Aricoris schneideri Siewert, Dolibaina, Mielke & Casagrande
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3764.4.10 |
|
publication LSID |
lsid:zoobank.org:pub:E9FA2F10-84AB-4E78-946A-0CAD85B129A2 |
|
DOI |
https://doi.org/10.5281/zenodo.6130509 |
|
persistent identifier |
https://treatment.plazi.org/id/03B30F39-FFC8-FFA1-C48C-2B255F6FFE55 |
|
treatment provided by |
Plazi |
|
scientific name |
Aricoris schneideri Siewert, Dolibaina, Mielke & Casagrande |
| status |
sp. nov. |
Aricoris schneideri Siewert, Dolibaina, Mielke & Casagrande sp. nov.
( Figs. 1–4 View FIGURES 1 – 8 ; 9–12)
Aricoris cinericia ; D’Abrera 1994, p. 1076 (male dorsal and female ventral). – Dolibaina, Mielke & Casagrande 2011. p. 348.
Diagnosis. Aricoris schneideri , sp. nov. belongs to the chilensis species group by its wing pattern and the morphology of the male and female genitalias. It can be associated with A. cinericia ( Figs. 5–8 View FIGURES 1 – 8 ;13–16) due to the dark brown elements on both dorsal wings. Even though, it is easily separated by the absence of any orange elements on forewing under side ( Figs. 1–4 View FIGURES 1 – 8 ), hind wing under side with the presence of a white discal band, from costal margin to 2A and a white postdiscal band from the end Sc+R1 vein to median area of 2A-anal margin, with the initial half strongly inclined laterally, then wavy anteriorly to 2A ( Figs. 2 and 4 View FIGURES 1 – 8 ).
Male description. Head: eyes brown with white marginal segment length, remaining dark brown caling; labial palpi white ventrally and laterally from basis to 2/3 of the second segment length, remaining dark brown, first segment short, second segment long, one and a half times the height of the eye with the initial third curved, third segment thin and anteriorly projected, a third of the second segment; frons densely covered with brown scales and center white, frontoclypeous white; antennae with 3/4 of the costal margin length, brown except for the white ventral surface and checkered with white scales at the basis of the segments in lateral internal view, club rufous brown, with white sparse scales on the ventral surface. Thorax: uniformly dark brown dorsally and white ventrally with a few cream scales; all legs brown externally and white internally.
Forewing, size and shape: length 14 mm (n=1); triangular, costal margin straight, apex thin, outer margin slightly wavy, more pronounced in M3 and in space CuA1–CuA2.
Forewing upper side ( Figs. 1 and 3 View FIGURES 1 – 8 ): ground color brown and light brown at submarginal area; fringes white with brown scales at the end of veins and at the space CuA2–2A; discal cell with white sparse scales at the basis, one faint white spot at the center and another white rounded at the end; four subapical white spots in spaces R2–R3, R3–R4, R4–M1 and M1–M2, the first two very small; two rectangular contiguous white spots at the base of M1–M2 and M2–M3; three discal isolated white spots between M2–M3, M3–CuA1 and CuA1–CuA2, the first two in the middle between the end discal cell and the outer margin; the spot in space CuA1–CuA2 remains below the center between the end discal cell and the spot between M3–CuA1; two superposed fading spots in CuA2–2A aligned with the end of the discal cell; all white spots anteriorly surrounding by dark scales; small circular dark submarginal spots in the spaces from apex to tornus.
Hind wing, shape: rounded, strongly convex from apex to median portion of outer margin with a smooth depression in CuA2–2A; tornus rounded.
Hind wing, upper side ( Figs. 1 and 3 View FIGURES 1 – 8 ): ground color brown; posterior half with elongated greyish scales from basis to median area; outer margin brown; fringes white with brown scales at the end of veins and at CuA2–2A; thin white band from apex toward the center of the wing to M3, constituted by four white spots and separated by the black veins; sparse white scales in M3–CuA1 and CuA1–CuA2.
Forewing, under side ( Figs. 2 and 4 View FIGURES 1 – 8 ): ground color brown; outer margin line thin and brown; fringes as on dorsal surface; costal margin until apical spots with sparse white scales; discal cell with three white spots, the first triangular at the base, the second rectangular transversal in the center and the last also rectangular just before the cell end; other rectangular spot at the base of space M1–M2, M2–M3; apical and discal spots in the same position of the dorsal surface but larger and sharper white; submarginal white band from near apex between R3–R4 to 2A, initially inclined toward the basis then more or less regular, divided by the dark veins, and widely disjunct by CuA1 and CuA2; marginal white band in the spaces between M2 to 2A, widely disjunct by the dark veins with the first spot inclined and subsequent spots parallel to the margin, with CuA2–2A spot linked with the submarginal white spot; remaining area of CuA2–2A also white with four brown spots; 2A to anal margin totally white.
Hind wing, under side ( Figs. 2 and 4 View FIGURES 1 – 8 ): ground color brown; outer marginal line thin and brown; fringes as on dorsal surface; basis brown with white scales; sub-basal band white, from costal margin to 2A, slightly inclined toward basis, proximally and distally bordered by two feeble bands; small, inclined white spot of Sc+R1–Rs, just between sub-basal and discal band; discal white band from end Sc+R1 to the center area of the anal margin, regular in Sc+R1 to initial third of Rs–M1, after strongly inclined toward end cell until M3, the inferior M2–M3 spot has a thin projection toward outer margin that connect the inferior portion of the discal band, from M3 to anal margin, with wavy margins; internally to discal white band in M3–CuA1, CuA1–CuA2 and in a small area that surround inferiorly CuA2 there are faint white mixed with brown scales; externally of the discal white band a brown band, darker betweenM3–2A, and externally to this, from end of M1 to 2A, a large ochre band and after this, an area with sparse white scales from M2 to anal margin, including curve white spots between M3–2A; dark brown rectangular spot in 2A–3A on the tornus.
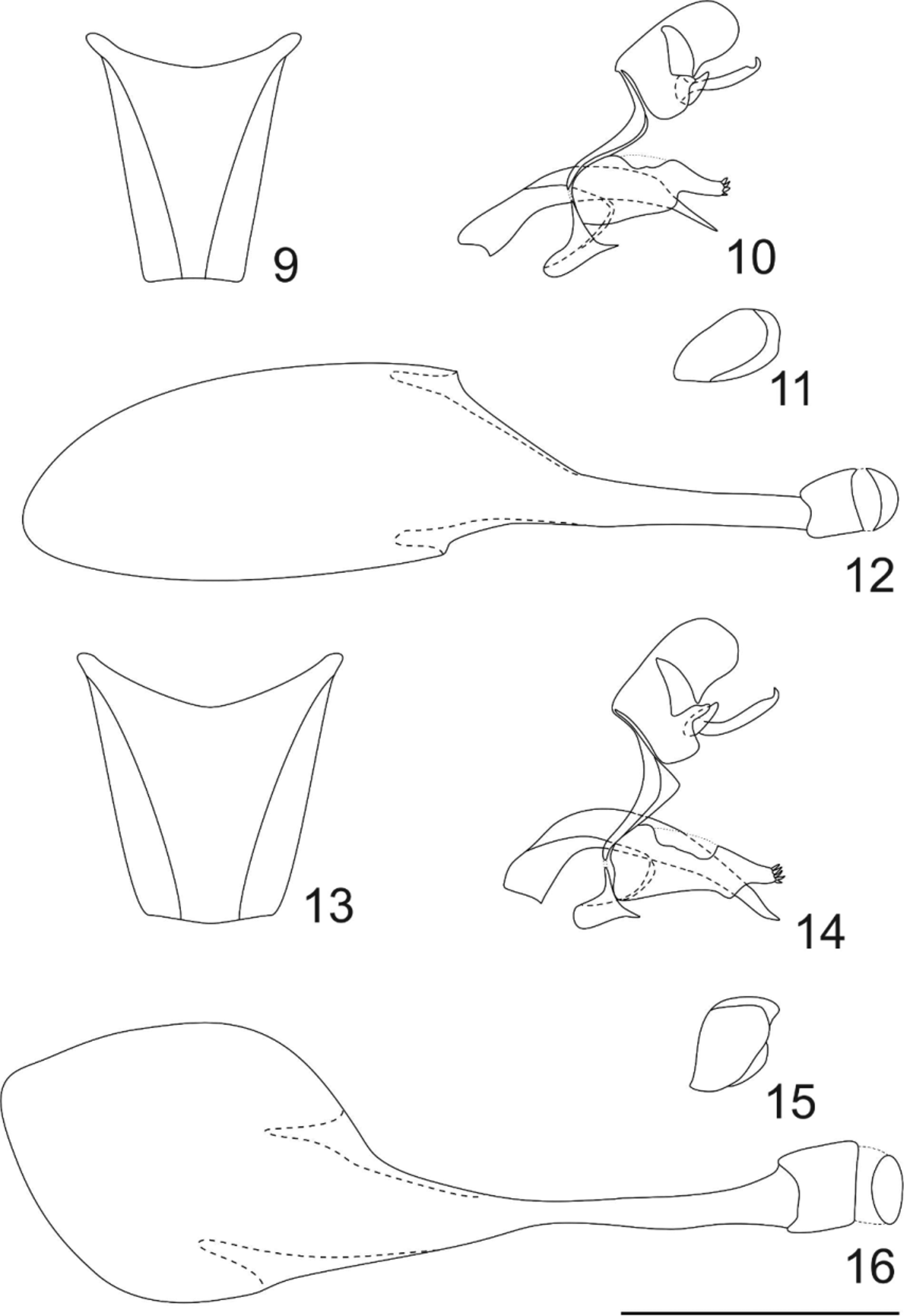
Abdomen: dorsally dark brown with sparse white scales from distal half to end, ventrally white. Eighth male sternite not exceeds beyond the pleural membrane ( Fig. 9 View FIGURES 9 – 16 ).
Genitalia ( Fig. 10 View FIGURES 9 – 16 ): tegumen rectangular, larger than long, ventral projection strongly sinuous, enlarged in the median portion and not fused to dorsal projection of saccus; uncus widely bifid (dorsal view), laterally rounded and larger than long; membranous triangular area between the tegumen and uncus wide; distal inferior projection of the tegumen that connect it with the gnathos thin and short; anterior projection of saccus short (longer than in A. cinericia ), dorsal projection thin and short, connected to ventral projection of the tegumen by a membranous area, posterior projection of saccus thin and short; gnathos bifid, thin and totally bent below the uncus, with a small concave area in distal portion and with end hook-shaped and upturned; valvae elongated and narrow, dorsally concave with rounded margins, distally with a superior tip, a half of the length than the anterior valvae portion and with four spines broad at the basis and slightly pointed at the end; aedeagus thick, twice the length of tegume+uncus and with the distal fourth thinned.
Female description: similar to male, but with wings more elongated and outer margin more convex (length 14.5 mm (n=2)).
Genitalia ( Figs. 11–12 View FIGURES 9 – 16 ): papilla analis twice longer than high, with a wide glabrous area; sterigma sclerotinized, formed by a ringed-shaped lamella antevaginalis and a sclerotinized pocket between ostium bursae and papilla analis; bursae copulatrix totally membranous, twelve times longer than sterigma; ductus bursae thinned until the insertion of signa, with half of bursae length; corpus bursae enlarged and long (half of bursae length) with a pair signa, long, asymmetric in length and with a conical median portion.
Etymology. This new species is named in honor of the late Hipólito Schneider, friend of some of the authors and also collector of this species and other riodinids from natural grassland in Guarapuava region, Paraná State.
Distribution. Aricoris schneideri sp. nov. is known for only four records from southern Brazil, all in natural grassland areas: Candói and Guarapuava in Paraná State and Coronel Bicaco and Jaguari in Rio Grande do Sul State. However, this new species was figured by D’Abrera (1994: 1076 p.) from Paraguay (male) and Misiones, Argentina (female) as A. cinericia .
Type material. Holotype male with the following labels: / HOLOTYPUS / GEN. PREP. DOLIBAINA 2012/ Candoi, Guarapuava, P[a]R[aná], Brasil, 1000 m, 29-III-1972, Moure & Mielke [leg.]/ DZ 23.417/ HOLOTYPUS Aricoris schneideri Siewert, Dolibaina, Mielke & Casagrande det. 2013/. Deposited at DZUP.
Allotype female with the following labels: /ALLOTYPUS/ Candoi, Guarapuava, P[a]R[aná], Brasil, 1000 m, 29-III-1972, Moure & Mielke [leg.]/ DZ 23.437/ ALLOTYPUS Aricoris schneideri Siewert, Dolibaina, Mielke & Casagrande , det. 2013/. Deposited at DZUP.
Paratypes: Brazil: Paraná: Candói, X-1982, Schneider leg. 1 ♀ (DZ 23.427); Rio Grande do Sul: Coronel Bicaco, 6-XII-1992, Moser leg. 1 ♂ ( CLAM); Jaguari, 16-I-2001, Moser leg. 1 ♂ ( CLAM).
Discussion. Through the features already mentioned in the introduction, Aricoris schneideri sp. nov. belongs to chilensis species group, which has three other valid species and, at least, ten names were used to describe the diversity of the group and that are currently considered synonyms ( Hall & Harvey 2002, Callaghan & Lamas 2004). Among these species, A. schneideri sp. nov. seems to be related to A. cinericia , especially with the general color pattern of the dorsal wings which includes a background color brown with some white elements, a distinctive characteristic of the other species from this group.
The main characters of the wings which distinguish this new species from A. cinericia are the absence of orange elements on the wings and the presence of a white discal band and another white postdiscal band on the ventral surface of the hind wing. This last characteristic occurs in other species of the chilensis species group, including most synonyms. However, A. umbrata (Giacomelli, 1928) , currently a synonym of A. chilensis , has a different pattern, because such bands are absent on the ventral surface of the forewing as in A. cinericia . This evidence could put in doubt the description of A. schneideri sp. nov. as a distinct species, as this would only represent a phenotypic form from A. cinericia . Nevertheless, some of the phenotypes which represent distinct synonyms of A. chilensis were currently found in sympatry, arising from distinct immatures and associated with different species of ants and host plants, and this fact could be a strong argument to suggest that some of the synonyms currently associated with A. chilensis correspond to valid species and should have reassessed its taxonomic status (L. A. Kaminski & L. Volkmann, in prep.).
Callaghan & Soares (2001) reviewed the ‘Cinericiiformes’ from Stichel (1910) and established two new synonyms to Aricoris cinericia, Haemearis precaria Schweizer & Kay, 1941 and Haemearis precaria ab. similis Schweizer & Kay, 1941, both from Casa Blanca, Paysandu, Uruguay. Still Callaghan & Soares (2001) found that the type series used by Schweizer & Kay (1941) or any other specimen belonging to this species were not found at the Museo de Historia Natural de Montevideo (MHNM), and through museum’s information, those specimens may have been lost. However, Schweizer & Kay (1941) provided images of the holotype and allotype of H. precaria and from the female holotype of H. precaria ab. similis and illustrated the male genitalia of H. precaria (from holotype or paratype), thus allowing the immediate association of A. cinericia with these names. Nevertheless, according to Callaghan & Soares (2001) the male specimen indicated as the holotype of H. precaria and figured by Schweizer & Kay (1941) is not conspecific with the female and could represent a male of A. chilensis . This information is probably due to the fact that Callaghan & Soares (2001) do not have the male of A. cinericia and, if the statement of the authors were confirmed, H. precaria would correspond to a synonym of A. chilensis and not to A. cinericia as proposed in the paper. However, the male illustrated by Schweizer & Kay (1941) represent A. cinericia and it has the same wing pattern that the male figured in the present paper ( Figs. 5–6 View FIGURES 1 – 8 ).
There is still a taxon, somewhat obscure, described by Schneider (1937) as a new species, Haemearis arenarum Schneider, 1937, from Rincon de la Ronda, Uruguay. During the phylogeny from the basal subtribes of Nymphidiini , Hall & Harvey (2002) provided a checklist with all valid species for the genus Aricoris and mentioned that they have not examined the type material from H. arenarum, but indicated that this could represent a synonym of some species already known in the genus. During the description of this species, Schneider (1937) mentioned that the individuals have an erratic flight, the male has a darker color on the surface of both wings and in the ventral surface of the forewing has orange elements in the middle of black and white spots, and on the ventral surface of the hind wing has some darker lines, but he does not mention the presence of white elements. According to him, the female is more common than the male and has the general orange pattern of the forewing. Clearly, the male and the female described by Schneider (1937) are not conspecific and should represent two distinct species. By having the wing pattern dorsally orange, the female could represent a different species occurring in the austral South America, while the male has features only found in A. cinericia . In addition, Schneider (1937) mentions that specimens were collected in a small “desert”, similar to the xerophile environment of the A. cinericia ’s records in Corrientes and Entre Rios (Núñez Bustos, comm. pers.). All these evidences shows that H. arenarum did not represent A. schneideri sp. nov., but a synonym of A. cinericia , however, the absence of any information about the number of individuals used by Schneider (1937) or from the type material preclude the assertion of the identity of this taxon.
| DZUP |
Universidade Federal do Parana, Colecao de Entomologia Pe. Jesus Santiago Moure |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |