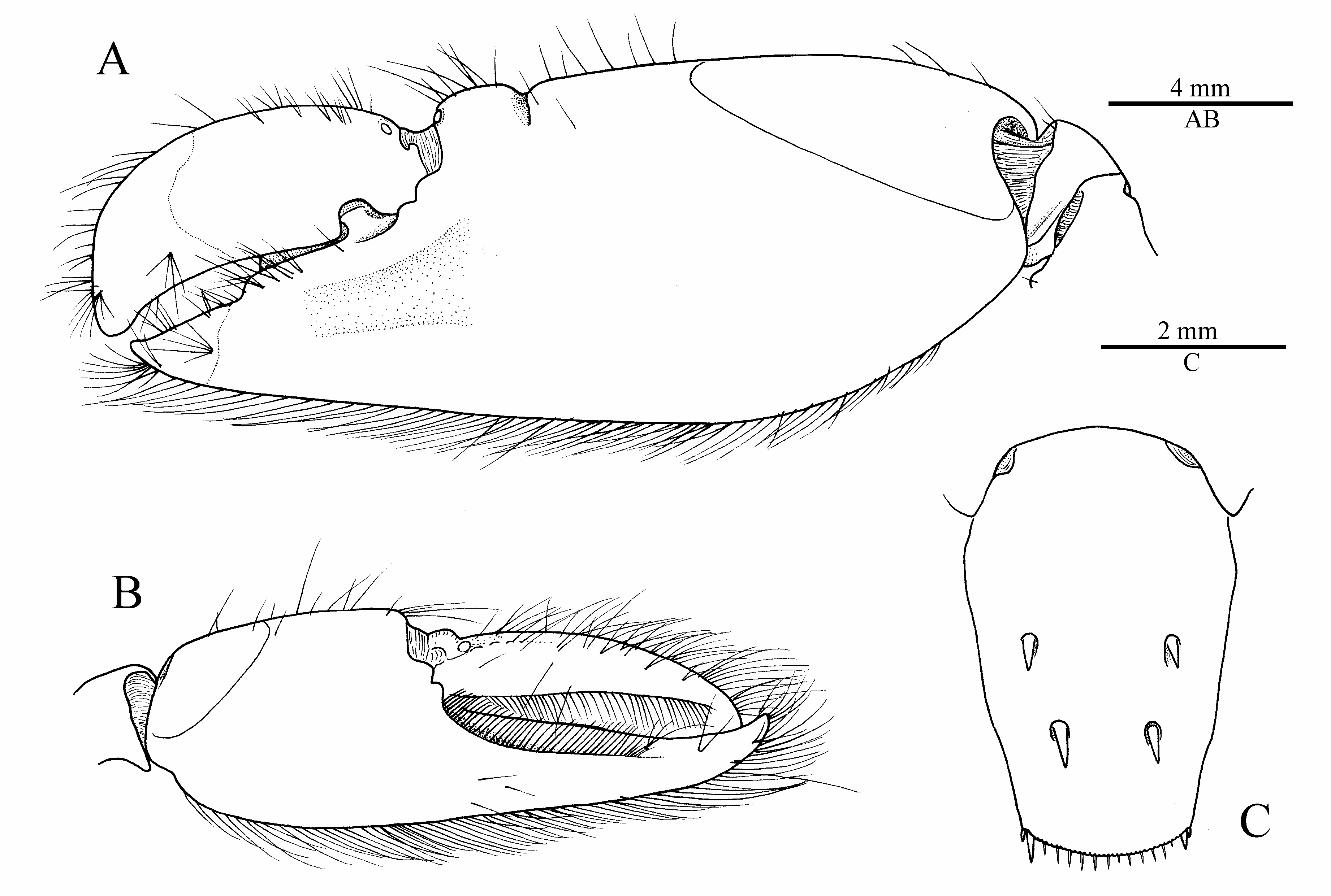
Description. Carapace not setose, not pubescent, pitted ( Fig. 4A, B View FIGURE 4 ). Rostrum well developed, wide, about 1.7 times as long as wide at base, subacute distally, just reaching distal third of first article of antennular peduncle, pointing straight-forward in lateral view; rostral carina well marked, rounded dorsally, gently sloping into welldemarcated adrostral furrows, continuing well beyond base of orbital hoods, gradually flattening, not reaching midlength of carapace ( Fig. 4A, B View FIGURE 4 ). Orbital hoods swollen, feebly projecting anteriorly in lateral view, unarmed; frontal margin between rostrum and orbital hood shallowly concave ( Fig. 4A, B View FIGURE 4 ). Pterygostomial angle rounded; cardiac notch well developed, deep ( Figs. 4B View FIGURE 4 , 7B View FIGURE 7 ).
Telson very broad, subrectangular, gently tapering distally, about 1.8 times as long as maximal width, with lateral margins not noticeably constricted; dorsal surface with two pairs of stout cuspidate setae both inserted far from lateral margin, first pair at about 0.5 telson length, second pair at about 0.7 of telson length; posterior margin broadly rounded, with row of short spiniform setae above plumose setae (less conspicuous or with slender spiniform setae in some specimens); posterolateral angles each with one pair of spiniform setae (typically, sometimes with only one spiniform seta), mesial ones stouter and two to three times as long as lateral ones [posterolateral spiniform setae somewhat aberrant in the illustrated paratype ( Fig. 4C View FIGURE 4 ), see Fig. 6C View FIGURE 6 for more typical condition].
Eyes well developed, with large, normally pigmented corneas ( Figs. 1A, B View FIGURE 1 , 7 View FIGURE 7 ). Antennular peduncle moderately elongate and stout; stylocerite feebly swollen laterally, distal part slenderer, ending in sharp point, latter not reaching distal margin of first article; ventromesial carina with large subtriangular tooth; second article about 2.7 times as long as wide; lateral antennular flagellum with secondary ramus fused to main ramus over most of its length, with numerous groups of aesthetascs distally, starting from about 14th subdivision ( Fig. 4A, B, D View FIGURE 4 ). Antenna with basicerite fairly stout, its distoventral margin armed with large, anteriorly projecting, sharp tooth; scaphocerite with moderately broad blade distinctly exceeded by sharp distolateral tooth, lateral margin shallowly concave; scaphocerite reaching distinctly beyond end of antennular peduncle and slightly beyond carpocerite ( Fig. 4A, B View FIGURE 4 ).
Mouthparts not dissected, generally typical for genus in external observation. Third maxilliped relatively stout; coxa with bluntly projecting lateral plate; antepenultimate article flattened ventrolaterally, with distinct ridge running parallel to dorsal margin on lateral surface and rugose mesial margin, about 3.9 times as long as high; penultimate article very short, cup-shaped, bulging ventrally, its distoventral margin with few moderately long setae, its dorsal margin with row of elongate setae; ultimate article densely furnished with rows of very long, stiff setae, dorsal and apical setae longest; arthrobranch well developed ( Fig. 4E View FIGURE 4 ).
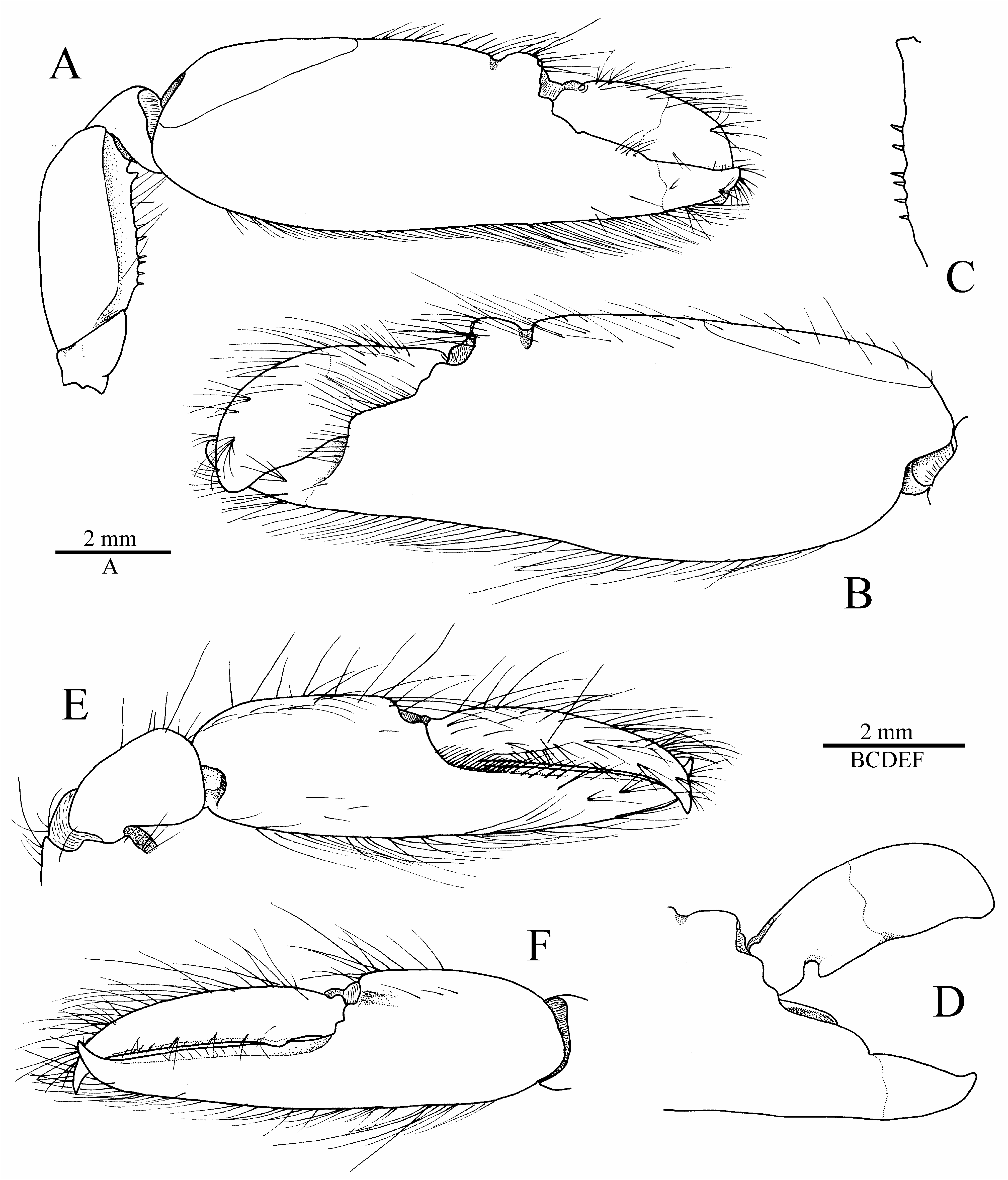
Male major cheliped robust; ischium short, stout, smooth; merus very stout, trigonal in cross-section, about twice as long as maximal width, distodorsal margin ending bluntly, ventromesial margin somewhat rugose, with more or less pronounced, blunt, distal tooth and row of five or six spiniform setae; carpus short, distally widening, cup-shaped; chela not particularly elongate, fringed with long setae along dorsal and ventral surfaces; palm strongly compressed, stout, subrectangular in cross-section, length / maximal height ratio 1.8–2.1 (lower in larger males); surfaces relatively smooth, without granules; dorsal margin with well-marked transverse groove subdistally, without marked longitudinal ridges; ventral margin almost straight; lateral surface with shallow depression distally, near base of pollex; fingers subequal in length, about half-length of palm, not twisted or significantly deviating from chela axis; dactylus distally rounded, plunger somewhat reduced, poorly demarcated from anterior cutting edge, latter slightly bulging; adhesive disks very small ( Fig. 5A–D View FIGURE 5 ; see also Fig. 6A View FIGURE 6 ). Female major cheliped generally similar to male major cheliped, smaller, weaker; chela with somewhat different proportions (see Fig. 7C View FIGURE 7 ).
Male minor cheliped with ischium short, smooth; merus similar to that of major cheliped in size and stoutness, trigonal in cross-section, distodorsal angle blunt, ventromesial margin slightly rugose distally, usually without distal tooth, armed with spiniform setae; carpus distinctly longer than that of major cheliped, distally widening, cupshaped; chela not elongate, not swollen, fringed with long setae along dorsal and ventral surfaces; palm compressed, subrectangular in cross-section, length / maximal height ratio 1.3–1.5 (lower in larger males); surfaces smooth, not granulated; dorsal margin without groove; ventral margin broadly convex; fingers subequal in length, about 1.3 times length of palm, not twisted, slightly gaping when closed, with balaeniceps crests lined with setae (better developed in larger males); adhesive disks greatly reduced ( Fig. 5E, F View FIGURE 5 ; see also Fig. 6B View FIGURE 6 ). Female minor cheliped generally similar, but with weaker chela, latter with balaeniceps ridges and setae on fingers poorly developed or lacking ( Fig. 7C View FIGURE 7 ).
Second pereiopod slender; ischium and merus subequal in length; carpus with five subarticles, first noticeably longer than second, ratio of carpal subarticles approximately equal to 3.0/2.5/1.0/1.0/1.7; chela longer than distalmost carpal subarticle ( Fig. 4F View FIGURE 4 ). Third pereiopod fairly robust; ischium with stout spiniform seta on ventrolateral surface; merus about 4.1 times as long as maximal width, ventromesial margin unarmed distally; carpus about 0.6 length of merus, much more slender than merus, unarmed; propodus subequal to carpus, setose, with at least 10 spiniform setae along ventral margin, including distal pair adjacent to dactylar base; dactylus slightly more than halflength of propodus, gradually curving distally, subspatulate, moderately broadened, flattened ventrally, with distinct longitudinal keel separating ventral and dorsal surfaces, with scarce setae ( Fig. 4G View FIGURE 4 ). Fourth pereiopod generally similar to third pereiopod, more slender. Fifth pereiopod much more slender than third and fourth pereiopods; ischium with small spiniform seta on ventrolateral surface; merus about 5.7 times as long as wide; carpus slightly more slender than merus, about 0.9 length of merus; propodus subequal to carpus, with rows of serrulate setae forming cleaning brush on distal third of ventrolateral surface, and at least eight spiniform setae along ventromesial margin, including distal pair adjacent to dactylar base ( Fig. 4H, I View FIGURE 4 ).
Second pleopod with appendices masculina and interna equal in length, former with long stiff setae ( Fig. 4J View FIGURE 4 ). Uropod with mesial and lateral lobes of protopod each ending in sharp (lateral) or blunt (mesial) distal tooth; exopod broad, broadly rounded distally, with short triangular distolateral tooth; diaeresis straight for most part, except for rounded lobe adjacent to slender spiniform seta, latter not reaching level of distal margin of exopod; endopod much narrower than exopod, with row of spiniform setae on distal margin ( Fig. 4K View FIGURE 4 ).
Colour pattern. General background colour white with pale yellowish tinge; carapace with several (three to four) broad bands, running transversely dorsally and more obliquely laterally, some broadly croissant-shaped; carapace flanks with very large brown to almost black spot (quickly fading in captured individuals); pleon with narrower, transversely-obliquely running, brown bands, usually two per pleonite; posterior end of carapace and first pleonite with conspicuous, white, dorsal “saddle”; fourth and fifth pleonites with transverse, whitish, dorsal patches; chelipeds whitish or greyish, with irregular, brown, green-brown or green-bluish patches; distal half of fingers hyaline-white; antennules and antennae whitish with brownish spots and streaks; antennular flagella pale yellow proximally, more intense yellow distally; antennal flagella pale yellow proximally, uncoloured distally; second pereiopods and walking legs greyish-blue with yellow markings around articulations; pleopods brownish yellow; uropods whitish with some brown-red bands or spots, mainly on endopod; telson yellow whitish proximally, with brown-red pattern distally ( Figs. 7 View FIGURE 7 , 8C View FIGURE 8 ; see also Anker & De Grave 2019: fig. 4C).
Etymology. This new species is named after Justin Scioli, PhD cand., the author’s great friend and collaborator in projects involving taxonomy and phylogenetics of alpheid shrimps.
Distribution. Western Pacific: confirmed records from Solomon Islands, Australia (Great Barrier Reef) and Vietnam (present study); photographic records from the Philippines (Palawan), Indonesia (Java, Flores, Bali), Papua New Guinea and Palau ( Debelius 2001; Randall et al. 2007; Kuiter & Debelius 2009; Hurt et al. 2021).
Ecology. Shallow coral reefs and adjacent reef flats and lagoons; associated with gobies, typically Cryptocentrus cinctus (Herre) , C. fasciatus (Playfair) , C. leptocephalus Bleeker and C. inexplicatus (Herre) , but also Ctenogobiops pomastictus ( Fig. 8C View FIGURE 8 ; see also Debelius 2001; Randall et al. 2007; Kuiter & Debelius 2009; Hurt et al. 2021); may also harbour the “commensal” palaemonid shrimp, Palaemonella aliska Marin, 2008 ( Marin 2008; Anker & De Grave 2019).
Remarks. Alpheus sciolii sp. nov. has a unique and unmistakable colour pattern ( Figs. 7 View FIGURE 7 , 8C View FIGURE 8 ), characterised by the dark-brown or olive-brown transverse banding and the presence of a very large dark spot on the flanks of the carapace. As in the previous species, the white “saddle” marking the limit between the carapace and the pleon is quite conspicuous. This colour pattern ( Fig. 7 View FIGURE 7 ; see also Kuiter & Debelius 2009, as Alpheus sp. 6 , and Minemizu 2013, as Alpheus sp. 2 ) is very different from the colour patterns of A. bellulus , A. macellarius , A. fenneri , A. mannarensis (see Miya & Miyake 1969; Debelius 2001; Kuiter & Debelius 2009; Minemizu 2013; Anker et al. 2015; Purushothaman et al. 2021; for A. bellulus see also Fig. 8F View FIGURE 8 ) and A. thompsoni sp. nov. ( Figs. 3 View FIGURE 3 , 8A, B View FIGURE 8 ).
The non-type male specimen of A. sciolii sp. nov. from Nha Trang, Vietnam, has noticeably larger and stouter chelipeds, with a markedly stronger balaeniceps condition on the minor chela fingers, compared to that of the complete male type specimens from the Solomon Islands and Australia, ( Fig. 6A, B View FIGURE 6 ), which may be simply due to its larger size (cl 14.5 mm vs. 6.6–9.3 mm). A large paratype male of similar size (cl 16.0 mm) from Heron Island is unfortunately lacking both of its chelipeds (see Anker & De Grave 2019: fig. 4C). The Nha Trang specimen also seems to have a darker colour (see Marin 2008: fig. 9b), although it is partly covered by detritus and sediment. In addition, a colour pattern very similar to that of A. sciolii exists in shrimps associated with different gobies, such as Amblyeleotris steinitzi (Klausewitz) (see Fig. 8D View FIGURE 8 ). Therefore, collection of additional material and a molecular comparison between the specimens of A. sciolii sp. nov. from Solomon Islands / Australia and Vietnam (or other parts of South-East Asia) are highly desirable.
The separation of A. sciolii sp. nov. from A. djeddensis is based on the same criteria as those used by De Man (1909) to distinguish A. djiboutensis from A. djeddensis , viz. the scaphocerite blade reaching well beyond the adjacent distolateral tooth in both A. sciolii sp. nov. and A. djiboutensis (vs. not reaching beyond it in A. djeddensis ), and the first subarticle of the second pereiopod carpus noticeably longer than the second in A. sciolii sp. nov. and A. djiboutensis (vs. as long as the second in A. djeddensis ). On the other hand, A. sciolii sp. nov. may be separated from A. djiboutensis by the much broader telson, which is around 1.8 times as long as maximal width in A. sciolii sp. nov. ( Figs. 4C View FIGURE 4 , 6C View FIGURE 6 ) vs. 2.1 times in A. djiboutensis (see De Man 1909: fig. 18). In fact, the telson of A. sciolii sp. nov. appears to be the broadest among the presently known species of the A. djeddensis – A. djiboutensis complex. Also noteworthy is that no snapping shrimp with the colour pattern of A. sciolii sp. nov. is known from the Red Sea ( Karplus et al. 1981) or western Indian Ocean ( Polunin & Lubbock 1977), from where A. djeddensis and A. djiboutensis were origially described. The Australian material reported as A. djiboutensis by Banner & Banner (1982) appears to contain more than one species (possibly including the true A. djiboutensis and A. sciolii sp. nov.) and will need to be carefully re-examined.
Alpheus sciolii sp. nov. can be distinguished morphologically from A. bellulus by the rostrum much narrower and longer (vs. shorter and subtriangular in A. bellulus ), the ventral margin of the major chela almost straight to slightly convex (vs. somewhat concave in A. bellulus ), and the telson 1.8 times as long as broad (vs. 2.5 times as long as broad) (see Miya & Miyake 1969: figs. 1, 2B, C); from A. macellarius by the distolateral tooth of the antennal scaphocerite pointing straight-forward (vs. curved mesially in A. macellarius ), the much stouter minor chela (which is very slender in A. macellarius ), and the much stouter third pereiopod merus, about four times as long as wide (vs. almost six times in A. macellarius ) (see Chace 1988: fig. 6e, f, i); from A. fenneri by the distolateral tooth of the scaphocerite reaching far beyond the blade (vs. not or very slightly overreaching the blade in A. fenneri ), the mesial surface of the major and minor chelae not granulated (vs. distinctly granulated in A. fenneri ), and the posterior margin of the telson unarmed between the mesial pair of the posterolateral spiniform setae (vs. with a row of spiniform setae in A. fenneri ) (see Bruce 1994: figs. 1C, M, N, 2C); from A. mannarensis by the distolateral tooth of the scaphocerite reaching far beyond the blade (vs. not reaching the anterior margin of the blade in A. mannarensis ), the second pereiopod carpus with the first subarticle slightly longer than the second (vs. with the second subarticle noticeably longer than the first in A. mannarensis ), the posterior margin of the telson unarmed between the mesial pair of the posterolateral spiniform setae (vs. with a row of spiniform setae in A. mannarensis ) (see Purushothaman et al. 2021: figs. 2B, E, 3G); and from the above-described A. thompsoni sp. nov. by the relative development of the distolateral tooth of the scaphocerite (cf. Figs. 1A View FIGURE 1 , 4A View FIGURE 4 ), the length-width proportions of the telson (cf. Figs. 1C View FIGURE 1 , 4C View FIGURE 4 ), the much stouter third maxilliped, without extremely elongate setae on the pendultimate article (cf. Figs. 1E View FIGURE 1 , 4E View FIGURE 4 ), and the much stouter walking legs (cf. Figs. 1G, I View FIGURE 1 , 4G, H View FIGURE 4 ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Alpheus sciolii
| Anker, Arthur 2022 |
Alpheus aff. djiboutensis
| Hurt, C. & Hultgren, K. M. & Anker, A. & Lemmon, A. R. & Moriarty Lemmon, E. & Bracken-Grissom, H. 2021: 2 |
Alpheus sp. 2
| Minemizu, R. 2013: 101 |
Alpheus sp. 6
| Kuiter, R. H. & Debelius, H. 2009: 152 |
Alpheus sp.
| Debelius, H. 2001: 153 |