Caecilia wilkinsoni, Fernández-Roldán & Lynch, 2023
|
publication ID |
https://doi.org/10.11646/zootaxa.5270.2.2 |
|
publication LSID |
lsid:zoobank.org:pub:8CDE156F-86BC-4EF8-B812-C51C70A431DA |
|
DOI |
https://doi.org/10.5281/zenodo.7864560 |
|
persistent identifier |
https://treatment.plazi.org/id/03B887B7-FF82-8D7B-81CB-1C917FF1FD17 |
|
treatment provided by |
Plazi |
|
scientific name |
Caecilia wilkinsoni |
| status |
sp. nov. |
Caecilia wilkinsoni sp. nov.
Figs. 1–6 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 ; Table 1 View TABLE 1 .
Caecilia tenuissima Taylor, 1973 View in CoL : Lynch (2000: 329)
{ urn:lsid:zoobank.org:pub:8CDE156F-86BC-4EF8-B812-C51C70A431DA }
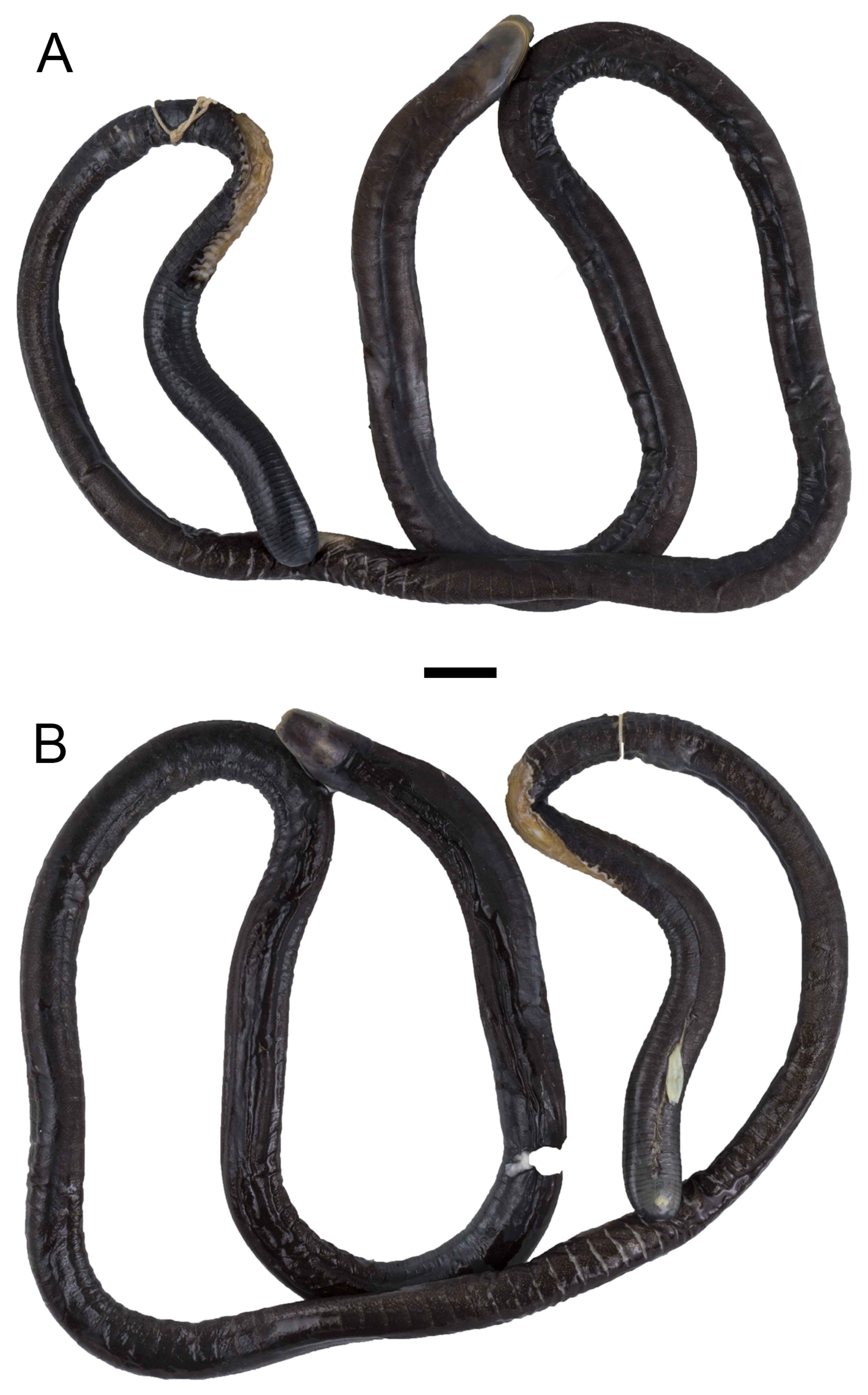
Holotype. ICN 58477, an adult male collected by Gladys Cárdenas-Arévalo and Rafael Ángel Moreno Arias at Quebrada El Hojal, Consejo Comunitario Unión del Río Rosario , vereda La Chorrera, Tumaco, Nariño, Colombia, 6 April 2006. 1° 45’ 52’’ N, 78° 45’ 56’’ W, 14 m. a.s.l. ( Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 ). GoogleMaps
Paratype. UVC 7686, an adult female obtained by Liliana Peláez at Estero San Antonio, corregimiento Flor de la Brisa, vereda Robles, municipio El Charco, Nariño, Colombia, 8 October 1984. 2° 26’ 48.1’’ N, 78° 11’ 44.5’’ W, 10 m. a.s.l. ( Figs. 4–6 View FIGURE 4 View FIGURE 5 View FIGURE 6 ) GoogleMaps .
Distribution. Currently Caecilia wilkinsoni sp. nov. is known only from the holotype and paratype localities of southwestern Colombia in the Pacific lowlands of the Litoral Pacífico, in Tumaco, and from Estero San Carlos, El Charco, Nariño, between 10– 14 m.a.s.l.
Diagnosis. Caecilia wilkinsoni sp. nov. differs from C. atelolepis , C. attenuata Taylor, 1968 , C. caribea , C. corpulenta Taylor, 1968 , C. crassisquama Taylor, 1968 , C. degenerata , C. guntheri , C. inca Taylor, 1973 , C. macrodonta , C. occidentalis , C. orientalis , C. pachynema (Günther, 1859) , C. pulchraserrana , and C. subdermalis in having (vs lacking) secondary grooves.
Caecilia wilkinsoni sp. nov. differs from congeners that have secondary annular grooves as follows. Caecilia disossea Taylor, 1968 (216–262 primary grooves and 16–34 secondary grooves) is the only member of the genus that has more primary and fewer secondary grooves than C. wilkinsoni sp. nov. (190–195 primary grooves and 51– 71 secondary grooves). Caecilia aprix also has more primary grooves (234) but an overlapping secondary groove count (58) with the new species. Caecilia bokermanni (180–192 primary grooves and 15–21 secondary grooves), C. gracilis Shaw, 1802 (183–204 primary grooves and 11–21 secondary grooves), C. occidentalis (186–221 primary grooves and 0–15 secondary grooves), and C. thompsoni (187–240 primary grooves and 26–42 secondary grooves) have overlapping counts of primary grooves and fewer secondary grooves than the new species.
Caecilia wilkinsoni sp. nov. has more primary annular grooves than C. abitaguae Dunn, 1942 (137–148 primary grooves and 0–5 secondary grooves), C. albiventris Daudin, 1803 (144–147 primary grooves and 45–53 secondary grooves), C. antioquiaensis (171 primary grooves and 4 secondary grooves), C. armata Dunn, 1942 (186 primary grooves and 92 secondary grooves), C. dunni Hershkovitz, 1938 (124 primary grooves and 67 secondary grooves), C. epicrionopsoides (139–163 primary grooves, 21–40 secondary grooves), C. flavopunctata Roze & Solano, 1963 (155 primary grooves and 27 secondary grooves), C. guntheri (111–132 primary grooves and 0–10 secondary grooves), C. isthmica (131–147 primary grooves and 12–21 secondary grooves), C. leucocephala (118–142 primary grooves and 17–45 secondary grooves), C. mertensi Taylor, 1973 (142 primary grooves and 48 secondary grooves), C. museugoeldi Maciel & Hoogmoed, 2018 (152 primary grooves and 26 secondary grooves), C. nigricans (157– 189 primary grooves and 32–62 secondary grooves), C. perdita (139–152 primary grooves and 64–83 secondary grooves), C. subnigricans (151–161 primary grooves and 17–31 secondary grooves), C. subterminalis Taylor, 1968 (170 primary grooves and 16 secondary grooves), C. tentaculata (122–137 primary grooves and 30–42 secondary grooves), and C. volcani Taylor, 1969 (112–124 primary grooves and 14–32 secondary grooves).
Caecilia nigricans (159–195 primary grooves, 27–65 secondary grooves, length in width 32.6–80.4 times)— another species from the Pacific lowlands and Cordillera Occidental of Colombia —has overlapping counts of primary and secondary grooves, and attenuation index values with C. wilkinsoni sp. nov. (190–195 primary grooves, 51–71 secondary grooves, length in width 65.6–74.7 times), but C. nigricans has a terminal shield or ‘’cap’’ at the posterior end of the body that is barely interrupted dorsoventrally by the last few grooves of the body vs an entirely segmented terminal portion of the body in the new species, meaning that only the small disk and vent are free of grooves in C. wilkinsoni sp. nov. ( Figs. 3 View FIGURE 3 , 6 View FIGURE 6 ). Dermal scales obtained at the posterior end of the body of Caecilia nigricans are subcircular vs subrectangular in C. wilkinsoni sp. nov. Moreover, in life, Caecilia nigricans is mostly blue but C. wilkinsoni sp. nov. is mostly black.
The new species is readily differentiated from C. tenuissima by having many more secondary grooves (51–71 vs 9). In addition, the new species has a head that is narrower than its mid-body width (vs noticeably wider than the body in C. tenuissima Taylor, 1973: 221 , fig. 32); though in our experience a head much wider than mid-body is a rare condition within the genus, and the holotype of C. tenuissima appears to be somewhat desiccated from available photographs, suggesting this condition might be artefactual. Another difference between the species might be that in C. tenuissima the eye is not visible externally, while in C. wilkinsoni sp. nov. the eye is visible through translucent epidermis. There are no transverse grooves on the collars of C. tenuissima according to the original description ( Taylor, 1973) but C. wilkinsoni sp. nov. has well-defined transverse grooves present on both collars. Squamation also differs between these two species, in that C. tenuissima has dermal scales only towards the terminus while C. wilkinsoni sp. nov. has dermal scales throughout its body.
Caecilia wilkinsoni sp. nov. (190–195 primary grooves, 51–71 secondary grooves, length in width 65.6–74.7 times) is superficially similar to C. thompsoni (187–240 primary grooves, 26–42 secondary grooves, length in width 62–100 times), an endemic species of the Magdalena Valley of Colombia and the adjacent Cordilleras Central and Oriental, as well as the largest caecilian in the World, achieving a notable total length of 1767 mm ( Arredondo-Salgar, 2007). However, the new species has more secondary annular grooves than C. thompsoni (51–71 vs 26–42) and has small, flattened (not protruding) narial plugs on the tongue (vs protruding narial plugs in C. thompsoni ).
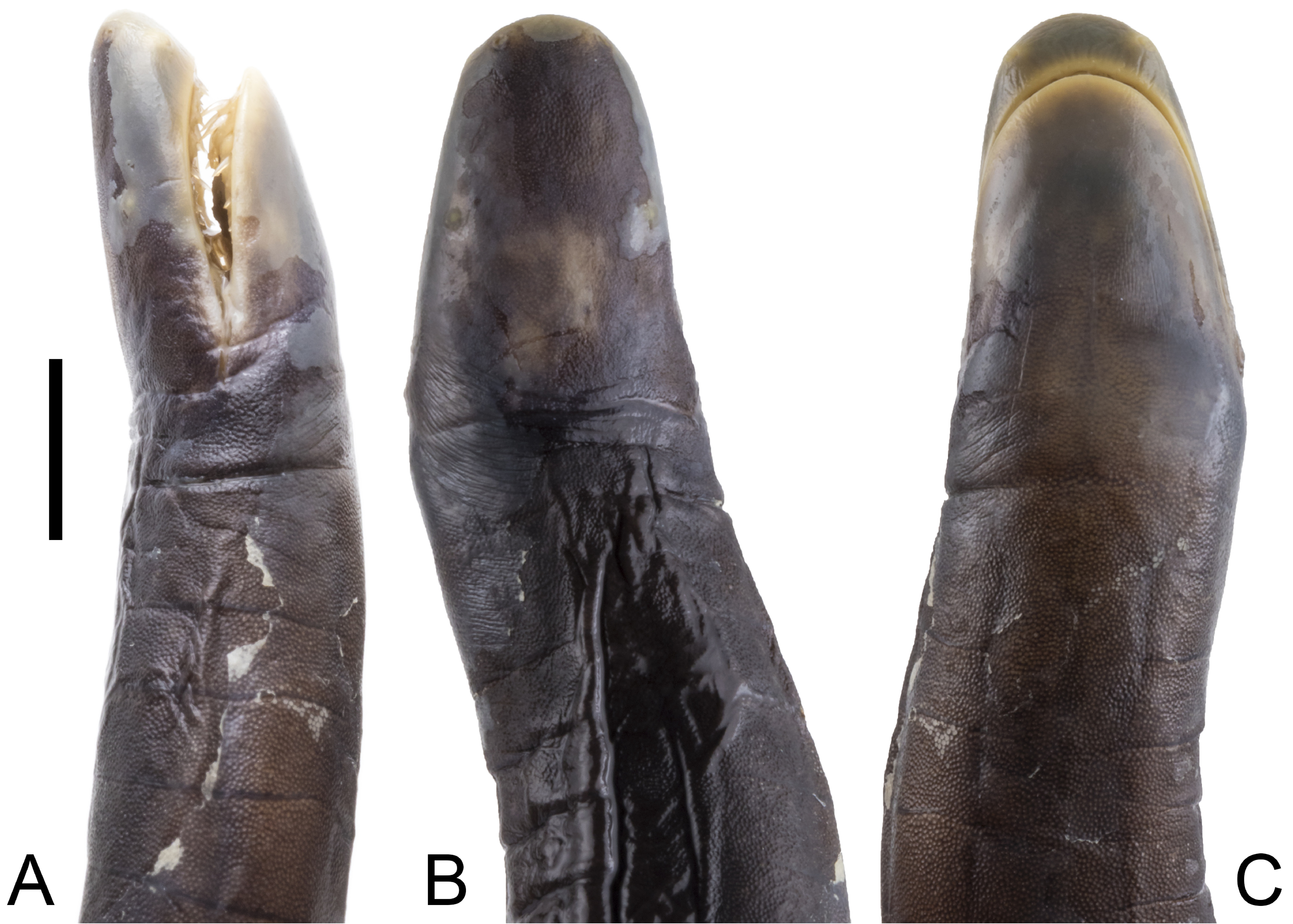
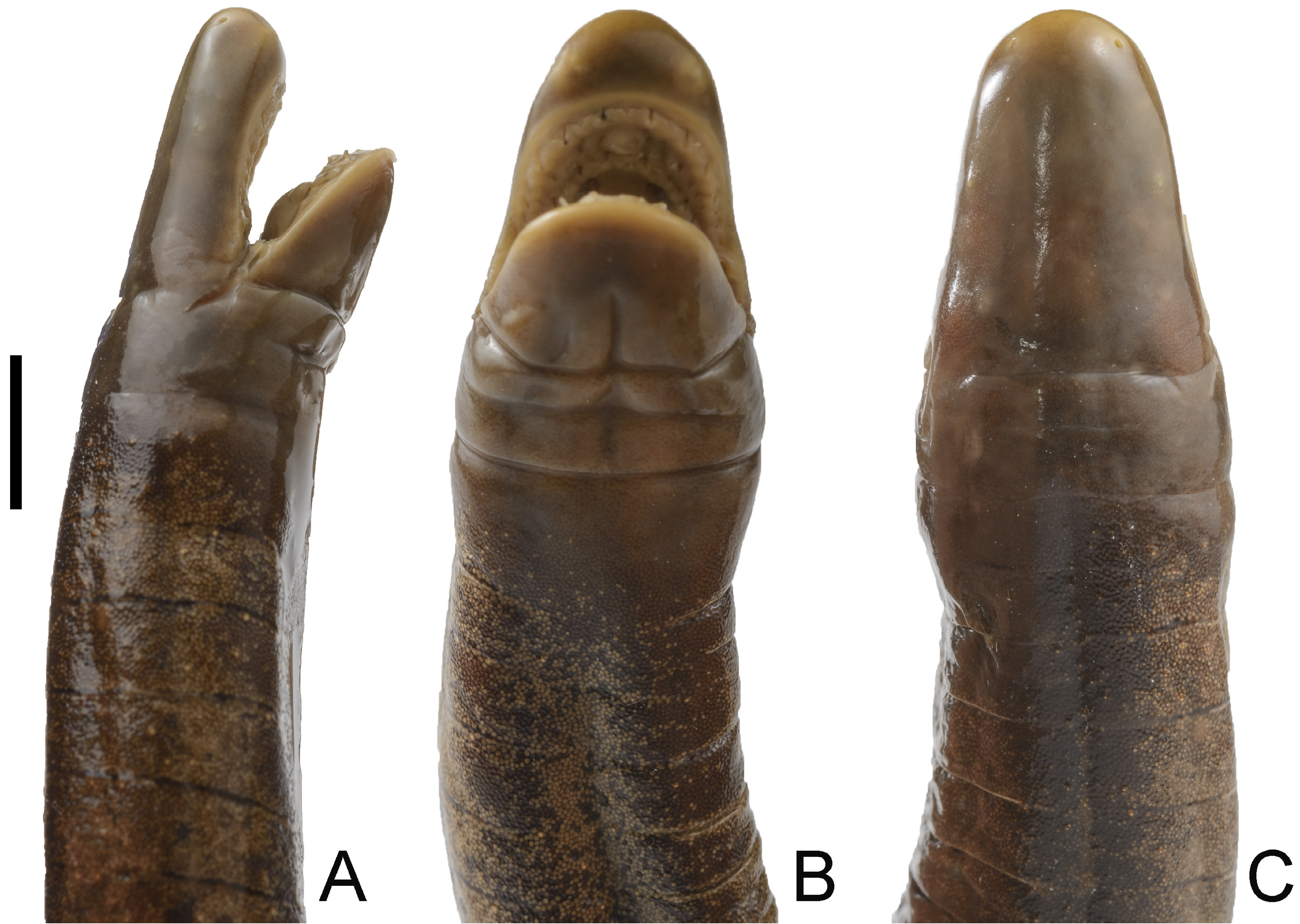
Description of the holotype. General condition of the specimen is good given that the individual was found dead, though it has two dorsal flesh wounds; one between primary grooves 116–121 and another between primary grooves 157–168. A ventral longitudinal incision was made between primary grooves 166–188 to search for sexual organs, and a few dermal pockets towards the terminus were opened to search for dermal scales. An adult male with a total body length of 590 mm and a mid-body width of 7.9 mm, length divided by width 74.7 times. Head (7.7 mm) slightly narrower than body. In lateral view, top of head slightly vaulted (not straight), upper margins of the mouth very slightly concave, lower margins of the mouth straight. Snout blunt in dorsal view, rounded in ventral view, but generally pointed (bullet-like) in profile, it extends beyond the mouth by 2 mm. Nostrils oval and broadening posteriorly, visible in dorsal view but not visible in ventral view, closer to tentacular opening than to eye, distance between nostril and tentacular opening 1.6 mm, distance between eye and nostril 4.5 mm, distance between nostrils 2.8 mm. Eyes small, 0.5 mm in diameter, covered by translucent epidermis and clearly visible in dorsal and lateral view but not visible in ventral view. Eye lies closer to the commissure of the mouth (4.2 mm) than to the nostril (4.5 mm). Distance between eyes 5.0 mm, less than distance from tip of snout to eye level (5.5 mm). Tentacular opening circular in outline, elevated above the skin, positioned below and posterior to the nostril, closer to margin of the mouth than to the nostril, barely visible in dorsal view. Collars well defined, the first is smaller than the second and bears a short groove dorsally, the second collar bears two incomplete grooves dorsally and dorsolaterally. The first and second nuchal grooves are complete, but the third nuchal groove incomplete dorsally and ventrally. Body width is even from collars (8.1 mm) to mid-body, where it then begins to taper, reaching a minimum body width of 6.5 mm at the fourth fifth of the total body length, before becoming abruptly stouter (8.1 mm) at the terminus, i.e. equivalent in width to the collars.
Primary grooves 195, only the last 10 prior to the vent completely encircle the body; secondary grooves 71, all on posterior half of body, the last 7 prior to the vent completely encircle the body. No terminal shield. Vent very small, and transverse, bordered by 6 denticulations anteriorly and 7 posteriorly, no anal glands in region surrounding vent. Dermal scales present as far anteriorly as the first primary groove, small (0.7 mm wide by 0.4 mm long), and oval; those closest to the terminus are very large (2.3 mm wide by 2.1 mm long), subrectangular, thicker and broader at the margin of inception with the dermal pocket. No trace of subdermal scales within the connective tissue of the skin.
All teeth monocuspid, pointed and slightly recurved, mandibular teeth considerably larger and more recurved than maxillary teeth. Four dental series are present: the premaxillary-maxillary series has 7-1-8 teeth, posterior ones notably smaller; the vomeropalatine series has 10-1-8 teeth, decreasing in overall size posteriorly; the dentary series has 8-8 teeth, very large, recurved, decreasing in overall size posteriorly, and show signs of tooth replacement; the inner mandibular series has 2-2 teeth, small, pointed, slightly oblique, and partially concealed by the gums. Choanae circular, 1.5 mm apart, diameter of each choana is 0.7 mm. Small, flattened, black-ish narial plugs present on the tongue, these are darker in contrast to the generally whitish color of the mouth. Coloration in life does not differ much from coloration in preservative. Main body coloration according to the field notes by the collectors of the holotype is dark purple on the dorsal surfaces, gradually becoming slightly olive on the ventral surfaces. Only the nostrils, tentacular openings, lips and vent have a contrasting pale cream color. Dermal scales are gray. No median ventral stripe.
Variation. In profile, the snout of the holotype is blunt but that of the paratype is rounded. The eye lies closer to the commissure of the mouth than to the nostril in the holotype, but equidistant between the commissure of the mouth and the nostril in the paratype. The vent is situated flush inside the surrounding disc in the holotype but situated in a slight depression (concave) in the paratype; the anterior margin of the vent of the paratype has two very small anal glands, unlike the holotype; the width of the disc surrounding the vent is smaller in the holotype but larger in the paratype. The paratype differs from the holotype by having 5 denticulations on the anterior and posterior margins of the vent vs 6 anterior denticulations and 7 posterior denticulations in the holotype. The attenuation index values indicate that the holotype is slenderer than the paratype (74.7 vs 65.6: Table 1 View TABLE 1 ). The gums of the paratype are very thick and conceal most of the teeth on all four dental series, with only their protruding ‘tips’ being noticeable; this condition is not present in the holotype. The choanae are circular in the holotype but subcircular in the paratype. Moreover, indications of teeth replacement in the form of dark, asterisk-like markings adjacent to emerging teeth were detected on the gums of the holotype but not the paratype. Lastly, it appears to us that the paratype has dried out at least once in the past 39 years and this made us chose the ICN specimen as the holotype.
Etymology. This species is named after Dr. Mark Wilkinson, Merit Researcher at the Natural History Museum in London, U.K., in recognition of his life-long dedication to the study of Gymnophiona . His numerous, highquality caecilian studies have provided a path for all aspiring ‘gymnophionologists’ to follow.
Natural history. Unknown, although the field notes associated with the holotype indicate that this individual was found dead along a road at 12:00 hours in the surroundings of a stream. The paratype was found alive approximately 50 cm below the surface.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Caecilia wilkinsoni
| Fernández-Roldán, Juan David & Lynch, John D. 2023 |
Caecilia tenuissima
| Taylor 1973 |