Oligobregma cruzae, Mendes & Paiva & Rizzo, 2024
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5424.1.4 |
|
publication LSID |
lsid:zoobank.org:pub:3DF7F8AA-BA4C-4C78-BB1E-450537B79CB6 |
|
DOI |
https://doi.org/10.5281/zenodo.10815158 |
|
persistent identifier |
https://treatment.plazi.org/id/03B887F7-3E02-6F78-F884-B1AF07F7FAC6 |
|
treatment provided by |
Plazi |
|
scientific name |
Oligobregma cruzae |
| status |
sp. nov. |
Oligobregma cruzae sp. nov.
https://zoobank.org/NomenclaturalActs/ 25783CDF-42E1-4126-98E7-8941C05A629C
Figures 4 View FIGURE 4 & 5 View FIGURE 5
Type material. UERJ8740 (Holotype; SANSED1 ; C7 R2 ; Lat: -25.033700, Long: -46.887400; 20 Jun 2019; 688 meters deep) . UERJ8233 (Paratype; SANSED1 ; B7 R1 ; Lat: -26.750800; Long: -46.074300; 18 Jun 2019; 700 meters deep) .
Description. Holotype complete, with 32 chaetigers, measuring 5 mm long, 1 mm wide on its expanded region and 0.25 mm wide on its narrowest region. Moderate sized species, Paratype measuring 6 mm long, 2 mm wide on expanded region and 0.5 wide on narrowest region, for 29 chaetigerous segments.
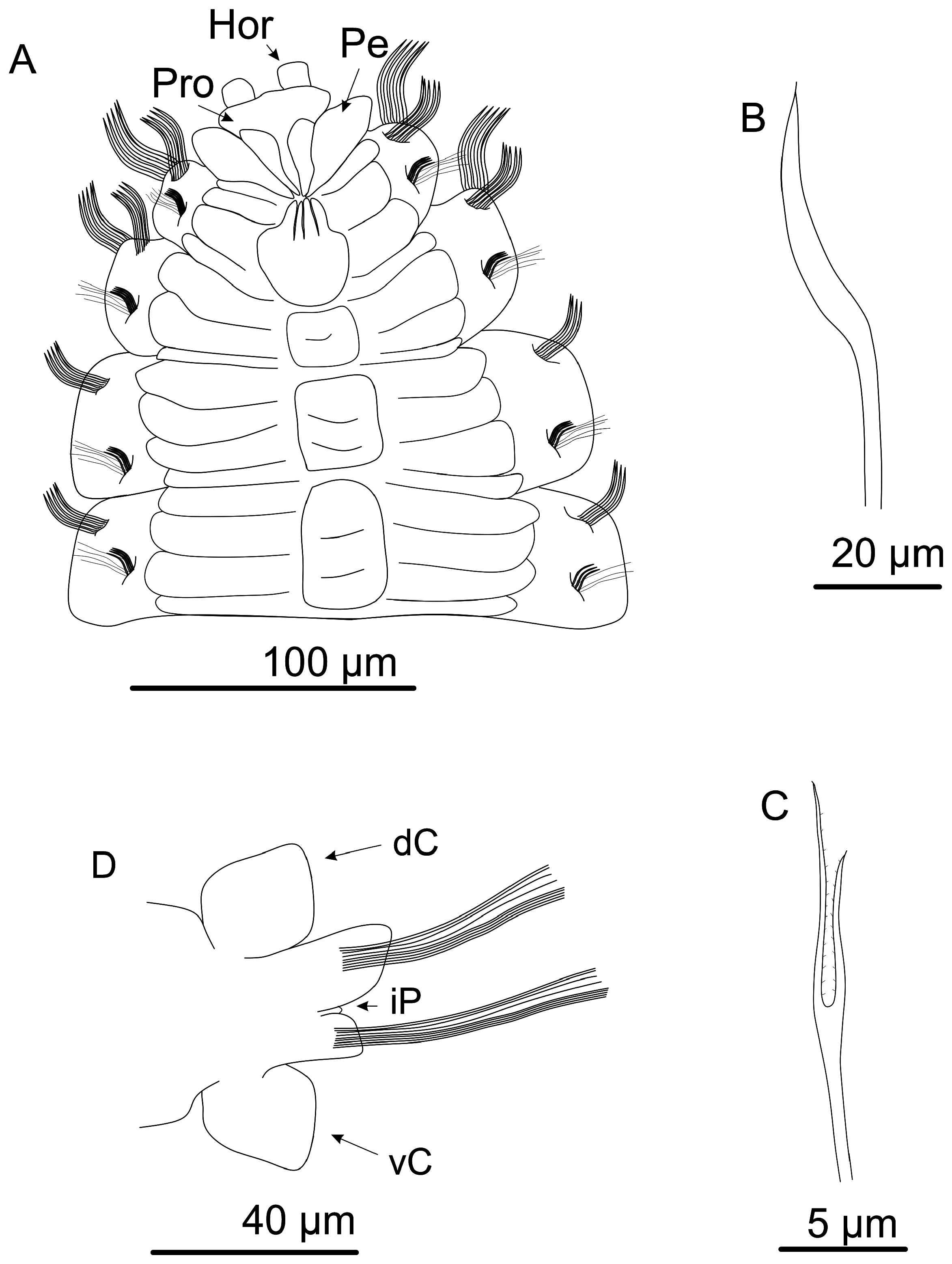
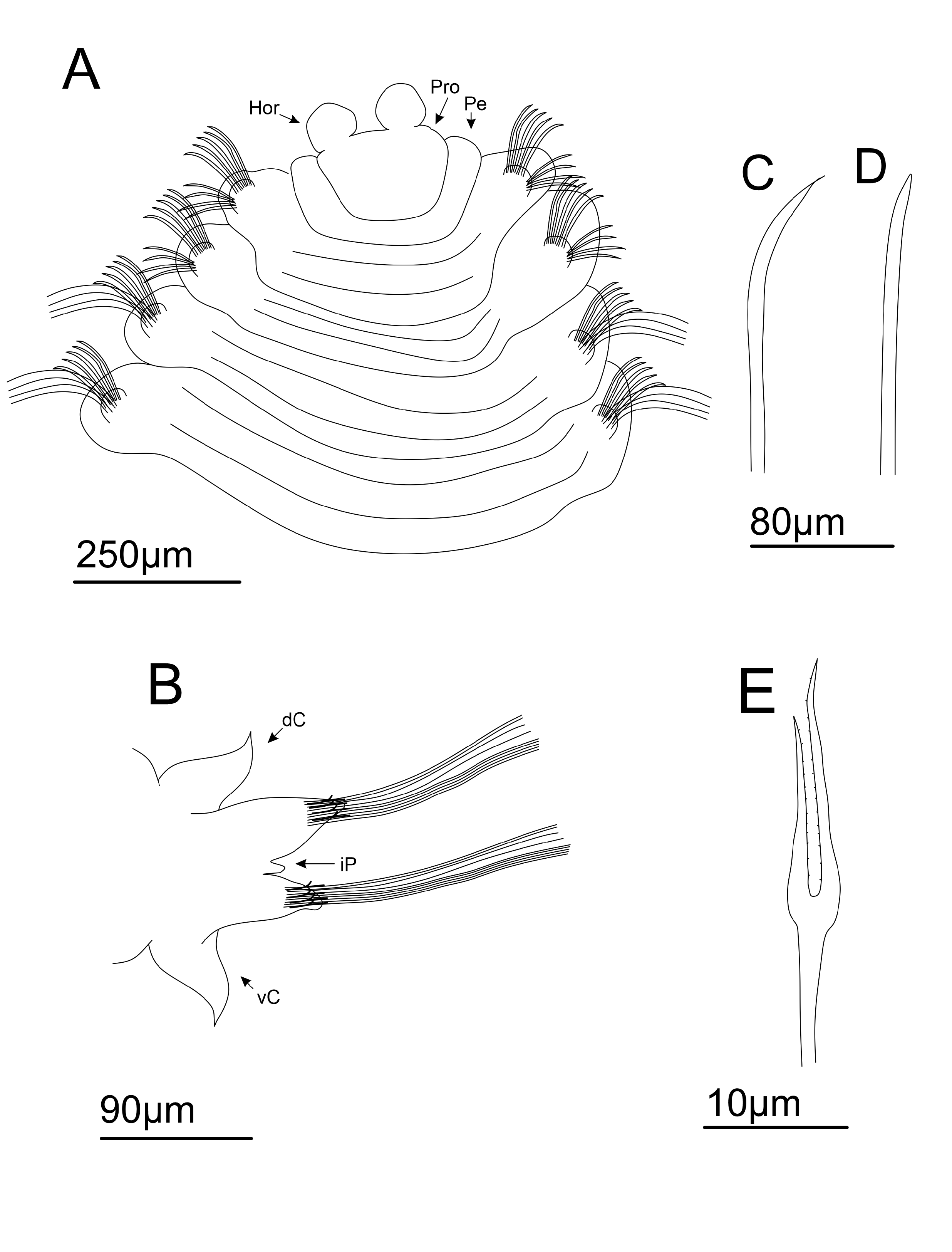
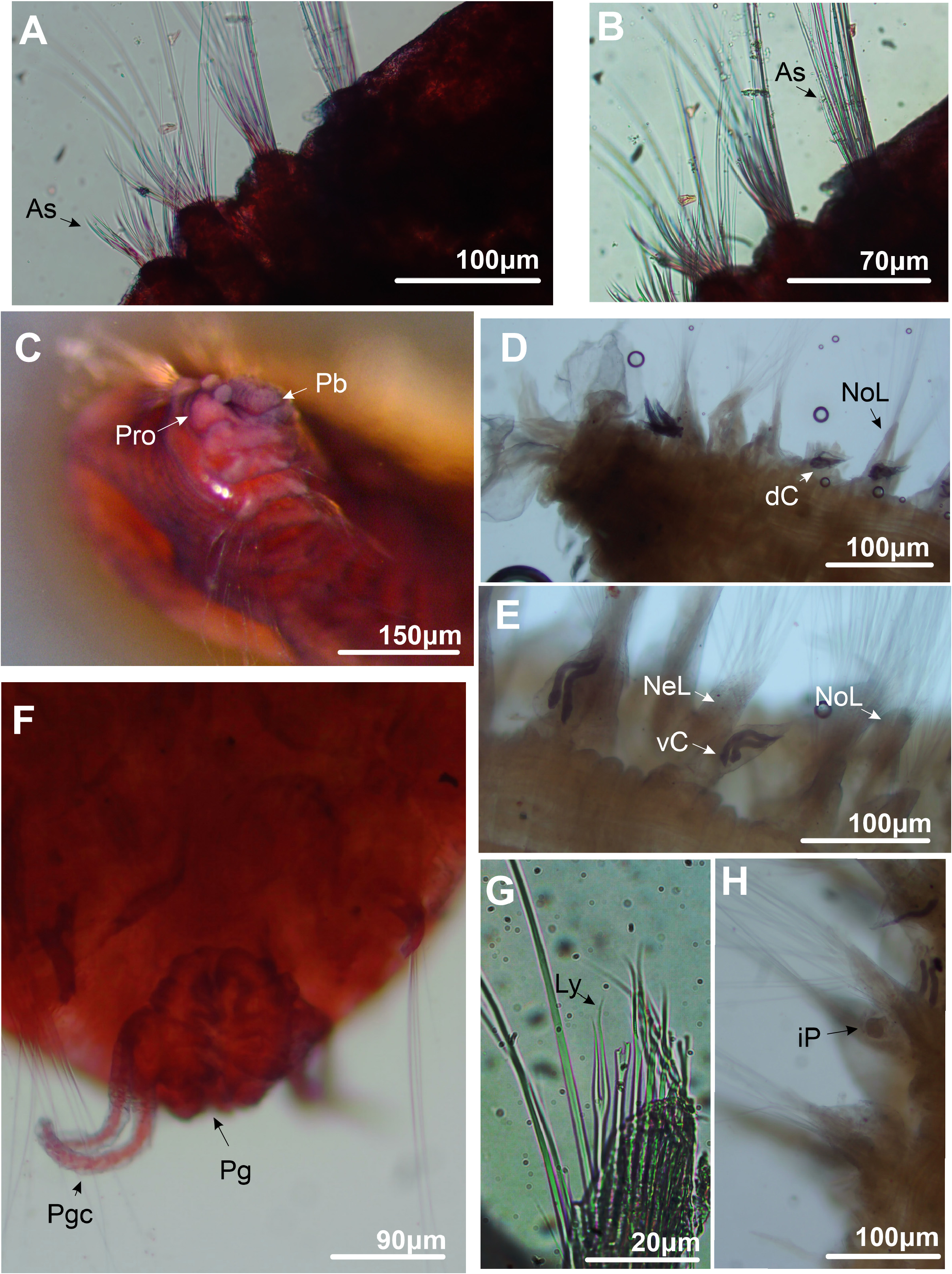
4. C. Anterior body in dorso-lateral view. D. Posterior end of the body in dorsal view. E. Mid body chaetigers in ventral view. F. Pygidium in dorsal view. G. Lyrate chaeta. H. Interramal papillae from midbody chaetiger in ventral view. Abbreviations: As = Acicular spine; dC = Dorsal cirrus; iP = Interramal papillae; Ly = Lyrate chaeta; Nel = Neuropodial lobe; Nol = Notopodial Lobe; Pb = Proboscis; Pro = Prostomium; Pgc = Pigidial cirrus; Pg = Pygidium; vC= Ventral cirrus.
Body arenicoliform, expanded over chaetiger 3–12. Colour in alcohol white to yellowish. Body surface covered by secondarily annulated rings. Secondary annuli smooth in both anterior and posterior regions.
Quadrangular prostomium, with two short, rounded horns, both projected upwards ( Figs 4A View FIGURE 4 ; 5C View FIGURE 5 ). Eyes absent. Nuchal organs not observed. Peristomium achaetous, uniannulated dorsally and ventrally ( Figs 4A View FIGURE 4 ; 5C View FIGURE 5 ). Proboscis crenulated ( Fig 5C View FIGURE 5 ). Ventral groove present from chaetiger 2 ( Figs 4A View FIGURE 4 ; 5C View FIGURE 5 ). Quadrangular uniannulated pads from chaetiger 2, forming a ventral mid-ridge up to end of body ( Fig. 4A View FIGURE 4 ). Each pad paired to a single chaetiger. Posterior pads smaller and narrower than anterior ones.
Dorsally, chaetiger 1–10 present one annulus connected to notopodial lobe, plus one superior and inferior annuli delimitating segment; from chaetiger 10 throughout, two secondary annuli connected to notopodial lobes, plus one superior and inferior annuli delimitating segment. Ventrally, chaetigers 1–3 with one annulus connected to neuropodial lobe, plus one superior and inferior annuli delimitating the segment. From chaetiger 4 throughout, two annuli connected to neuropodial lobe, plus one superior and inferior annuli delimitating segment.
Dorsal and ventral cirri present from chaetiger 14 ( Fig. 4B View FIGURE 4 ). Interramal papillae present ( Fig. 5H View FIGURE 5 ); rounded, in midbody, to “finger-like”, in posterior body, filled with granular content ( Fig. 5H View FIGURE 5 ). Dorsal and ventral cirri triangular to lanceolate ( Figs 1B View FIGURE 1 ; 5D–E View FIGURE 5 ), reaching their larger size on midbody chaetigers, becoming thinner on posterior chaetigers. Strong black tubular glands may be present in all cirriferous chaetigers; sparsed and less numerous initially ( Fig. 5E View FIGURE 5 ), then filling all internal space posteriorly ( Fig. 5D View FIGURE 5 ).
Parapodial lobes reduced in anterior chaetigers, becoming evident from midbody to posterior chaetigers ( Figs 4B View FIGURE 4 ; 5D–E View FIGURE 5 ). Notopodial and neuropodial lobes of same length on anterior to midbody chaetigers. From midbody chaetigers, notopodial lobe sometimes reaching a longer length than neuropodial lobe; emerging as a broad basis transitioning to a broad rounded tip; neuropodial lobe asymmetrical, emerging as a thin basis abruptly transitioning to a pointed tip on its dorsal face, forming a “knife-like” structure ( Fig. 5E View FIGURE 5 ). On posterior-most chaetigers, notopodial and neuropodial lobes with similar shape and length; both asymmetrical, emerging as a thinner projection transitioning abruptly to a pointed tip ( Fig. 4B View FIGURE 4 ); with transition occurring on ventral side of notopodial lobe and dorsal side of neuropodial lobe, both forming a pointed structure. At end of body, parapodial cirri and lobes reduce their size and become thinner ( Fig. 5D View FIGURE 5 ).
Acicular spines present from chaetiger 1–4 on noto and neuropodia ( Figs 4A, C–D View FIGURE 4 ; 5A–B View FIGURE 5 ). On notopodia, chaetigers 1–2 present two anterior rows of strong curved acicular spines ( Figs 4C View FIGURE 4 ; 5A View FIGURE 5 ), plus a posterior row of capillary chaetae; numbering eight spines on anterior row and 7–8 spines on posterior row. Chaetiger 3–4 present one anterior row of eight straight and weaker acicular spines, plus two posterior rows of capillaries ( Figs 4D View FIGURE 4 ; 5B View FIGURE 5 ). On neuropodia, chaetiger 1 with two rows of five straight acicular spines, plus a posterior row of capillary chaetae. Chaetigers 2–4 with one row of five straight acicular spines, weaker than chaetiger 1, plus two rows of capillary chaetae. Acicular spines on noto and neuropodia of chaetiger 4 transitional ( Fig. 5B View FIGURE 5 ).
Short spinous chaetae absent. Lyrate chaetae present on neuropodia from chaetiger 5 ( Fig 4E View FIGURE 4 ; 5G View FIGURE 5 ), numbering six in each fascicle on anterior chaetigers, reaching 11 in number on midbody chaetigers, then reach 5–6 in number on posterior chaetigers; always with unequal tynes (tyne’s ratio = 1.2), grouped in a single row anterior to capillaries. Capillaries organized in one row on chaetigers 1–2, then two rows from chaetiger 3 up to posterior chaetigers. Pygidium uniannulated, with a short terminal margin ( Fig. 5F View FIGURE 5 ); terminal margin lobated, with two pairs of pygidial cirri, first pair projected dorso-laterally and following pair projected ventro-laterally. Pygidial cirri long and thin ( Fig. 5F View FIGURE 5 ).
Remarks. Oligobregma cruzae sp. nov. is morphologically similar to O. bathyala Blake, 2023 , O. brasierae Wiklund, Neal, Glover, Drennan, Rabone & Dahlgren, 2019 , O. lonchochaeta Detinova, 1985 , O. quadrispinosa Schüller & Hilbig, 2007 , O. tani Wiklund, Neal, Glover, Drennan, Rabone & Dahlgren, 2019 and O. whaleyi Wiklund, Neal, Glover, Drennan, Rabone & Dahlgren, 2019 by the presence of acicular spines from chaetigers 1–4 on notopodia, lyrate chaetae with unequal tynes and conspicuous rounded horns on prostomium. However, they differ in other relevant aspects as discussed below.
The parapodial glands nature in O. bathyala resembles O. mucronata and O. cruzae sp. nov., by being strong and tubular. Oligobregma brasierae and O. whaleyi also present internal glands within parapodial cirri, but they are distributed in small patches, with a gold coloration; differing from O. bathyala , O. mucronata and O. cruzae sp. nov. Oligobregma tani presents conical cirri, always smaller than parapodial lobes, differing from O. bathyala , O. brasierae , O. quadrispinosa , O. whaleyi and O. cruzae sp. nov. Moreover, the parapodial glands nature are still unknown in O. tani .
Oligobregma bathyala presents asymmetrical, “nipple-like” triangular parapodial cirri, terminating in a rounded tip. In contrast, O. cruzae sp. nov. presents a more elevated triangular to lanceolate cirri, not asymmetrical, like O. mucronata . Oligobregma bathyala differs from O. mucronata and O. cruzae sp. nov. by lacking acicular spines on neuropodia of chaetiger 4. Importantly, although O. mucronata shares some similarities with O. cruzae sp. nov. on parapodial glands, prostomium and parapodial cirri format, this species belongs to a different subgroup within Oligobregma , with species presenting acicular spines only from chaetigers 1–3, whereas O. cruzae sp. nov. belongs to the subgroup with acicular spines present from chaetiger 1–4.
Oligobregma bathyala , O. whaleyi and O. cruzae sp. nov. present more than 10 lyrate chaetae on body chaetigers. However, O. whaleyi is the most dissimilar among them, with lyrate chaetae emerging from chaetiger 11, whereas they emerge in O. bathyala from chaetiger 4–5 and in O. cruzae sp. nov. from chaetiger 5. Finally, O. bathyala presents short spinous chaetae on notopodia of chaetigers 2–3. This type of chaetae is absent in O. brasierae , O. quadrispinosa , O. tani , O. whaleyi and O. cruzae sp. nov.
Oligobregma bathyala , O. whaleyi and O. cruzae sp. nov. present more than 10 lyrate chaetae on body chaetigers. However, O. whaleyi is the most dissimilar among them, with lyrate chaetae emerging from chaetiger 11, whereas they emerge in O. bathyala from chaetiger 4–5 and in O. cruzae sp. nov. from chaetiger 5. Finally, O. bathyala presents short spinous chaetae on notopodia of chaetigers 2
Regarding O. lonchochaeta , it is possible to highlight some differences in comparison to O. cruzae sp. nov., related to the emergence order of lyrate chaetae and body annulation. In O. cruzae sp. nov., the lyrate chaetae emerge from chaetiger 5 and the body has one annulus connected to notopodial lobe, plus one superior and inferior annuli delimitating the segment from chaetigers 1–10; whereas in O. lonchochaeta the lyrate chaetae emerge from midbody chaetigers and the body is triannulated on chaetigers 1–4.
Distribution. O. cruzae sp. nov. was collected living on continental slope of Santos oceanographic basin, from a depth range of 688–700 meters deep, in muddy sediment.
Etymology. The specific epithet “ cruzae ” was chosen to honor the arachnologist Dr.Amanda Cruz Mendes from Rio de Janeiro State University (UERJ) and National Museum of Brazil (MNRJ). For her important contributions to Brazilian Arachnology, to the formation of biologists at UERJ, and to celebrate our friendship.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.