Myotis soror, Ruedi, Manuel, Csorba, Gábor, Lin, Liang- Kong & Chou, Cheng-Han, 2015
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3920.2.6 |
|
publication LSID |
lsid:zoobank.org:pub:8B991675-0C48-40D4-87D2-DACA524D17C2 |
|
DOI |
https://doi.org/10.5281/zenodo.3860404 |
|
persistent identifier |
https://treatment.plazi.org/id/03BB8796-3D51-5D24-A8EB-F2BF1748FC32 |
|
treatment provided by |
Plazi |
|
scientific name |
Myotis soror |
| status |
|
Myotis frater Allen, 1923 View in CoL
Synonymy. Myotis frater Allen, 1923 . Type locality Yenping, Fukien Province, China. Myotis sp. 3: Lin et al. 2004. Vernacular, unavailable name.
Myotis sp. 3: Cheng et al. 2010. Vernacular, unavailable name.
Myotis sp. 3: Ruedi et al. 2013. Vernacular, unavailable name.
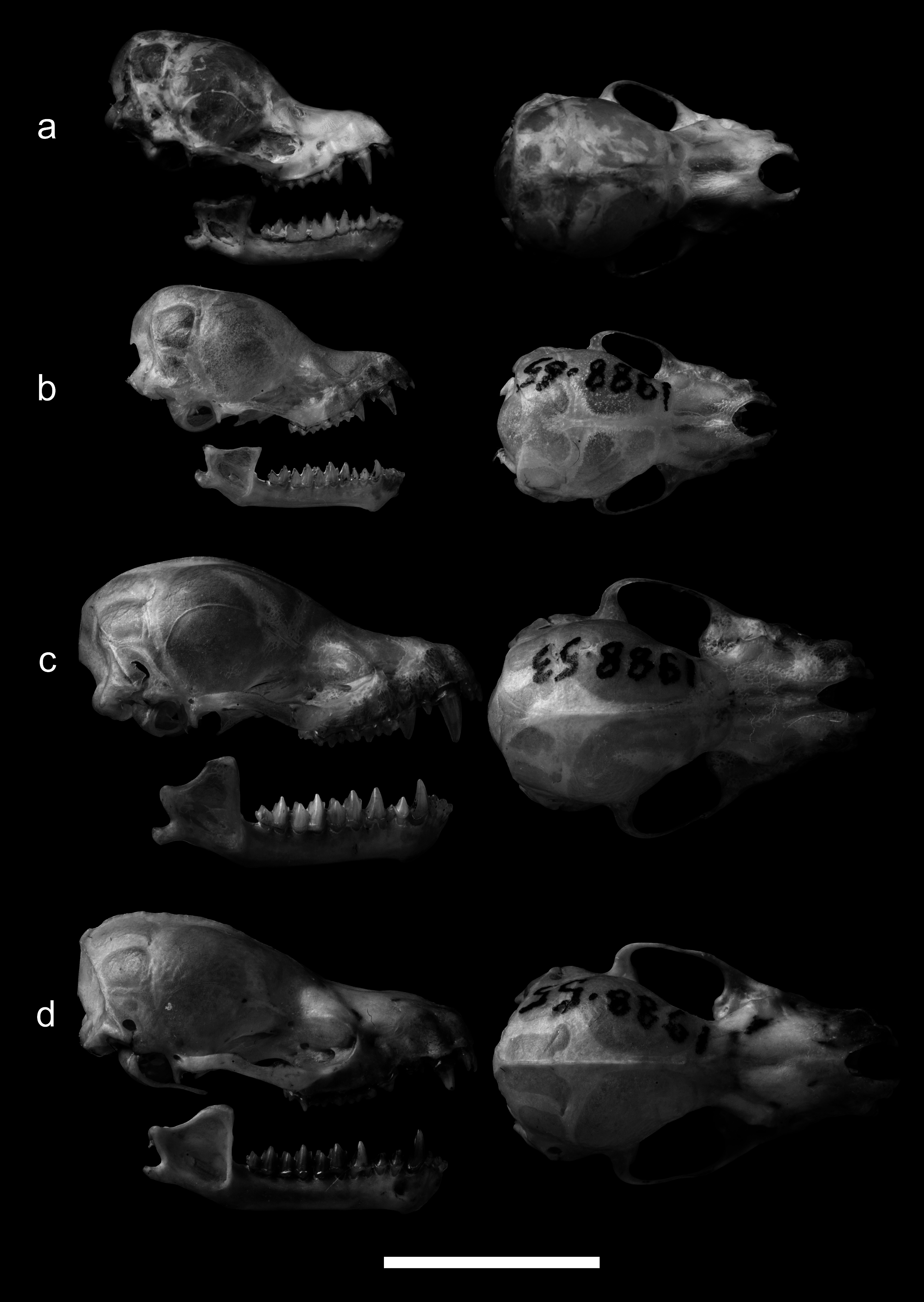
Taxonomic remarks. The angular skull, short toothrow and displaced upper middle premolar differentiate the taxa of the frater complex from other Myotis species ( Tsytsulina & Strelkov 2001), but this group is morphologically a polytypic species complex comprising various forms, with variation in skull profiles and in extension of displacement of the second upper premolar. Tsytsulina and Strelkov (2001) separated the larger, pale desert form isolated in western Asia as a distinct species, M. bucharensis Kuzyakin, 1950 . They commented on the taxonomy of the other, more boreal forms ( longicaudatus , kaguyae and eniseensis), but did not compare them with material from the type locality in SE China, where M. frater s. s. was originally described by Allen (1923). Morphological comparisons of skulls ( Figs. 9 View FIGURE 9 b, 9c) indicate that M. frater from Taiwan are very similar to the type of frater from adjacent China and thus represent the same species. Another specimen, described here as a new species in Taiwan, M. soror sp. n., also belongs to the frater species complex, but differs in external and cranial morphology ( Figs. 8 View FIGURE 8 a, 8b, 9) and is genetically divergent from its congeners ( Fig. 3; Table 5 View TABLE 5 ). Previous molecular analyses ( Kruskop et al. 2012) further showed that the subspecies eniseensis and longicaudatus , although geographically isolated and morphologically separable, differ only slightly at the mitochondrial COI gene (about 2%), suggesting that both belong to a single boreal species. Our phylogenetic reconstructions ( Fig. 3) further indicate that specimens from Manchuria (i.e., representing longicaudatus ) and Hokkaido (i.e., representing kaguyae ) also differ minimally from each other at another mitochondrial gene (less than 1% at Cyt b), again suggesting that all three taxa living in boreal and temperate forests are subspecies of a single species. These molecular data further suggest that bats living in more tropical regions (e.g., those sampled in Taiwan and nearby China) differ markedly in their Cyt b gene sequences (>13%) and in the size of the baculum ( Tsytsulina & Strelkov 2001), and thus are clearly distinct from all other boreal taxa ( Fig. 3). The genetic differences are comparable to those separating the two sympatric species in Taiwan ( M. soror sp. n. and M. frater , 11.4%; Table 5 View TABLE 5 ). Besides M. soror sp. n., we thus recommend treating M. frater s. s. from Taiwan and adjacent China as a species distinct from the other, more temperate taxa in this group. According to this new taxonomic arrangement and to designate the boreal-temperate species, M. longicaudatus Ognev, 1927 is the oldest available name, which has priority over kaguyae Imaizumi, 1956 and eniseensis Tsytsulina and Strelkov, 2001.
Owing to relatively important morphological variability of this taxon in the Japanese archipelago, more comprehensive genetic sampling across Hokkaido and Honshu is still needed to confirm that a single taxon is living throughout this archipelago.
Distribution. The known distribution of M. frater s. s. as understood here is restricted to Fujian Province (formerly Fukien) in eastern China, and Taiwan.
Measurements. See Table 4.
External morphology. Medium sized bat with a dense, soft pelage that extends well over the underparts of the tail and wing membranes, up to the elbows and around the anal region. The dorsal color is dark brown, without gloss, whereas the underparts are lighter, creamy brown, most of the hair base being darker brown. The wing membranes are broad and attached near the base of the outer toe, at the distal end of the metacarpus (see illustration in Cheng et al. 2010). Feet are relatively small, much shorter than half tibia length. Ears are short, relatively angular in shape, the front edge being vertical to three-quarters of it length, then bending sharply backwards at an angle of 45°; the rear edge has a distinct notch at mid-height; the inner side of the conch along the front edge is hairy, especially at its base, where long hairs form a comb-like structure; the outer surface of the ear is naked (see illustration in Cheng et al. 2010), unlike in M. soror sp. n. The calcar is long (half of the free edge of the uropatagium), with a narrow but unkeeled lobe. The uropatagium is particularly large, and is sparsely haired on the underside, near the anal region. The tragus is relatively long (longer than notch height), nearly straight but bending forwards and lacking a distal lobe. The external morphology resembles that of M. soror sp. n., except for color (richer cinnamon and frosted aspect of fur in the later), but differs from this new species by having longer tibia (20 mm instead of 17 mm) and slightly longer tail (about the same as head and body length, instead of being shorter in M. soror sp. n.).
Skull morphology. The outlines of the skull viewed from above are angular, fitting almost in a cube. In profile, the short rostrum, abruptly raised frontal part of the braincase and angular occipital region are characteristic of the M. frater complex ( Fig. 9 View FIGURE 9 ). The upper canines are strong and bear a typical, deep groove along their labial edge. The incisors are short but robust. The second upper premolar is displaced inwards and invisible in profile (see the type of frater , Fig. 9 View FIGURE 9 b), but the extent of this intrusion is variable within the same population.
Natural history. In Taiwan, this forest dwelling bat is widely distributed, from low to higher elevations. It was found roosting in tree holes, but never in caves. Lactating females were found from June to August, depending on altitude. Males started showing enlarged testis in August. In Yenping (Fujian) three specimens (including the type) were taken in holes of live bamboo stems in a mountain area at an altitude of about 1000 m ( Allen 1923).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |