Felis concolor Linnaeus, 1771
Currier, Mary Jean P., 1983, Felis concolor, Mammalian Species 200 (10), pp. 230704-230704 : 1-6
|
publication ID |
https://doi.org/ 10.2307/3503951 |
|
DOI |
https://doi.org/10.5281/zenodo.10165916 |
|
persistent identifier |
https://treatment.plazi.org/id/03B86D2D-FFA0-FF81-6B70-4556A776FEC1 |
|
treatment provided by |
Juliana |
|
scientific name |
Felis concolor Linnaeus, 1771 |
| status |
|
Felis concolor Linnaeus, 1771 View in CoL
Mountain Lion
Felis concolor Linnaeus, 1771:522 . Type locality restricted to Cayenne, French Guiana, by Goldman ( Young and Goldman, 1946).
Felis couguar Kerr, 1792:15 1 . T ype locality North and South Carolina, Georgia, Pennsylvania; restricted to Pennsylvania by Nelson and Goldman (1929).
Felis puma Molina, 1782:295 . Type locality vicinity of Santiago, Chile.
Felix (sic) oregonensis Rafinesque, 1832:62 . Type locality Oregon, by restriction ( Nelson and Goldman, 1932) to Ohanapecosh River, Mount Rainier National Park, Pierce County, Wash· ington.
Felis californica May, 1896:22 . Type locality Kern Co., California.
Felis coryi Bangs, 1899:15 . Type locality wilderness back of Sebastian, Florida.
Felis hippolestes Merriam, 1897:21 9 . Type locality western United States (Wind River Mountains, near basin Wind River, Fremont Co., Wyoming).
Felis bangsi Merriam, 1901:595 . Type locality Dibulla, department of Magdalena, Colombia.
Felis aztecus, Merriam, 1903:73 ; used as a full species, originally proposed as a subspecies of Felis hippolestes .
Felis arundivaza Hollister, 1911: 1 76 . Type locality 12 miles SW Vidalia, Concordia Parish, Louisiana.
Felis improcera Phillips , 191 2:85. Type locality CalmaIIi, Baja California, Mexico.
CONTEXT AND CONTENT. Order Carnivora , Family Felidae . Subfamily Felinae . The genus Felis includes about 29 species. The subgenus Puma (here recognized following Young and Goldman, 1946) includes one species, Felis concolor . Thirty subspecies are generally recognized ( Young and Goldman, 1946):
F. c. aerocodia Goldman, 1943:230 . T ype locality Descalvados, Matto Grosso, Brazil.
F. c. anthonyi Nelson and Goldman, 1931:209 . Type locality Playa del Rio Base, Monte Duida, Territory of Amazonas, Venezuela.
F. c. araucanus Osgood, 1943:77 . Type locality "Fundo Mairenuhue," Sierre Nahuelbuta, west of Angol, Malleco, Chile.
F. c. azteca Merriam, 1901:592 . Type locality Colonia Garcia, about 60 mi SW Casas Grandes, Chihuahua, Mexico.
F. c. bangsi Merriam, 1901:595 , see above.
F. c. borbensis Nelson and Goldman, 1933:52 4 . Type locality Borba, Rio Madeira, Amazonas, Brazil.
F. c. browni Merriam, 1903:73 . Type locality Colorado River, 12 mi below Yuma, Arizona.
F. c. cabrerae Pocock, 1940:308 . T ype locality La Rioja, Province of La Rioja, northern Argentina.
F. c. californica May, 1896:22 , see above.
F. c. capricornensis Nelson and Goldman, 1929:346 . Type locality Piracicaba, Sao Paulo, Brazil.
F. c. concolor Linnaeus, 1771:522 , see above.
F. c. coryi Bangs, 1899: 15 , see above ( arundivaza Hollister a synonym).
F. c. costaricensis Merriam, 1901:596 . Type locality Boquerte, Chiriqui, Panama.
F. c. couguar Kerr, 1792:151 , see above.
F. c. greeni Nelson and Goldman, 1931:211 . Type locality Curraes Novos, Rio Grande do Norte, Brazil.
F. c. hippolestes Merriam, 1897:219 , see above.
F. c. improcera Phillips, 1912:85 , see above.
F. c. incarum Nelson and Goldman, 1929:347 . Type locality Piscocucho, Rio Urubamba, Department of Cuzco, Peru.
F. c. kaibabensis Nelson and Goldman, 1931:209 . Type locality Powell Plateau, Grand Canyon National Park, Arizona.
F. c. mayensis Nelson and Goldman, 1929:350 . Type locality La Libertad, Department of Peten, Guatemala.
F. c. missoulensis Goldman, 1943:299 . Type locality Sleeman Creek, about 10 mi SW Missoula, Montana Co., Montana.
F. c. olympus Merriam, 1897:220 . Type locality Lake Cushman, Olympic Mountains, Washington.
F. c. oregonensis Rafinesque, 1832:62 , see above.
F. c. osgoodi Nelson and Goldman, 1929:348 . Type locality Buena Vista, Department of Santa Cruz, Bolivia.
F. c. patagonica Merriam, 1901:598 . Type locality Lake Pueyrredon, Territory of Santa Cruz, Argentina.
F. c. pearsoni Thomas, 1901: 188 . Type locality Santa Cruz, about 70 mi from coast, southern Argentina.
F. c. puma Molina, 1782:295 , see above.
F. c. soderstromii Lönnberg, 1913:2 . Type locality Nono, Mount Pichincha, Ecuador.
F. c. stanleyana Goldman, 1936: 137 . Type locality Bruni Ranch near Bruni, Webb Co., Texas.
F. c. vancouverensis Nelson and Goldman, 1932: 105 . Type locality Campbell Lake, Vancouver Island, British Columbia.
DIAGNOSIS. The mountain lion is the largest species in the genus Felis , as restricted to exclude the pantherines. Size varies among the subspecies, but males generally weigh between 55 and 65 kg, and females between 35 and 45 kg. Total length is generally between 2.2 and 2.3 m in males, and between 2.0 and 2.1 m in females. Its feet resemble those of F. geoifroyi , F. yagouaroundi , F. viverrinus , and F. silvestris more than those of the pantherines (Pocock, 1917 a). Its claws are retractile, but the claw-sheaths do not fully encase the claws as in the pantherines, thus resembling the claws of F. geoffroyi , F. yagouaroundi , F. viverrinus , and F. silvestris (Pocock, 1917 a). The tail is long, cylindrical, and typically about one-third of the animal's total length. The ears are short and rounded. The dorsal color is light grayish brown to dark reddish brown. The lateral muzzle, backs of ears, and tip of tail are dark brown or black. The chin, medial muzzle, and ventral area are creamy white.
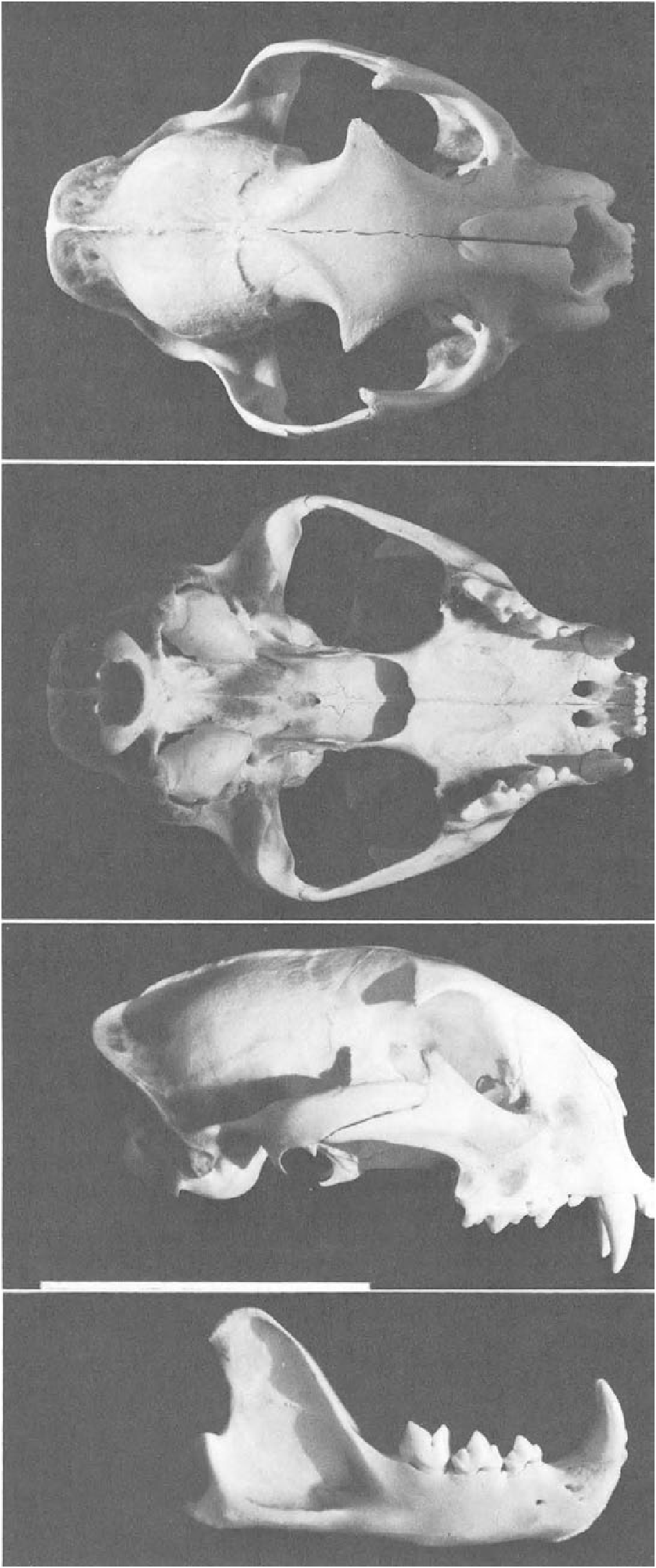
GENERAL CHARACTERS. The mountain lion is large and slender and has short, muscular limbs ( Fig. 1 View FIGURE 1 ). The pelage is of medium texture, characteristically short year-round in tropical forms, but growing longer and thicker in the winter in temperate forms. The young are black-spotted in three irregular dorsal lines and transverse rows. These spots are vivid up to the animal's third or fourth month of life. The eye color is blue in young kittens and turns grayish brown to golden in adults. The pupils are round. The skull ( Fig. 2 View FIGURE 2 ) is short, rounded, and has a sagittal crest, resembling the skull of F. caracal in shape ( Pocock, 1917 a). The partition dividing the tympanic bulla is low, forming a small outer chamber and large inner chamber, much as in F. nebulosa and others, but differing from the condition in Uncia uncia (Pocock, 19 16a). Teeth of F. concolor are, in general, like those of other felids, but lack the lateral longitudinal grooves on the canines that are present in many other felids ( Young and Goldman, 1946). Further distinctions are detailed in Pocock (191 7 a) and Young and Goldman (1946).
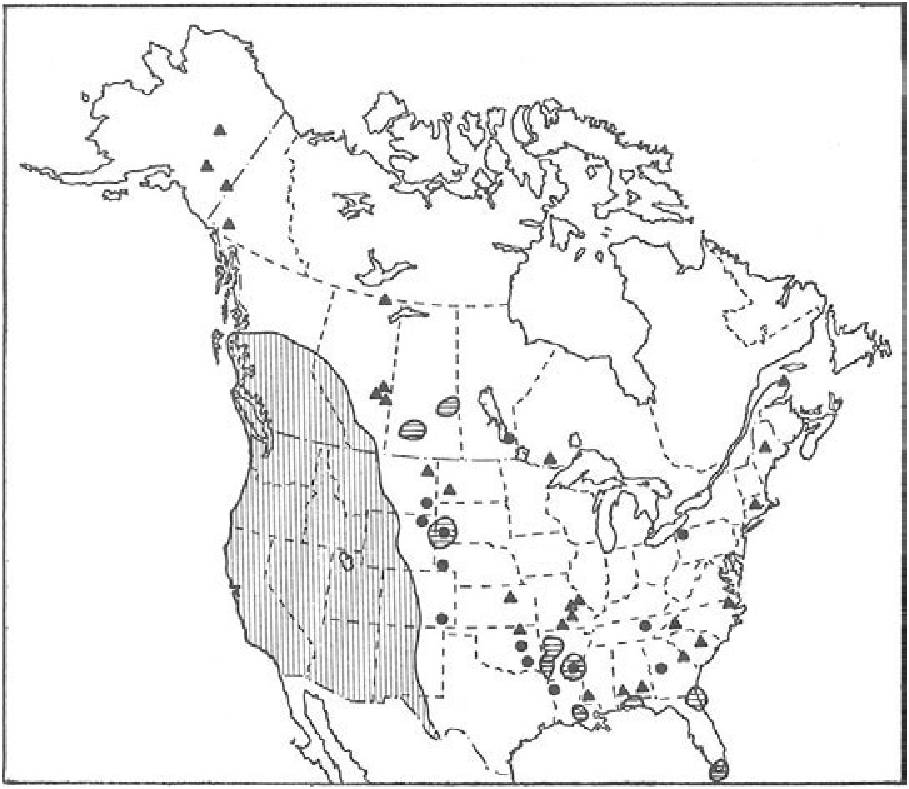
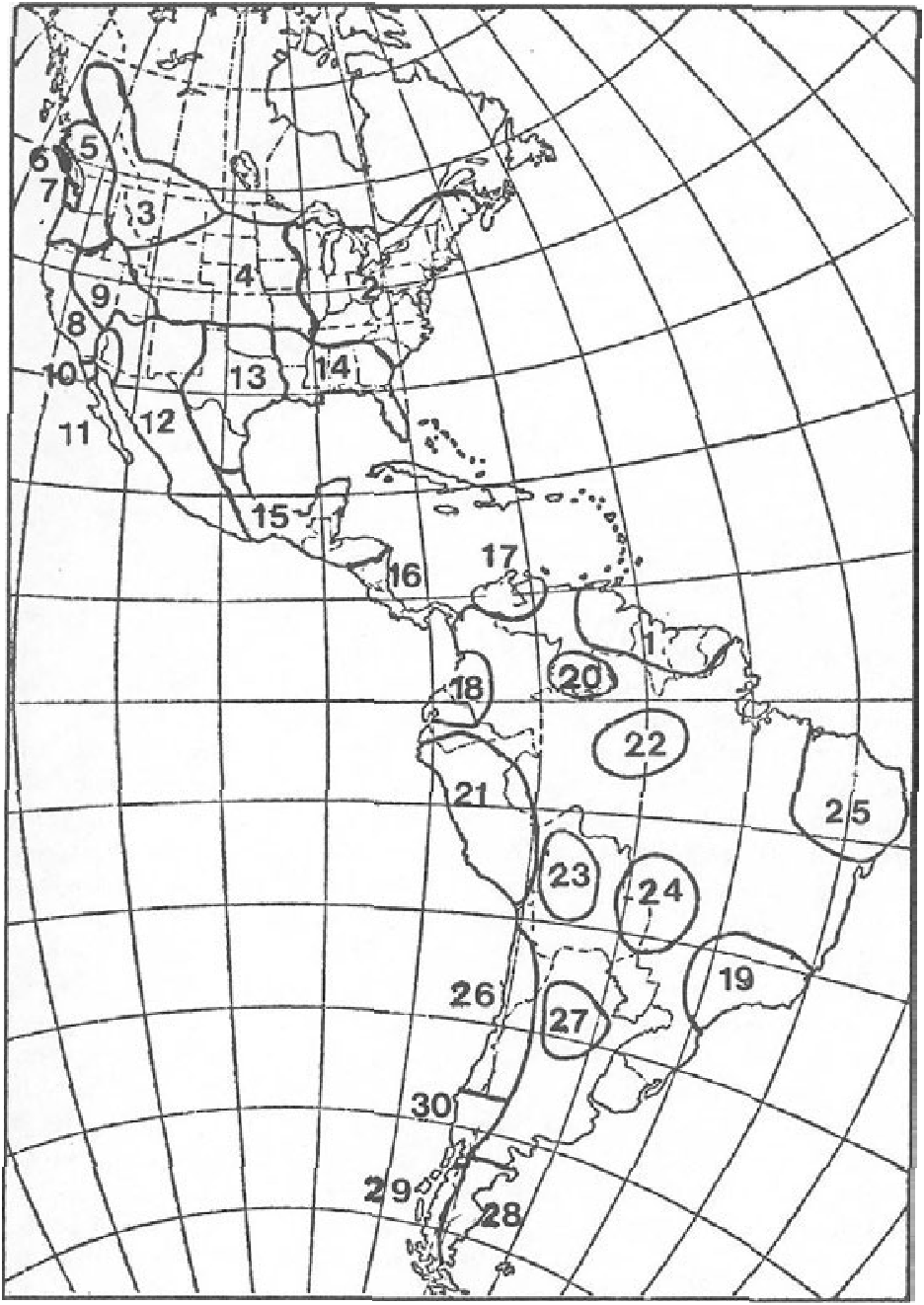
DISTRIBUTION. At one time mountain lions ranged from northern British Columbia to southern Chile and Argentina, and from coast to coast in North America ( Young and Goldman, 1946). Hunting pressure and changes in land management practices in western United States and Canada have restricted their range mainly to mountainous, relatively unpopulated areas, although isolated populations may exist elsewhere. They are probably similarly restricted by hunting and development in Central and South America. The current distribution of the mountain lion in North America is given in Fig. 3 View FIGURE 3 , and their distribution prior to 1946 in North and South America is shown in Fig. 4 View FIGURE 4 .
FOSSIL RECORD. Felis concolor has rio close living relatives, but mountain lion-like animals existed in the past. Remains of F. daggetti Merriam, 1918 , found in the La Brea tar pits, indicate a Pleistocene felid a little larger than a very large mountain lion. It had a more massive cranium, and the coracoid process of the mandible was more backwardly curved than that of F. concolor (Young and Coldman, 1946) . F. bituminosa Merriam and Stock, 1932 (= F. hawveri Stock, 1918 ?) was closer to the modern mountain lion in size, but quite different in cranial details. Simpson (1941) suggested that F. bituminosa was merely the female of F. daggetti . Goldman ( Young and Goldman, 1946) suggested that the line of evolution proceeded from F. daggetti through F. bituminosa to F. concolor . Two other mountain lion-like forms found in eastern United States ( F. inexpecta Cope, 1899 , and F. longicrus Brown, 1908 ) may represent different races of F. daggetti ( Simpson, 1941) . Glass and Martin (1978) demonstrated the close relationship between F. concolor and F. lacustris Gazin, 1933 (a late Pliocene felid from the western United States that was slightly smaller than F. concolor ), based on tooth measurements. They believed that the relationship among all of the aforementioned species warranted reevaluation.
FORM. Hair length is extremely variable according to clio mate and season ( Young and Goldman, 1946). Guard hairs from the mid-dorsal region of one specimen of F. c. hippolestes reached a maximum length of 39 mm. The maximum diameter at the distal shaft was 115 II- with regular or irregular-waved mosaic scales; in the basal region, smooth and distant or smooth and intermediate margins were present; at the tip, irregular-waved scales with cre nate-rippled margins were present. The hairs were grayish brown from the basal end up to one-fourth or one-third the shaft length, then black for about one-half the shaft length. This was followed by a 2- to 14-mm light brown band, ending in a black tip of from 2 to 5 mm. The hairs were oval in cross section ( Spence, 1963). Underfur is fine and kinky near the base. Shedding occurs in temperate forms in the spring.
Eight mammae are present, but apparently only six are functional in the female ( Lechleitner, 1969).
The feet are digitigrade with five toes on each forefoot. The pollex, or first toe, is small and set above the others. Each hindfoot has four toes. A sharp, retractile claw is found on each toe ( Lechleitner, 1969).
The hyoid apparatus is held close to the base of the skull and not imbedded in the muscles of the throat ( Pocock, 1916b). The jaws are heavy.boned and structured so no backward-forward motion is possible ( Young and Goldman, 1946). The clavicle, as in other felids, is better developed than in most carnivores ( Young and Goldman, 1946). Dentition is i 3 /3, c 1 /1, P 3/2, m 1 / 1, total 30. The tongue is covered with many rough papillae. The muscles of the jaws and legs are well developed.
The simple stomach can hold up to 10 kg (Hornocker, 1970) and the caecum is small.
FUNCTION. Long guard hairs and fine kinked underfur enable mountain lions in temperate regions to conserve heat in winter. The agouti hair pattern is common among mammals and probably aids in camouflage. The stationary hyoid apparatus permits purring, but not roaring. Sharp, retractile claws, heavy-boned jaws with no backward-forward motion, and well-developed jaw and leg muscles are necessary for the type of hunting employed by the mountain lion, as is a developed clavicle that allows more strength and flexibility of the forequarters (see ECOLOGY). The mountain lion licks itself clean with its rough tongue as do other felids. Massive muscles give the mountain lion strength. Currier and Russell (1982) analyzed blood from 22 free-ranging, non-kitten mountain lions cap· tured in Colorado and 43 captive mountain lions, and reported mean values for 28 blood properties.
ONTOGENY AND REPRODUCTION. Mountain lions are polygamous, but the same lions may mate year after year because of the stability of their home areas ( Hibben, 1937; Seidensticker et aI., 1973).
Gestation periods, based on the time from last day of mating to parturition,last from 82 to 96 days ( Eaton and Verlander, 1977; Rabb, 1959: Young and Goldman, 1946). Female mountain lions can come into estrous any time of the year, but most births are between April and September in the northern hemisphere ( Eaton and Verlander, 1977; Robinette et al., 1961).
Litter size ranges from 1 to 6 ( Robinette et al., 1961; Young and Goldman, 1946). The average number of fetuses in 66 preg· nant, wild females examined by Robinette et al. (1961) was 3.4; the average litter size of 13 1 females with kittens that weighed up to 23 kg was 3.0; the average litter size of 3 7 females with kittens larger than 23 kg was 2.2. The average litter size of 4 1 litters of mountain lions in western United States was 2.4 kittens ( Ashman, 1975; Currier et al., 1977; Hornocker, 1970; Seidensticker et al., 1973; Shaw, 1977; Sitton and Wallen, 1976).
If the litter is born dead or removed within 24 h, the female will usually come into estrous within a few weeks (Eaton and Ver· lander, 1977; Rabb, 1959). Unlike most larger felids, female mountain lions generally do not come into estrous soon after the death or removal of the litter if they raised the kittens for more than a few days ( Eaton and Verlander, 1977).
A mountain lion weighs approximately 400 g at birth (Vole, 1972; Young and Goldman, 1946). Its coat is densely spotted and its eyes and ears remain closed for one to two weeks after birth ( Eaton and Verlander, 1977; Young and Goldman, 1946).
Primary incisor teeth first appear at age 10 to 20 days, followed by the canines (20 to 30 days) and premolars (30 to 50 days) ( Currier, 1979; Eaton and Verlander, 1977; Volf, 1972). Perrnanent incisors start replacing primary teeth at about 5 \1 months of age. The permanent canines first appear at month 8, and for a short time both permanent and primary canines are present ( Currier, 1979).
Weight gain following birth is rapid. A weight of 1 kg is attained in 10 to 20 days, and at weaning (age 1 to 2 months), a kitten weighs 3 to 4 kg ( Currier, 1979; Eaton and Verlander, 1977). Males and females weigh about the same. Individual variation is greater than variation due to sex for about 30 weeks (Rob. inette et al., 1961). Adult weight is attained between the ages of 2 and 4 years.
Eye color of mountain lion kittens is initially blue. Within 4 months most of the iris is brown, and by age 9 months the iris has begun to change to golden ( Currier, 1979).
The vivid black spots on a mountain lion at birth fade rapidly between age 12 and 14 weeks, probably the age a kitten starts to accompany its mother on hunts. These marks are still discernible at age 1 year ( Currier, 1979). The stripes on the upper foreleg are still visible in some mountain lions at age 3 years (Currier, pers. observ.).
A mountain lion stays with its mother until age 1 \1 to 2 years (Hornocker, 1970; Seidensticker et aI., 1973). Age at sexual rnaturity of females is 2 to 3 years ( Eaton and Verlander, 1977; Rabb, 1959; Young and Goldman, 1946), but a mountain lion in the wild will probably not mate until it has established a home territory or area (Hornocker, 1970; Seidensticker et al., 1973).
Female mountain lions sometimes have a bloody discharge during estrous, usually associated with only the first estrous ( Eaton and Verlander, 1977). Estrous lasts from 4 to 12 days, with an average duration of 8 days ( Eaton and Verlander, 1977; Rabb, 1959). Rabb reported that a female from Chicago Zoological Park came into an 8- to Ll-day estrous, and when not mated, came into estrous again in 2 weeks. After 6 regular cycles without mating she had a 2-month lull (June to August) before coming into estrous again.
Frequency of copulation during estrous is variable. The highest frequency observed by Eaton and Verlander (1977) was 9 times in 1 h. A single copulatory act usually lasts less than 1 min ( Eaton and Verlander, 1977; Rabb, 1959). From 52 observed mated estrous periods, Eaton and Verlander (1977) calculated that the chance of conception per mated estrous was 6 7 %.
Female mountain lions can remain reproductively active to at least an age of 12 years, and males to at least an age of 20 years ( Eaton and Verlander, 1977). Mountain lions have lived longer than 20 years in captivity, but 12 years of life is probably old for a freeranging mountain lion ( Young and Goldman, 1946).
ECOLOGY. The distribution of mountain lions is probably limited in the Western Hemisphere by one or more of the following three factors: human interference, lack of prey, or lack of stalking cover. Mountain lions have been reported from sea level to 4,000 m, and from desert areas to the tropical rain forests of South America. Since they can catch and eat many different kinds of animals, they are probably not limited by lack of any given prey species ( Spalding and Lesowski, 1971; Young and Goldman, 1946).
Because of this adaptability, generalizations about the species as a whole are difficult to make.
Although mule deer ( Odocoileus hemionus ) generally make up about 75 % in winter and 60 % in summer of the bulk of a mountain lion's diet in western North America ( Robinette et al., 1959; Young and Goldman, 1946), lions are highly opportunistic and will take advantage of whatever food source is available ( Smith, 1981). Items reported eaten by mountain lions are listed in Table 1 View TABLE 1 . The percentage of empty stomachs reported from dead mountain lions varied from 10 % ( Spalding and Lesowski, 1971) to 64 % ( Connolly, 1949), but about 30% ( Robinette et al., 1959) is probably more realistic, although the actual figure is highly dependent upon diet. This suggests that an average mountain lion eats only 6 days out of 9.
Hornocker (1970) calculated that an adult mountain lion would have to kill and utilize from 860 to 1,300 kg of large prey animals per year (5 to 7 elk or 14 to 20 deer). In warm weather, large carcasses probably decompose before full utilization by a mountain lion, but this bias is offset by the larger percentage of smaller animals in the diet in the summer months ( Young and Goldman, 1946).
Mountain lions kill proportionately more old males and very young deer and elk than are found within the population as a whole (Hornocker, 1970; Spalding and Lesowski, 1971). The older deer are probably more vulnerable because of infirmities, the males are probably more vulnerable because of their solitary habits, and young animals are probably more vulnerable because they are small. Half of the deer and elk killed by lions and examined by Hornocker (1970) were in poor condition whereas 40% of those that he randomly killed in the same area were in poor condition, which suggests a slight, but possibly not significant bias in prey selection. Hibben (1937) found a similar bias in New Mexico.
A mountain lion generally brings down larger prey by maneuvering to within about 15 m, then leaping on its back within a few strides and breaking the animal's neck with a powerful bite below the base of the skull ( Hibben, 1937; Young and Goldman, 1946). It then drags its kill to a secluded spot before eating from it. The unconsumed portion is usually covered with whatever substrate is available, usually leaves, sticks, or pine needles, and is usually returned to later.
If a mountain lion is able to maneuver within striking distance of its prey, its chances for successfully bringing the animal down are great. Hornocker (1970) recorded track data that indicated 37 out of 45 attempts were successful.
After an extensive predator-prey study, Hornocker (1970) concluded that elk and deer populations were limited by winter food supply rather than predation by lions, but that lion predation dampened prey oscillations and distributed the deer and elk more widely over the available range.
Mountain lions take livestock more frequently in the southwestern than in the northwestern United States ( Christensen and Fischer, 1976). Shaw (1977) hypothesized that the number of cattle killed by mountain lions varies inversely with the number of deer available.
Where they coexist with mountain lions, grizzly bears, wolverines, and jaguars are possible competitors ( Young and Goldman, 1946). Coyotes, black bears, and bobcats and other smaller felids probably compete with mountain lions for smaller mammals and sometimes for deer throughout most of the mountain lion's range.
An age-estimation method for mountain lions has recently been developed by the author ( Currier, 1979), but has been applied on only one population in southern Colorado. That population was subjected to heavy hunting pressure, particularly on adult males because of trophy hunting (generally adult males usually attain trophy size). Age distribution of the females was greater than that of the males, but declined after age 5 years. The oldest female was estimated to be 13 years old. Only five adult males were caught, and two of them were killed by hunters during the study. The estimated ages in years of the adult males at time of capture were 2, 2, 6, 6, and 8. The adult male: adult female: juvenile ratio was 0.3: 1.0:0.5. Hornocker (1970) reported a ratio in an unhunted mountain lion population in Idaho of 0.75:1.0:1.4.
It is likely that the main cause of mortality of mountain lions in western North America, and probably in South America, is hunting by humans. Natural, non-hunting mortality is probably heaviest during three periods of the mountain lion's life cycle: postnatal, immediately after independence, and old age. Adult male mountain lions sometimes kill kittens (Hornocker, 1970). Unattended kittens are also vulnerable to attack by other predators. Newly independent mountain lions that have not yet established home areas do not hunt as efficiently as resident lions. Old mountain lions are less efficient hunters because of physical deterioration.
Mountain lions are subject to accidental deaths throughout their lifetimes. Collisions with motor vehicles are the most common cause of accidental deaths and probably will increase as man encroaches into mountain lion habitat. Other types of accidents result from encounters with prey ( Currier, 1976; Gashwiler and Robinette, 1957; Hornocker, 1970), falls from cliffs (Hornocker, 1970), and drownings ( Macgregor, 1974; Sitton and Wallen, 1976). Probably lightning, rockslides, poisoning by venomous reptiles, postpartum complications, and choking also kill some mountain lions ( Russell, 1978).
Mountain lions are solitary. The only social unit that endures more than a few days is the maternal bond of a female and her kittens. Females with small kittens avoid interactions with other mountain lions, but as the kittens approach independence and the female approaches estrous, she tolerates contact with other mountain lions of either sex. When she fully enters estrous, a male will usually join and travel with her until estrous is completed ( Seidensticker et al., 1973). Males may be found together immediately after independence from the mother, but only rarely as established adults.
Intraspecific relationships determine the maximum crowding tolerated by mountain lions and establish a maximum density of one mountain lion every 25 to 50 km2 ( Currier, 1976). At or below this density, home range or area size is probably dependent upon prey density and stalking cover in relation to prey density (prey vulnerability) ( Seidensticker et al., 1973). Home area size varies from season to season and year to year. The home area of one male radio-tracked by Seidensticker et al. (1973) was 145 km2 during winter-spring of 1970- 71. It increased to 293 km2 that summerfall, then decreased to 96 km2 the following winter-spring. The summer home area of some mountain lions is in a different location from the winter area, thus involving a migration, but for some mountain lions it is merely an enlargement of the winter area. The home area of males is generally larger than the home area of neighboring females.
Seidensticker et al. (1973) found that home areas of adult males in an unhunted population do not overlap, but home areas of adult females sometimes partially or entirely overlap with each other or with an adult male. Sitton and Wallen (1976) found a greater degree of overlapping home areas in a region that had been heavily hunted until 2 years prior to their study, but unhunted during the study. Overlapping home areas may be due to social disruption from hunting, or to topography ( Hopkins, 1981).
Mountain lions are exceptionally free of ectoparasites, probably due to their solitary nature, low densities, and mobile habits. Occasionally fleas ( Arctopsylla setosa ), ticks ( Dermacentor variabilis , Ixodes ricinus , and I. cookei in North America, and Amblyomma cajennense , Boophilus microplus , and Dermacentor cyaniventris in South America), and lice ( Trichodectes felis in South America) infest mountain lions ( Young and Goldman, 1946).
Tapeworms ( Taenia omissa ), obtained from eating the immature stages in lungs or pericardium of deer ( Odocoileus spp.), are the most common internal parasites, although they are not widespread (Hornocker, 1970; Leiby and Dyer, 1971; Sitton and Wallen, 1976). Flukes ( Heterophyes heterophyes ) ( Davis and Libhe, 1971), and nematodes ( Trichinella spiralis ) ( Worley et al., 1974; Zimmerman, 1971) also have been reported. The roundworm Filaroides striatum has been reported in mountain lions in Brazil ( Young and Goldman, 1946). One case of piroplasmosis caused by the protozoan Babesialla felis has been reported in a captive mountain lion ( Howe, 1971). One probable case of rabies has been recorded ( Storer, 1923), and Bittle (1970) acknowledged the occurrence of feline panleukopenia in mountain lions. There is some evidence that arthritis occurs in old animals ( Connolly, 1949; Hornocker, 1970). Anthrax has been reported in mountain lions that have eaten infected meat ( Miller, 1971).
Two subspecies of mountain lion, F. c. coryi and F. c. couguar, have been declared endangered (U.S. Fish and Wildlife Service, 1974). The mountain lion was bountied in 9 western states (not in Alaska, Wyoming, or Nevada), and by the provinces of British Columbia and Alberta. The bounty programs varied in duration between 1843 and 1970, but averaged almost 50 years in each state or province. Although the state did not bounty mountain lions in Texas, counties did. In 1970, two counties still paid a bounty, and one remained in 1974 ( Nowak, 1976). The mountain lion was declared a game animal in Colorado and Nevada in 1965, in Washington and British Columbia in 1966, in Oregon and Utah in 1967, in California (but is currently protected by a legislative moratorium) and Alberta in 1969, in Arizona in 1970, in New Mexico and Montana in 1971, in Idaho in 1972, and in Wyoming in 1973. It is still considered a predatory animal in Texas and receives no protection.
The mountain lion was bountied intermittently in Florida during the 1800's. From 1950 to 1958 it was considered a game animal, and in 1958 it became fully protected. The mountain lion is fully protected in the following states and provinces: Alabama, Arkansas, Connecticut, Delaware, Georgia, Illinois, Kentucky, Louisiana, Manitoba, Maryland, Massachusetts, Missouri, New Brunswick, New Hampshire, New Jersey, New York, North Carolina, Oklahoma, South Carolina, Tennessee, and Virginia. As of 1976, there was no legal classification and no protection of mountain lions, except in agreement with the federal government, by the following states and provinces (lions are federally protected in states followed by an asterisk, because part of the original range of the endangered subspecies occurred there): Alaska, Indiana *, Iowa, Kansas, Maine*, Michigan*, Minnesota, Mississippi*, Nebraska, North Dakota, Northwest Territories, Nova Scotia, Ohio*, Ontario, Pennsylvania*, Quebec, Rhode Island*, Saskatchewan, South Dakota, Vermont *, West Virginia*, Wisconsin*, and Yukon ( Nowak, 1976).
Mountain lions readily breed in captivity and are, therefore, often recipients of birth control implants to control overpopulation problems in some zoos. Unfortunately, many captive mountain lions originated from indiscriminate crossbreeding of different subspecies, so pure strains of the endangered subspecies are not readily available. A breeding program for the endangered F. c. coryi (Florida panther) is being attempted at the Rare Feline Breeding Compound in Florida by R. Baudy ( Downing, 1979), but three of the four males are well over 20 years old and the fourth is believed to be sterile.
Mountain lion pelts are not commercially valuable, although both North and South American Indians formerly made extensive use of them. Mountain lion claws and teeth are sometimes used for ornamentation.
The main methods of studying mountain lions have been observation of sign and capture and tagging. Mountain lions are generally tracked with two to four experienced hounds, then immobilized with phencyclidine hydrochloride (0.5 mg/lb) or a derivative injected from a dart shot from a Cap-Chur gun (Palmer Chemical and Equipment Co., Douglasville, Georgia 30134, USA), and marked with either a nylon rope collar and ear tattoo or a radio collar ( Ashman, 1975; Currier et aI., 1977; Donaldson, 1975; Hornocker, 1970; Seidensticker et aI., 1973; Shaw, 1977; Sitton and Wallen, 1976).
Numerical estimates of population density based on tracks have been attempted ( Currier, 1976; Koford, 1978; Kutilek et al., 1980), but accurate estimation is difficult. Seidensticker et aI. (1973) were able to mark essentially the entire resident population on their 520 km2 area, but this was not possible in most studies. Johnson and Couch (1954) developed a formula for a minimum population estimate based on lions killed: N = 3.3K, where N = minimum population and K = number of lions killed each year. Nowak (1976) estimated the total population of mountain lions in the United States and Canada to be 16,000.
BEHAVIOR. Reproductive behavior in the mountain lion is typical of felids. When a female is in estrous, she vocalizes freely, frequently rubs against nearby objects, and often exhibits lordosis and treading ( Rabb, 1959). A male responds vocally with similar yowls ( Rabb, 1959), sniffs the female's genital area, and tests her condition with Flehmen (vomeronasal response) (Eaton and Verlander, 1977). After a period of courtship, which primarily involves the male docilely following the female, an attempted mounting by the male is met by either defensive snarls and hisses or by allowed copulation. Prior to intromission, the male often grasps the female's neck fur. Copulation is brief but frequent (see REPRODUCTION AND ONTOGENY). The female seeks a secluded place to have her young, but no bedding is prepared.
Communication between adult mountain lions is largely visual and olfactory. When a female is in estrous, auditory and tactile communication are also important. Adult males and infrequently adult females make scrapes in their home areas ( Musgrave, 1926; Smith, 1981). Scrapes are small piles of substrate kicked up by the hindfeet. Seidensticker et aI. (1973) measured 86 scrapes and found them to be 15 to 46 em long, 15 to 30 em wide, and 3 to 5 em deep. Most were found where topography yielded easy passage: on the downhill side of trees, near mouths of canyons, in draws, and on ridges. While tracking lions, they found the lion might go for many kilometers without scraping, or make two scrapes within a few hundred meters. Hibben (1937) stated that a male will scrape frequently when courting a female. Feces or obvious urine were only associated with about 20% of the scrapes; however, detection of urine was difficult, so it may be much more prevalent. Feces were sometimes found unassociated with a scrape, usually near a kill site (Seidensticker et aI., 1973). Both males and females visit scrape sites and sometimes change course abruptly after the visit, suggesting that information is transferred from one lion to another ( Hornocker, 1969).
Communication between mother and offspring is mainly tactile (licking, rubbing) and vocal. Young mountain lions give a loud, chirping whistle that serves to direct the mother's attention to the kitten ( Eaton and Verlander, 1977; Rabb, 1959). Adult mountain lions have a low-pitched squeal that also appears to function in attention-getting ( Rabb, 1959). Like smaller cats, but unlike the large, roaring cats, mountain lions can show contentment by purring both during inspiration and expiration of breath (see FORM AND FUNCTION). Mountain lions in captivity also make a variety of meows and barks which probably do not occur as frequently in more solitary wild mountain lions. The occurrence of the fabled "scream" is much debated. For example, Seidensticker et aI. (1973) did not witness it in eight years of work with wild and captive mountain lions.
Many postures and habits of the mountain lion are typical of felids. It cleans itself by licking (see FORM AND FUNCTION). It laps water with its tongue and tears chunks of meat from a carcass with its sharp premolars and molars. Lions swim only when necessary, although they are not so averse to water as are domestic cats. Posture and facial expressions are similar to those described by Hemmer (1972) for the snow leopard. The greeting posture of captive mountain lions is standing with the tail curved upwards, and is accompanied by a short "rnra" sound (Currier, pers. observ.). Annoyance or anger is indicated by a hiss or growl accompanied by a flattening of the ears against the skull ( Bogue and Ferrari, 1974). Mountain lions remain playful throughout their lives, particularly when a female is in or approaching estrous ( Young and Goldman, 1946).
GENETICS. The mountain lion has 19 pairs of chromosomes as do most felids. Eighteen of these pairs are metacentric or submetacentric and one is acrocentric or subacrocentric; the total number of chromosome arms is 37 (most felids are 19-17-2-36) ( Robinson, 1976). Hsu et al. (1963) suggested that one pair of small acrocentric chromosomes was eliminated in mountain lions through pericentric inversion. The X chromosome is medium-sized and metacentric and the Y chromosome is small and submetacentric ( Wurster and Benirschke, 1968).
Of the 15 coat color mutant genes known in the domestic cat ( F. domesticus ), the mountain lion probably exhibits three forms: non-agouti (the yellow or brown band is absent from agouti hairs resulting in a black-appearing coat), albinism, both reported by Young and Goldman (1946), and nonextension of black in agouti hairs, resulting in yellowish or reddish coat color ( Robinson, 1976).
REMARKS. The number of recognized genera in Felidae remains debatable. Simpson (1945) listed three genera: Felis , Panthera , and Acinonyx . Kretzoi (1929) listed more than 60 genera. A few authors recognize Puma as a separate genus for the mountain lion ( Glass and Martin, 1978; Hemmer, 1978; Pocock, 1917 b), but Felis is generally accepted ( Simpson, 1945; Young and Goldman, 1946).
Other vernacular names for the mountain lion include cougar and puma.
TABLE 1. PREY ITEMS REPORTED TAKEN BY MOUNTAIN LIONS (RUSSELL, 1978; SPALDING AND LESOWSKI, 1971; YOUNG AND GOLDMAN, 1946).
| Large | Small | Domestic animals | Other items |
|---|---|---|---|
| Mule deer | Snowshoe hare | Sheep | Turkey |
| White-tailed deer | Other rabbits | Cattle | Ruffed grouse |
| Wapiti | Pika | Horse | Fish |
| Bighorn sheep | Marmot | Burro | Insects |
| Moose | Skunk | Goat | Grass |
| Mountain goat | Ground squirrels | Pig | Berries |
| Pronghorn | Pine squirrel | Dog | Rhea |
| Peccary | Flying squirrel | Cat | |
| Porcupine | Rock squirrel | Chicken | |
| Beaver | Pocket gopher | Peafowl | |
| Badger | Woodrat | ||
| Armadillo | Cotton rat | ||
| Bear | White-footed | ||
| Bobcat | mouse | ||
| Mountain lion | Meadow vole | ||
| Coyote | Raccoon | ||
| Pampas deer | Fox | ||
| Huemul | Coatamundi | ||
| Guanaco | Agouti | ||
| Brocket |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Felis concolor Linnaeus, 1771
| Currier, Mary Jean P. 1983 |
Felis improcera
| Phillips 1912 |
Felis arundivaza
| Hollister 1911: 1 |
Felis aztecus
| Merriam 1903: 73 |
Felis bangsi
| Merriam 1901: 595 |
Felis coryi
| Bangs 1899: 15 |
Felis hippolestes
| Merriam 1897: 21 |
Felis hippolestes
| Merriam 1897 |
Felix (sic) oregonensis
| Rafinesque 1832: 62 |
Felis couguar
| Kerr 1792: 15 |
Felis puma
| Molina 1782: 295 |
Felis concolor
| Linnaeus 1771: 522 |
Felis californica May, 1896:22
| Linnaeus 1758 |