Ramisyllis multicaudata, Glasby & Schroeder & Aguado, 2012
|
publication ID |
https://doi.org/ 10.1111/j.1096-3642.2011.00800.x |
|
publication LSID |
lsid:zoobank.org:pub:630EEDBE-8D69-435C-BA8C-3B0425524255 |
|
DOI |
https://doi.org/10.5281/zenodo.10544481 |
|
persistent identifier |
https://treatment.plazi.org/id/EA147877-1605-48CC-839C-7EA512261874 |
|
taxon LSID |
lsid:zoobank.org:act:EA147877-1605-48CC-839C-7EA512261874 |
|
treatment provided by |
Marcus |
|
scientific name |
Ramisyllis multicaudata |
| status |
sp. nov. |
RAMISYLLIS MULTICAUDATA View in CoL SP. NOV.
( FIGS 1–9 View Figure 1 View Figure 2 View Figure 3 View Figure 4 View Figure 5 , 11 View Figure 11 ; TABLES 2, S 1, S 2)
Material examined: Holotype: Darwin Harbour , Channel Island (type locality), about 200 m north of bridge, 12°33.2′S, 130°52.4′E, coll. C. Glasby, 8 November 2006 (in two pieces, head end mounted for SEM and posterior end in ethanol; both NTM W23745) GoogleMaps . Paratypes: Darwin Harbour, Channel Island , same collection data as for holotype, one specimen ( LACM-AHF on SEM stub; number unavailable), one specimen ( NTM W23752), one specimen ( NTM W23750) GoogleMaps ; Darwin Harbour , same collection data except coll. C. & B. Glasby, 28 October 2007, one specimen ( NTM W23753), one specimen ( NTM W23749), one specimen ( NTM W23747), one specimen ( NTM W23746) GoogleMaps ; Channel Island, ‘ Town Hall’ , 12°33.74′S, 130°51.67′E, 10–20 m depth, coll. C. & B. Glasby, 9 September 2004, one specimen ( NTM W23748), one specimen ( NTM W23751) GoogleMaps ; Dudley Point , 12°24.87′S, 130°49.27′E, low water spring tide level, coll. B. Glasby, 30 September 2011, one specimen ( NTM W23790). Non-types (all lacking heads): Darwin Harbour, same collection data as holotype, NTM W23766 GoogleMaps ; Channel Island , same collection data, except coll. C. & B. Glasby, 28 October 2007, one specimen ( NTM W23767) GoogleMaps ; Channel Island, ‘ Town Hall’ , 12°33.74′S, 130°51.67′E, 10–20 m depth, coll. C. & B. Glasby, 21 September 2003, NTM W23760 GoogleMaps , coll. C. & B. Glasby, 9 September 2004 NTM W23759, NTM W23763 ; Channel Rock , 12°24.94′S, 130°47.04′E, 13 m, coll. B. Glasby, 5 September 2003, NTM W23761, NTM W23768 GoogleMaps ; Stevens Rock , 12°29.102′S, 130°47.111′E, 14 m, coll. B. Glasby & party, 7 May 2002, NTM W23757 GoogleMaps , coll. B. Glasby & party, 22 August 2003, NTM W23758, NTM W23762 ; Sandy Island (aka Crocodile Island ), 12°35.272′S, 130°52.262′E, 6 m, coll. C. & B. Glasby, 9 September 2004, NTM W23764 GoogleMaps ; Sandy Island , 12°35.272′S, 130°52.262′E, 6 m, coll. B. Glasby & R GoogleMaps . Willan , 5 June 2006, NTM W23765 . Bynoe Harbour, Spencer Point, near Indian Island , 12°35.48′S, 130°31.29′E, NTM W23754 GoogleMaps . Wessel Islands, eastern Arnhem Land, off Rimbija Island , 3.2 km west off Cape Wessel , 11°45.82′S, 136°43.65′E, NTM W23756 GoogleMaps . Wigram Island, the English Company Islands, eastern Arnhem Land , 11°00.41′S, 136°43.65′E, NTM W23755 GoogleMaps .
Comparative material: Syllis ramosa , syntype BMNH 1885 :12:1:150–154, Philippines, near Cebu, 95 fathoms, Challenger expedition; Japan, Sagami Bay , 100 fathoms, one specimen (NSM unreg.).
The direction of the first branch only is given, as subsequent ones alternate.
Etymology: Name derived from the Latin, multus for many, and caudata, feminine, for tailed.
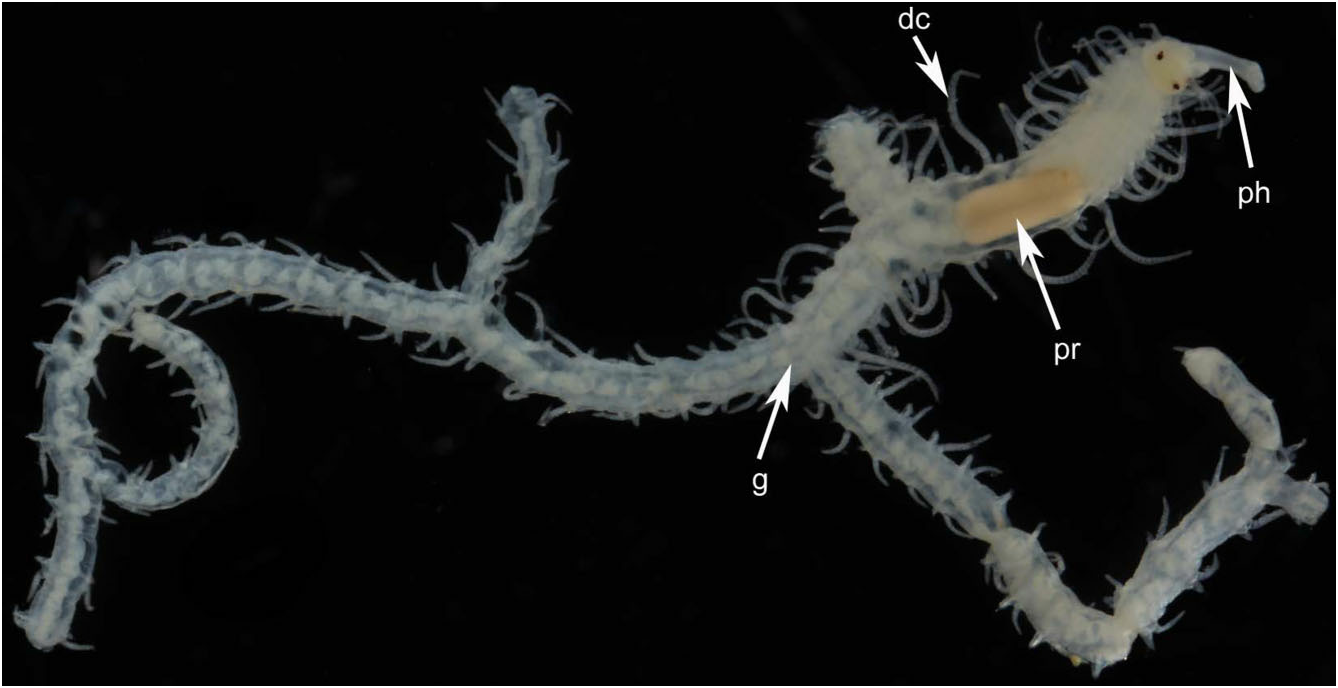
Description: All specimens incomplete. Body width about 1 mm throughout; length varies depending upon path taken to a particular posterior end, up to 3–4 cm when stretched out; segments of several distinct morphologies. Holotype with five orders of branching producing 2 n - 1 or 16 terminal branches, where n = 5 branch nodes (specimen incomplete); the complete worm therefore probably had hundreds, possibly thousands, of terminal branches. First branch appears on the left or right side between segments 14 and 20, and branching develops in individuals with as few as 26 segments ( Table 2; Figs 1 View Figure 1 and 2A–C View Figure 2 ).
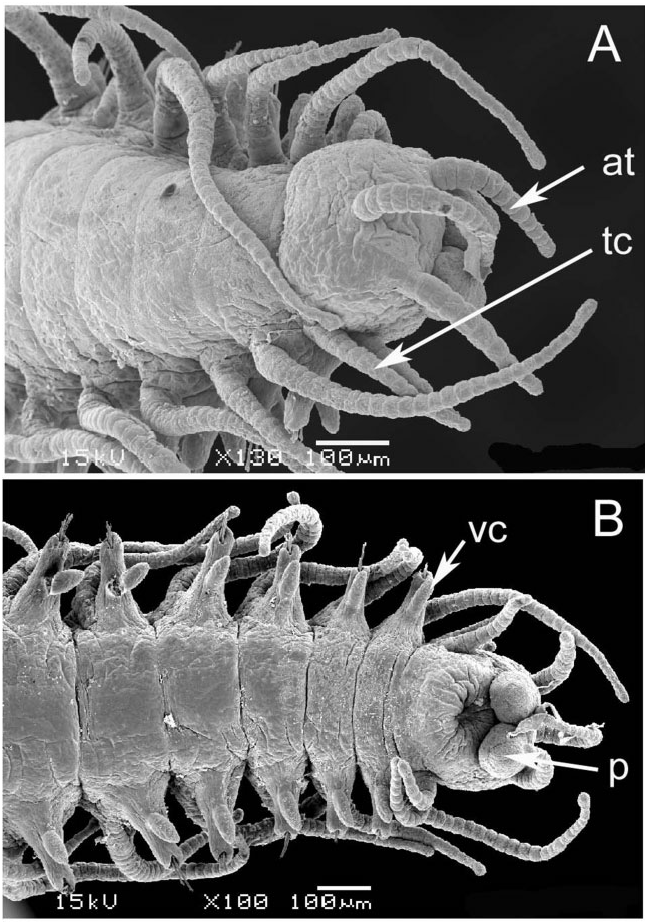
Prostomium with two pairs of eyes, anterior pair ventral and lateral to posterior pair; antennae articulated, of equal length, median one placed behind lateral ones; palps small, oval shaped, anteroventrally directed ( Fig. 3A, B View Figure 3 ). Tentacular cirri articulated, of equal length. Pharynx slender, about one-quarter the width of proventricle, tooth absent in adults, present in juveniles ( Fig. 4 View Figure 4 ). Proventricle prominent, barrel shaped, lying between chaetigers 12–14 in holotype ( Fig. 2A View Figure 2 ) and 11–17 (other material); it lies further forward (segments 3–5) in juveniles ( Figs 2C View Figure 2 and 4 View Figure 4 ). Segments anterior to first branch node have elongate dorsal cirri with 17–30 articles. Dorsal cirri slender anteriorly, showing slight length–position alternation; pattern may be altered in post-proventricle segments (after segment 11 in holotype), resulting in dorsal cirri of markedly different sizes being borne on the two sides of a single segment ( Fig. 5A, B View Figure 5 ); symmetrical alternation pattern can reappear posteriorly ( Fig. 5C View Figure 5 ). Pygidial cirri articulated, resembling smaller dorsal cirri of posterior segments ( Fig. 5C View Figure 5 ).
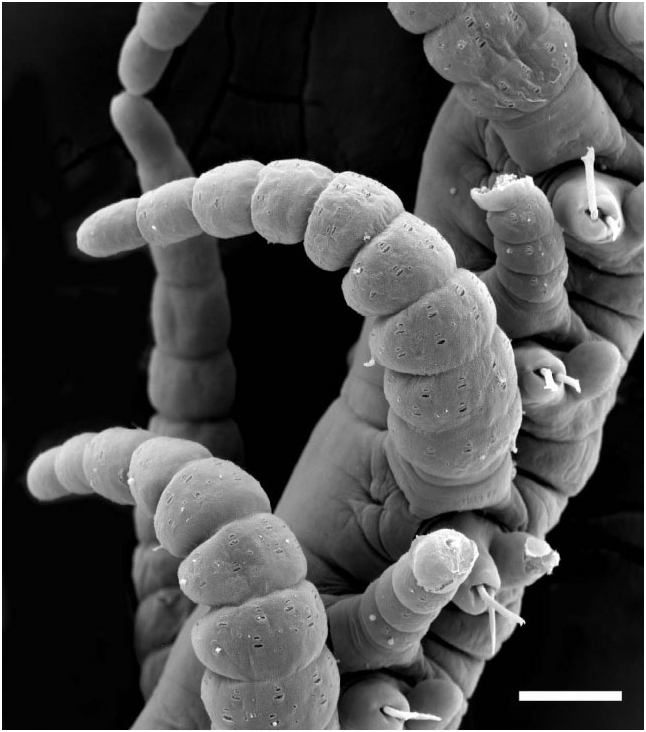
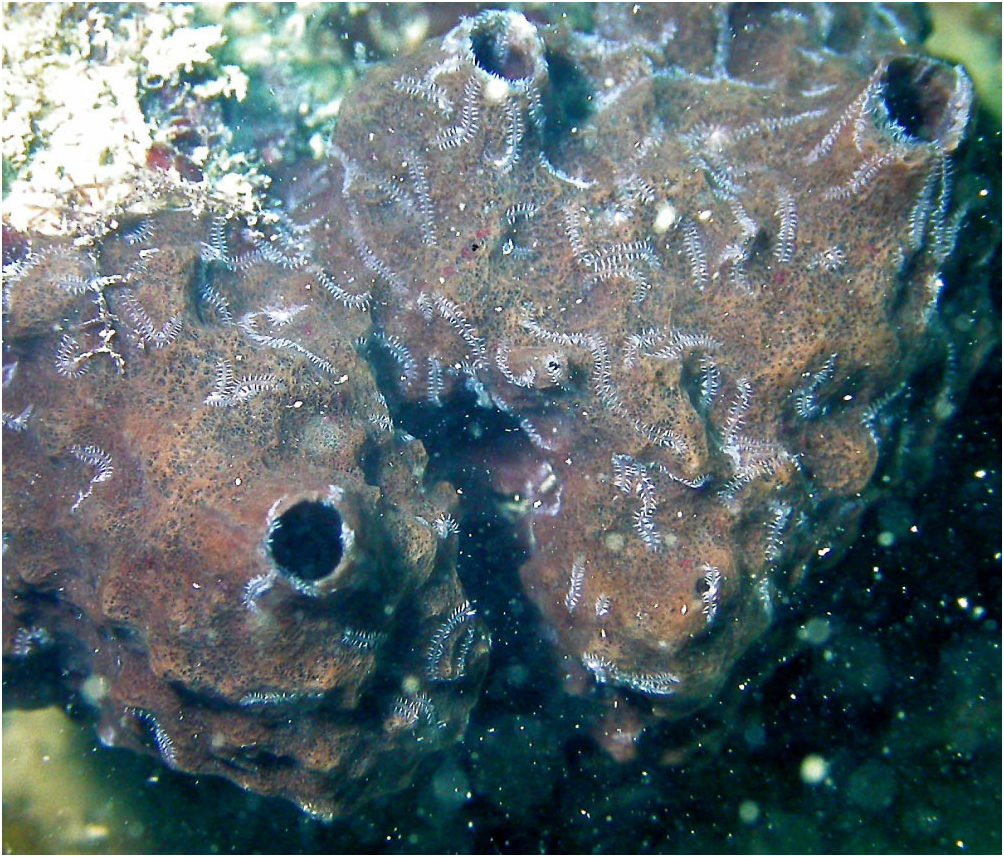
Segment form and branching pattern differ depending on location in host. Segments in internal regions of host highly variable in length (200–700 Mm; series of longer ones tending to span largest gaps in sponge tissue), largely devoid of pigment, dorsal cirri slender and short. First branch from segments 14–20 on right side (holotype) or left side (most other paratypes), and subsequent ones usually emerging on alternate sides of body ( Table 2); branches emerging more or less at right angles from between parapodium and anterior face of posterior septal wall of originating segment ( Fig. 6A, B). Posterior segments at or near surface of host shorter (about 100 Mm in length), bearing dorsal cirri that include large, highly pigmented, thick ones that arch over the dorsal surface in preserved specimens ( Fig. 7 View Figure 7 ), and are stretched horizontally on the surface of the host in life ( Fig. 11 View Figure 11 ), alternating with shorter unpigmented cirri; branching remains alternate, although with fewer segments between oped parapodia and musculature; the heads of both sexes have two pairs of well-developed dorsal (posterior) and ventral (anterior) eyes, and lack palps, antennae, and tentacular cirri ( Fig. 9 View Figure 9 ). The parapodia bear similar tomahawk-shaped chaetae as the parent stock, except that the angle and relative size of distal teeth differ slightly ( Fig. 8B View Figure 8 ). Regeneration of the posterior end (pygidium) of parent-stock stalks may occur before detachment of the stolon, as suggested by what appears to be minute developing anal cirri ( Fig. 9 View Figure 9 ). We are unsure about where fertilization takes place, the fate of the stolons (and stolon stalks), and the form of the larvae (no larvae were found in the sponges examined). The smallest juveniles found ranged in size from 2.2–2.5 mm in length and 28–29 segments; they were unbranched. The smallest branched specimen was 2.7 mm in length with 26 segments along the primary axis ( Fig. 2C View Figure 2 ; Table S2).
branches; branching pattern tending towards pinnate in stolon-bearing regions of body (see below).
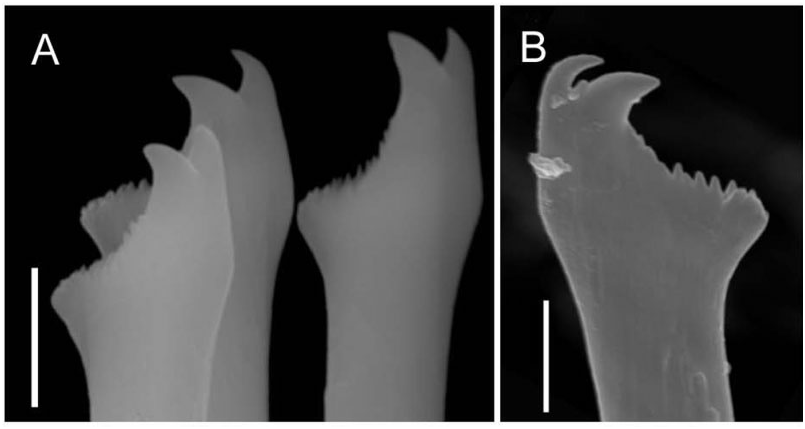
Each parapodium comprises an articulated dorsal cirrus, a small subconical unarticulated ventral cirrus, and slightly larger neuropodia, bearing two or three simple chaetae and a tapered, blunt-tipped acicula. Articles of most dorsal cirri covered with prominent arrays of pits and perforated plates ( Fig. 7 View Figure 7 ); they may occur in groups of three or four, up to about 24. Chaetae tomahawk shaped, bifid distally, prominent subdistal spur, and series of denticles between teeth and spur ( Fig. 8A View Figure 8 ); chaetal shape constant along body.
Reproduction: Male and female stolons are produced de novo as reproductive structures, which is typical of gemmiparous schizogamy. However, unlike typical gemmiparity, in which stolon segments originate from a proliferation zone immediately in front of the stolonal pygidium ( Garwood, 1991), the stolonal segments in R. multicaudata gen. et sp. nov. are produced at the end of a specialized short branch: the stolon stalk ( Fig. 2C View Figure 2 ). Stolon stalks have between six and 13 segments; they develop toward the external (posterior) segment region ( Fig. 2B View Figure 2 ), but are not observed to be exposed at surface in life; male stolons are similar in width to stalk, about half the width of egg-laden females, and have relatively better devel- Distribution and habitat: Coastal waters of the ‘Top End’ of northern Australia from low water spring tide level to at least 20 m; endosymbiont of Petrosia sp. (purple and white forms).
Remarks: At the generic level the new taxon is most similar to Parahaplosyllis Hartmann-Schröder 1990 , sharing with this taxon the presence of three antennae, small, oval-shaped palps that are free to the base, and tomahawk-shaped chaetae ( Table 3). It differs from this genus in the dendriform body, alternating form of the dorsal cirri, in lacking a trepan, and in adults a pharyngeal tooth, and in having only a single type of parapodial chaeta ( Parahaplosyllis has two); however, recent studies suggest that the presence or absence of a pharyngeal tooth is sometimes variable at the species level, as a result of ontogenetic loss ( Aguado & San Martín, 2009 and references therein). Further comparisons of key features between the new genus and other syllid genera having simple chaetae are shown in Table 3.
At the species level, R. multicaudata gen. et sp. nov. bears an obvious resemblance to S. ramosa , especially in the dendriform body and the asymmetrical pattern of the dorsal cirri. The new taxon may be distinguished from previously described forms of S. ramosa in coloration, parapodial and chaetal morphology, and in having a greater diversity of segment types, as discussed below. However, it is likely that the name S. ramosa encompasses more than one species.
Illustrations of the type material of S. ramosa contain a number of enigmatic features ( McIntosh, 1885: plates 34A and 8, the head) compared with the typical features of the genus Syllis . Most strikingly, the illustration shows no sign of a proventricle, usually considered to be a defining feature of the Syllidae ( Aguado & San Martín, 2009) ; nor is the median antenna, typical of many genera, including the genus Syllis , indicated. Palps are not illustrated and the text suggests that they may be vestigial (‘a minute and flattened lobe appears and it is possible that this is the homologue of the palpus’). Finally, neither figure nor text is clear on whether or not the four eyes typical of many syllids, including Syllis , are present. Izuka’s (1912) description of the head of a Japanese specimen does not extend to the proventricle, of which no mention is made, but indicates that the head is that of a typical sylline syllid, including two pairs of eyes, and elongate, conical palps, which differ from the short ovoid palps of the new species and from McIntosh’s illustration. As the S. ramosa material we received from Japan did not include a head, we cannot add to the description of the head nor confirm the accuracy of McIntosh’s observations. The longer dorsal cirri of McIntosh’s S. ramosa contain about 26 articles (28 in the Japanese specimens: Izuka, 1912), and the shorter dorsal cirri contain about 15 (both reports): many more than the cirri of the new species ( Table S1). Furthermore, the chaetae of S. ramosa are more slender, hooked at the tip, and have a distinct articulation, compared with the simple, robust, tomahawk-shaped ones of R. multicaudata gen. et sp. nov.
Oka (1895) reported that, in Japanese material, branches form on an intercalary segment that appears between two existing segments, and grow out from this on both sides of the worm. McIntosh (1885) illustrated no examples of this type and explicitly stated that it was not evident in his material. He reports that branches may arise by the replacement of one parapodial dorsal cirrus with a zone of cell proliferation, which ultimately develops into a new branch of the worm. This form of development is also sketched and discussed by Okada (1937). We have not observed this process in any of our material: branching in R. multicaudata gen. et sp. nov. clearly occurs as an outgrowth of the body wall behind a parapodium; no dorsal cirri are involved ( Fig. 6B) and no paired branches have been observed. Furthermore, the ‘vestigial’ palps described by McIntosh differ significantly from the more normal palps illustrated by Izuka (1912) from Japanese material.
Okada (1937) notes that in his Japanese S. ramosa the stolon stalk is composed of a variable number of segments, all of which are typical in their structure. They thus differ from McIntosh’s (1885: pl. 33, Fig. 11 View Figure 11 ) description of the type material, in which the stolon stalks are formed of four segments with parapodia either incompletely developed or (in the two segments closest to the body) absent. Okada expresses doubt that the condition described by McIntosh actually occurs; McIntosh does not report how many such stalk–stolon combinations he observed. The segments of comparable stalks of R. multicaudata gen. et sp. nov. all contain well-developed parapodia.
The single photo published by Read (2001) of a headless portion of a branching worm collected from deep water off New Zealand looks heavier bodied than the other S. ramosa . Read observed that many stolons were present, but free of the stalks and ‘congregated in the hollow tube that forms the basal stalk of the sponge’, and that their mode of egress from this location in the sponge is not apparent. The New Zealand specimens formed a reddish pigmented mass. Japanese specimens were also reported to be red in life ( Imajima, 1966). Both the New Zealand and Japanese specimens were found in a species of the hexactinellid sponge Crateromorpha .
PHYLOGENETIC POSITION INFERRED FROM
| NTM |
Northern Territory Museum of Arts and Sciences |
| R |
Departamento de Geologia, Universidad de Chile |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |