Potamonautes licoensis, Daniels & Bittencourt-Silva & Muianga & Bayliss, 2020
|
publication ID |
https://doi.org/ 10.5852/ejt.2020.716 |
|
publication LSID |
lsid:zoobank.org:pub:7C4A53D7-B8F2-4E8F-85B3-41C6EEE56E97 |
|
DOI |
https://doi.org/10.5281/zenodo.4323836 |
|
persistent identifier |
https://treatment.plazi.org/id/0DCF9EC6-858E-4E0F-BFFA-CE17095DCCE7 |
|
taxon LSID |
lsid:zoobank.org:act:0DCF9EC6-858E-4E0F-BFFA-CE17095DCCE7 |
|
treatment provided by |
Valdenar |
|
scientific name |
Potamonautes licoensis |
| status |
sp. nov. |
Potamonautes licoensis View in CoL sp. nov.
urn:lsid:zoobank.org:act:0DCF9EC6-858E-4E0F-BFFA-CE17095DCCE7
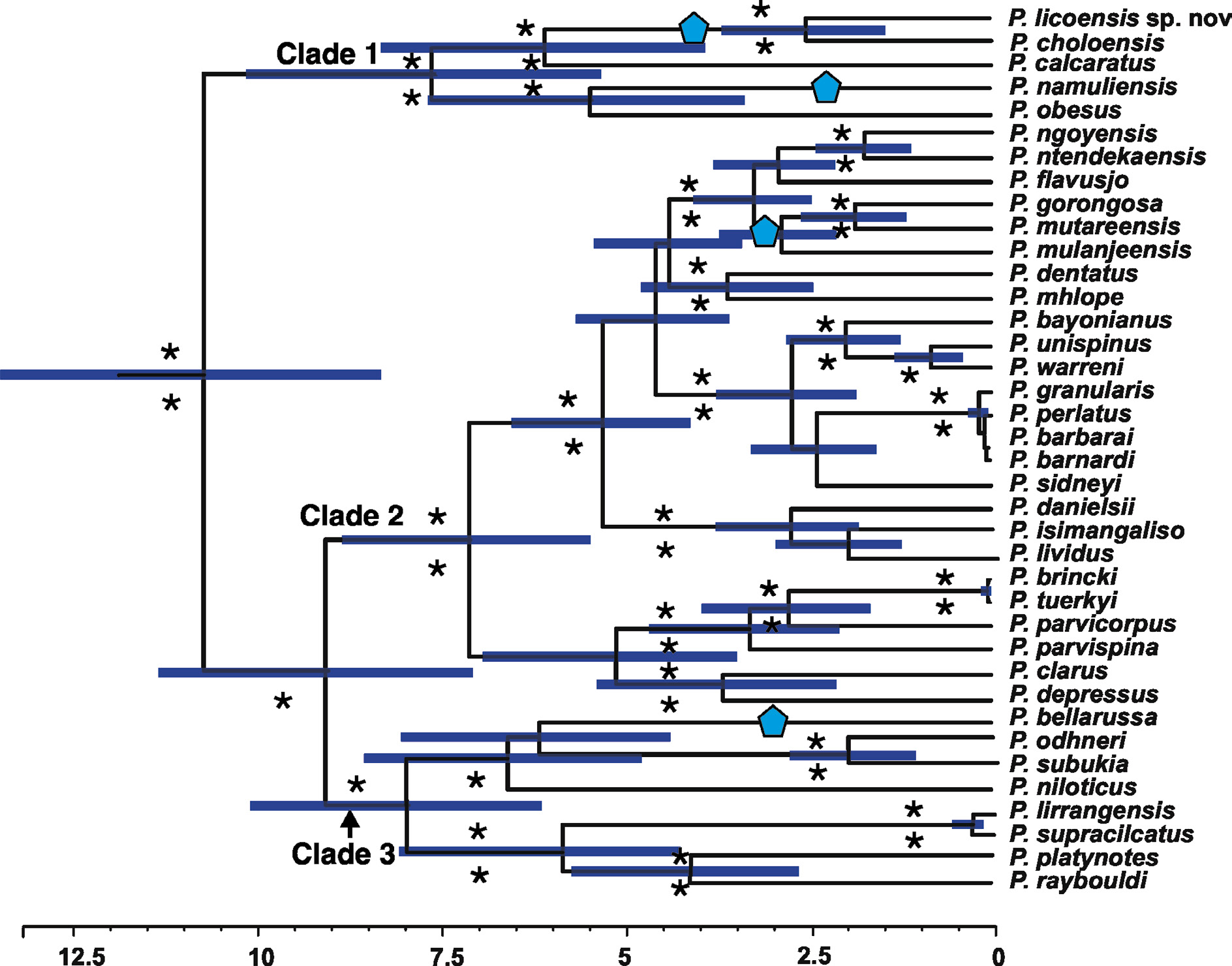
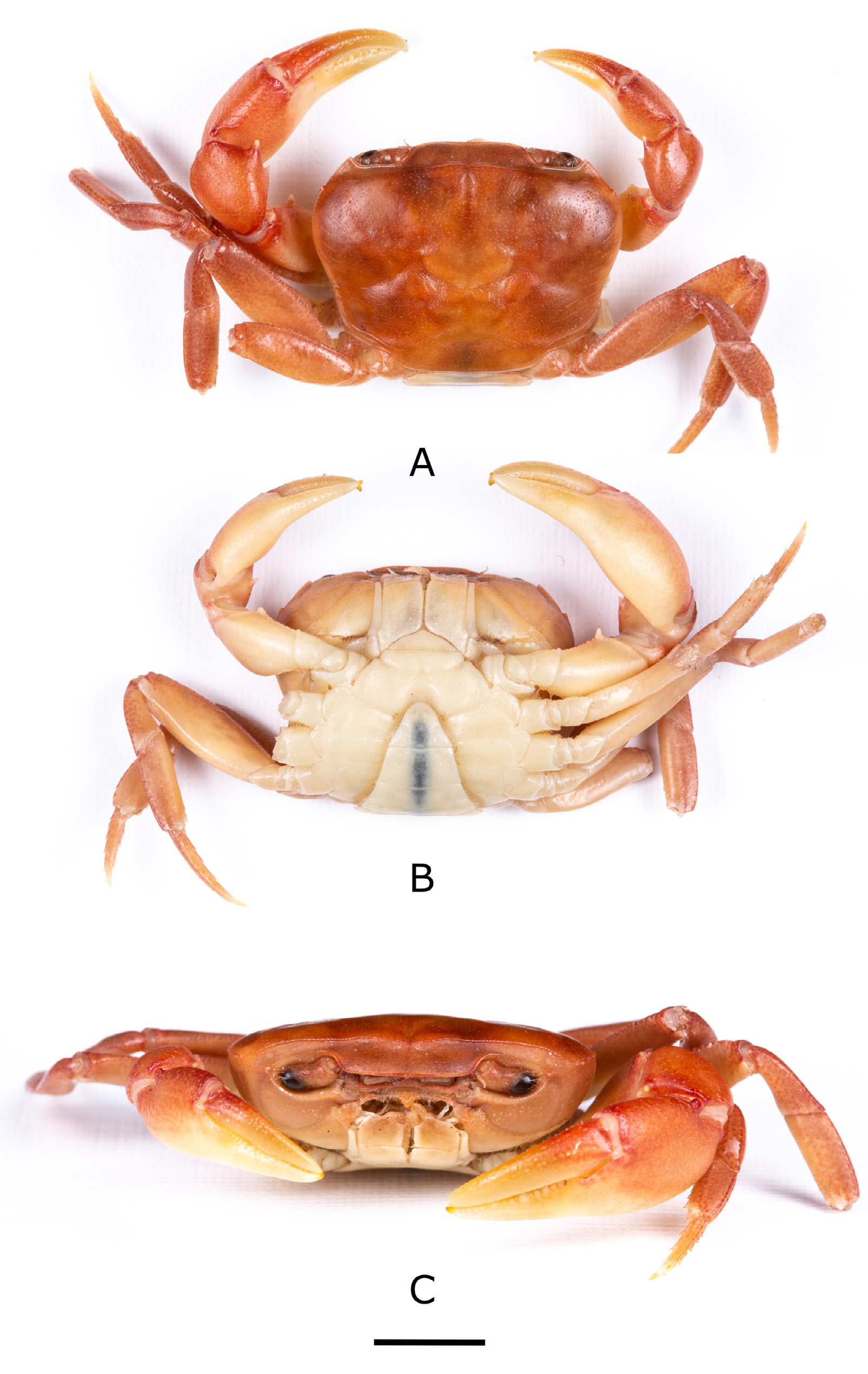
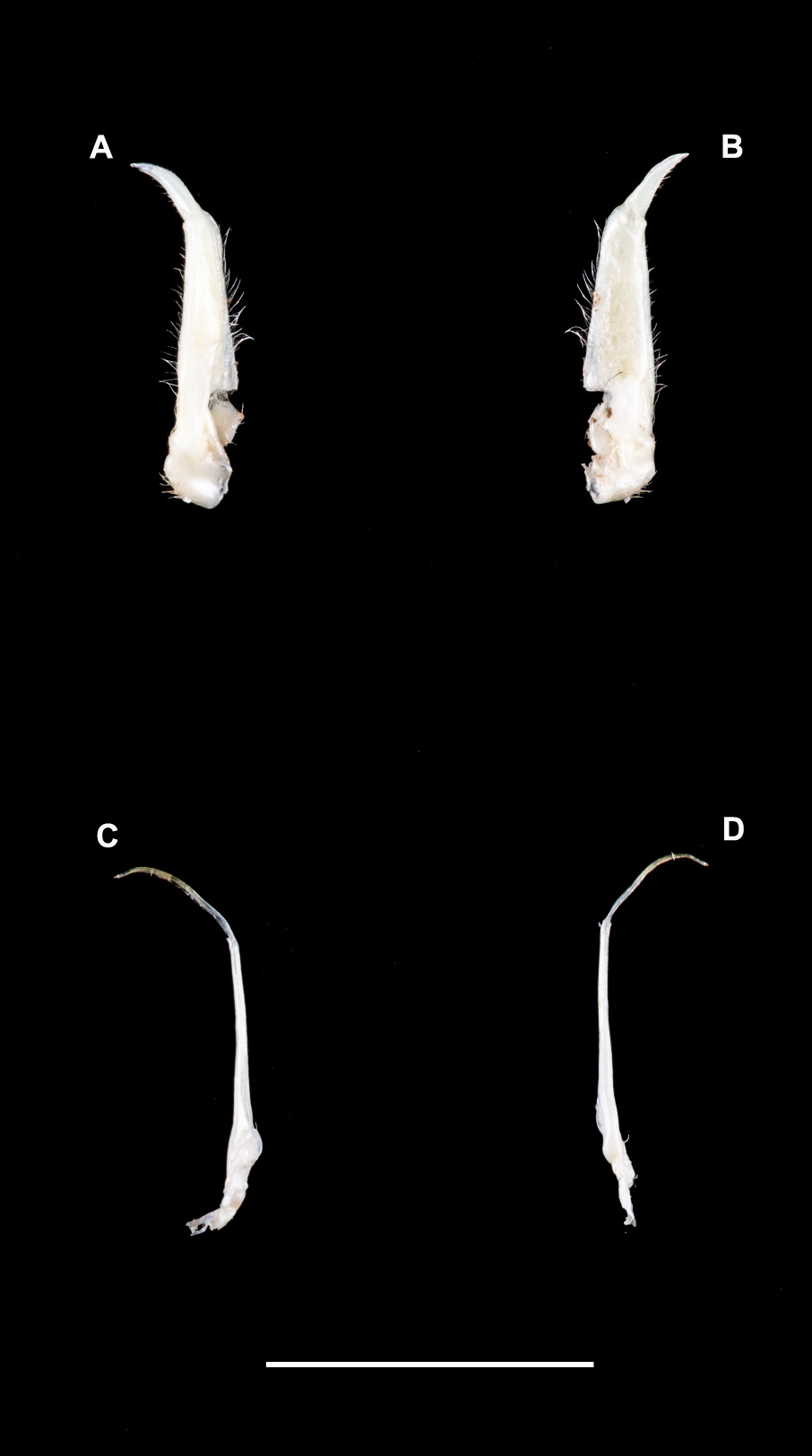
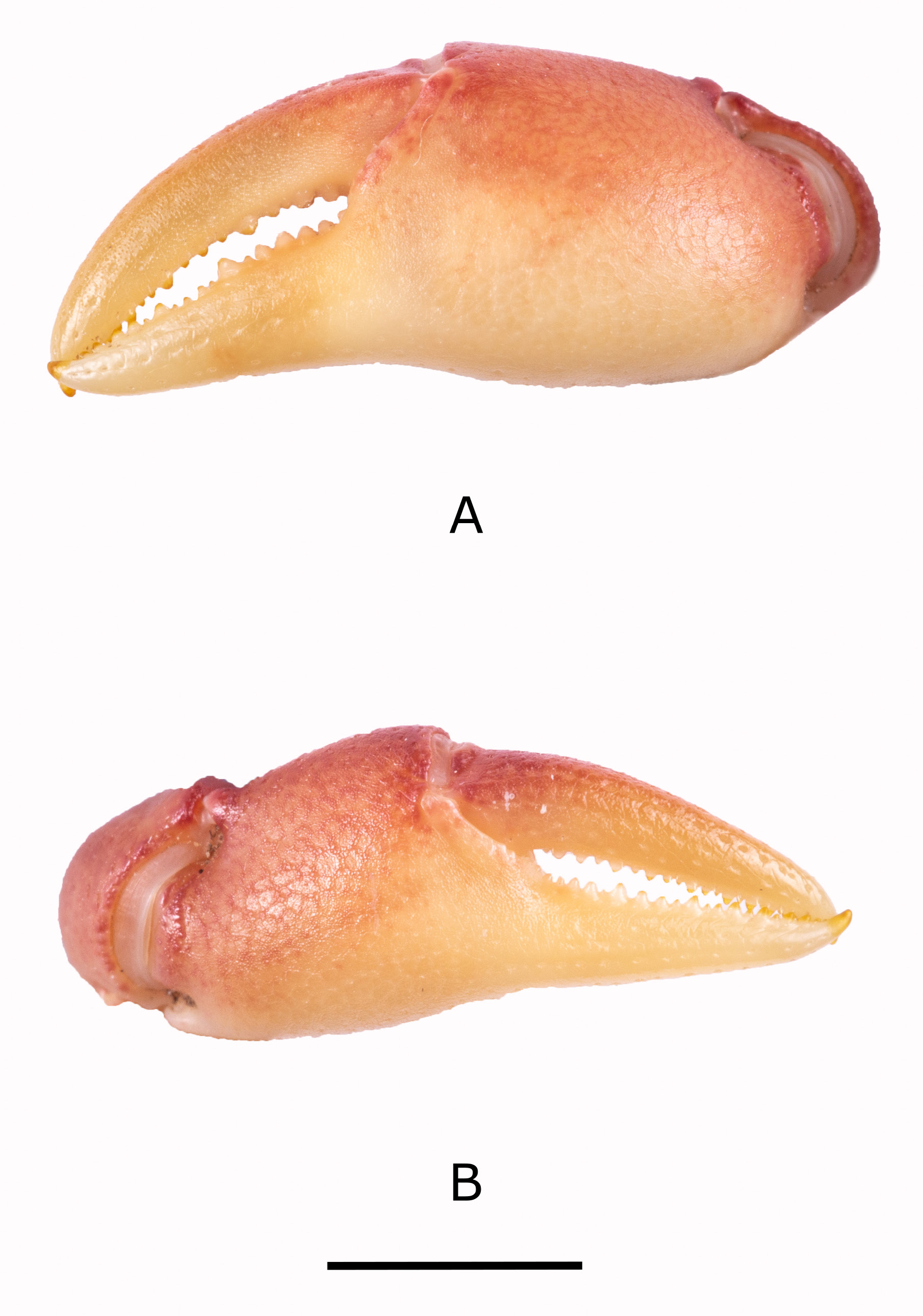
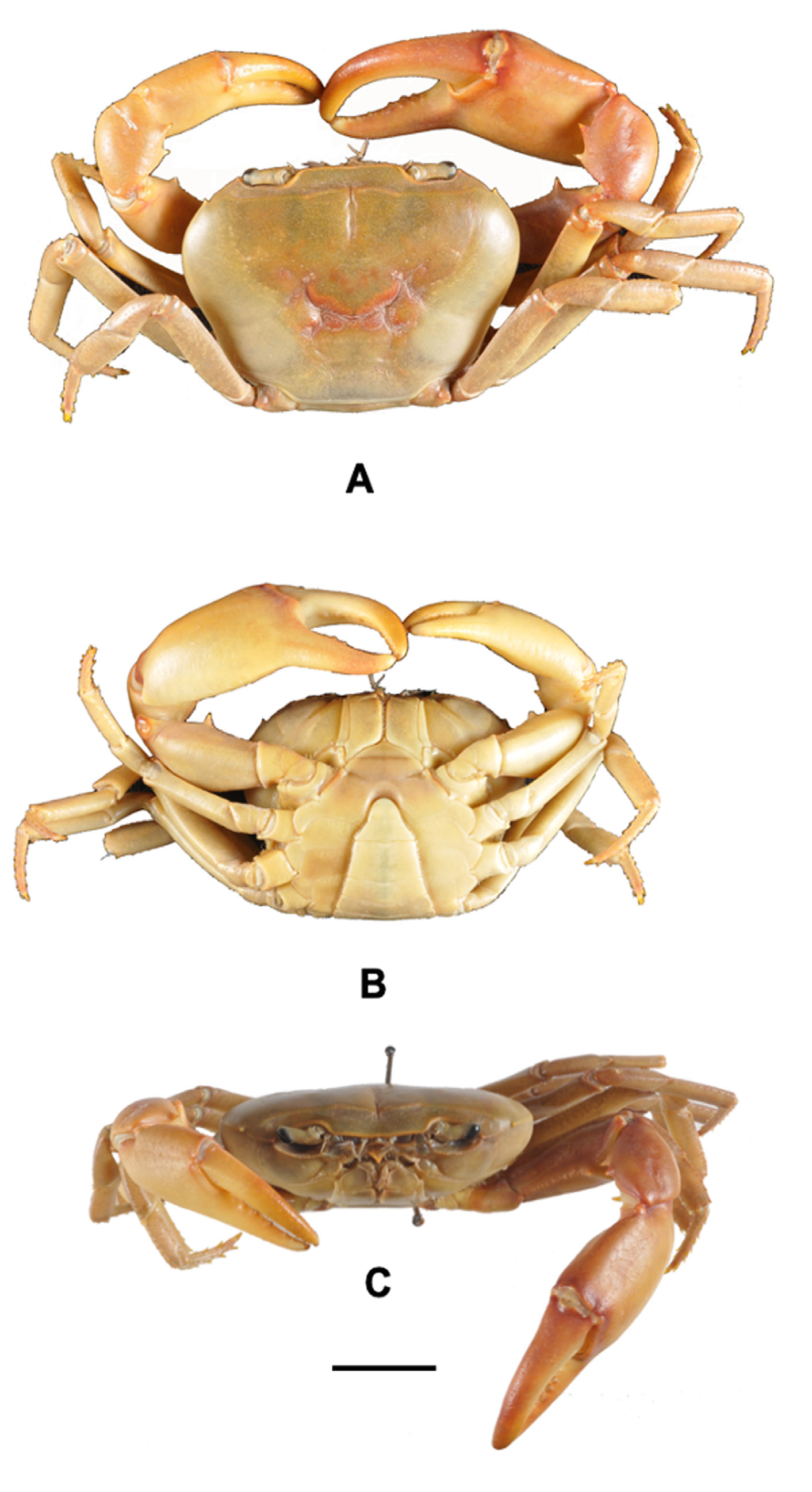
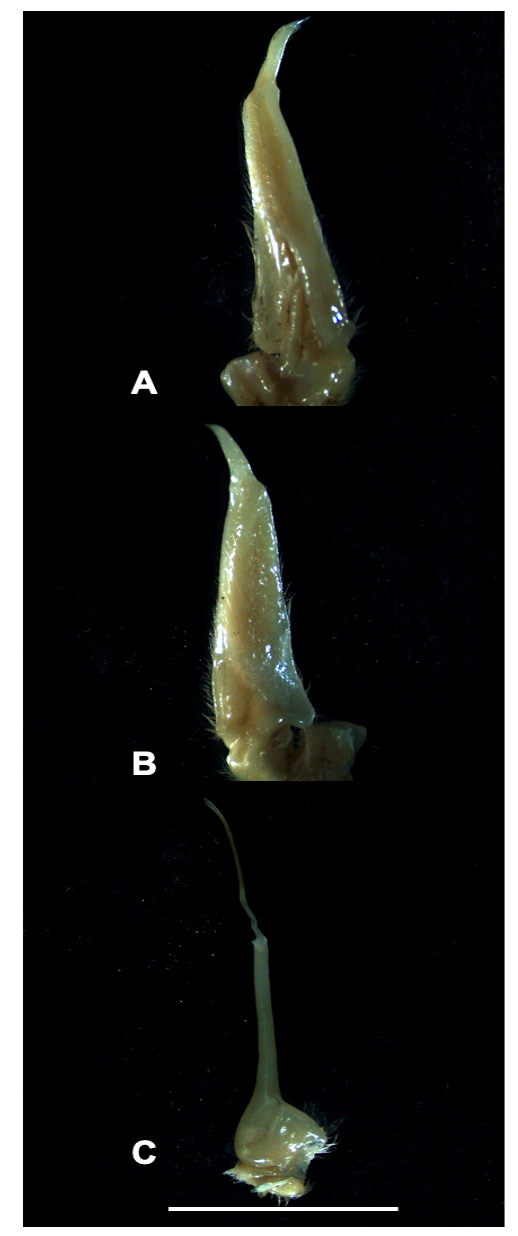
Figs 1 View Fig , 2 View Fig A–C, 3A–D, 4A–B, 5A–B, 8; Table 1 View Table 1
Diagnosis
Carapace: highly flat ( CH /CL = 0.44) ( Table 1 View Table 1 ); postfrontal crest well-defined, complete, lateral ends meeting anterolateral margins; epigastric crests faint, median sulcus between crests short, not forked posteriorly; exorbital, epibranchial teeth reduced to granules; anterolateral carapace margin with small tooth epibranchial ( Figs 2 View Fig A–C, 5A). Third maxilliped: ischium with distinct vertical sulcus ( Fig. 3C View Fig ); s3/s4 complete, V-shaped, deep, midpoint almost meeting anterior margin of sterno-pleonal cavity; margins of s4 low, not raised ( Fig. 2B View Fig ). Cheliped: dactylus (moveable finger) slim, highly arched, enclosing oval interspace, with three larger teeth interspersed by smaller teeth along length; propodus (fixed finger) with four larger teeth interspersed by smaller teeth along length ( Fig. 2 View Fig A–C); carpus inner margin distal tooth large, pointed, proximal tooth reduced to granules ( Fig. 3A View Fig ); medial inferior margin of merus lined with series of small granules terminating distally at small, low distal meral tooth, lateral inferior margin smooth. G1 terminal article: ¼ rd length of subterminal segment; first third straight in line with longitudinal axis of subterminal segment, middle part directed outward at 45°, widened by raised rounded ventral lobe, tip curving sharply upward ( Fig. 3 View Fig A–B).
Etymology
Named for Mount Lico, from where the species was first collected.
Material examined
Holotype
MOZAMBIQUE • ♂ adult; Zambezia Province, top of Mount Lico; -15.79196° S, 37.36057° E; 900 m a.s.l.; 9 Sep. 2019; Julian Bayliss leg.; forest streams; SAM C-A091399 . GoogleMaps
Paratype
MOZAMBIQUE • ♂ adult; Zambezia Province, top of Mount Lico; 5 May 2018; Julian Bayliss leg.; SAM C-A091400 .
Other material examined
MOZAMBIQUE – Zambezia Province • 2 ♀♀ adults, 1 ♂ adult, 4 juvs; top of Mount Lico; 10 Sep. 2019; Julian Bayliss leg.; forest streams; SAM C-A091401 • 1 ♂ adult, 1 ♀ adult, 1 juv.; Mount Lico ; 16 May 2018; Julian Bayliss leg.; SAM C-A091402 • 1 ♂ adult, 5 juvs; Mount Lico base stream; 9 Sep. 2019; Gabriella Bittencourt-Silva leg.; SAM C-A091402 ; 2 ♀♀ adults, 3 juvs ; Mount Lico base stream; 16 May 2018; Vanessa Muianga leg.; SAM C-A091403 .
Description
Based on male holotype (CWW 22.89 mm, Table 1 View Table 1 ). Carapace with small distinct tooth on the anteriolateral margins; widest anteriorly, narrowest posteriorly (CWP/CL 0.60); flattened ( CH /CL 0.43) ( Fig. 2A View Fig ); front broad, ¼ CWW (FW/CWW 0.37); urogastric, cardiac grooves distinct, other grooves faint or missing; postfrontal crest complete, anterolateral margin posterior to epibranchial tooth granulated, meeting epibranchial teeth; epigastric crests faint, median sulcus between crests short, forked posteriorly; exorbital, epibranchial teeth each reduced to granule; anterolateral margin between exorbital, epibranchial teeth faintly granulated, curving slightly outward, lacking intermediate tooth; ( Fig. 2 View Fig B–C) branchiostegal wall vertical sulcus faint, meeting longitudinal sulcus, dividing branchiostegal wall into 3 parts, suborbital, dorsal pterygostomial regions granulated, hepatic region smooth; suborbital margin faintly granulated. Third maxilliped: filling entire buccal frame, except for respiratory openings; exopod with long flagellum, ischium with faint vertical groove ( Fig. 4A View Fig ). Epistomial tooth large, triangular, margins lined by large granules. Mandible: palp two-segmented; terminal segment simple; tuft of setae at junction between segments. Sternum: s1, s2 fused; s2/s3 deep, completely crossing sternum; s3/s4 complete, V-shaped, deep, midpoint almost meeting anterior margin of sterno-pleonal cavity; margins of s4 low, not raised. Cheliped: dactylus (moveable finger) slim, arched, enclosing oval interspace, with three larger teeth interspersed by smaller teeth along length; propodus (fixed finger) with four larger teeth interspersed by smaller teeth along length ( Fig. 4 View Fig A–B); carpus distal tooth large, pointed, proximal tooth small but distinct, followed by granule; both inferior margins of merus lined by series of small granules, distal meral tooth small, pointed. Pereopods: walking legs slender, pereopod 3 longest, pereopod 5 shortest; dorsal margins of pereopods with fine sharp bristles, dactyli of walking legs ending in sharp point, with rows of spine-like bristles along segment. Pleon: outline broadly triangular with straight margins. G1 terminal article: short (⅓ rd length of subterminal segment), curving away from midline, first third straight in line with longitudinal axis of subterminal segment, middle part directed outward at 45°, widened by low raised rounded ventral lobe, tip curving gently upward. G1 subterminal segment broad at base, tapering to slim junction with terminal article distally where these two parts have same width, ventral side of segment with heavily setose margins; with setae-fringed flap covering lateral half of segment; dorsal side of segment smooth, no flap, with broad membrane on the dorsal side of suture marking junction between terminal, subterminal parts ( Fig. 3 View Fig A–B). G2: terminal article long, flagellum-like, 0.5 times as long as of subterminal segment ( Fig. 3 View Fig C–D).
Size
A small-bodied species (CL = 17.55 mm; CWW = 22.89 mm) ( Table 1 View Table 1 ), typical of mountain stream living freshwater crabs.
Colour in life
Carapace dark to light brown in living specimens ( Fig. 5B View Fig ).
Type locality
Mount Lico, Zambezia Province, Mozambique.
Habitat
Primary rain forest streams on top of Mount Lico, Zambezia Province, Mozambique. Frequently found under small stones in first order streams ( Fig. 5A View Fig ).
Distribution
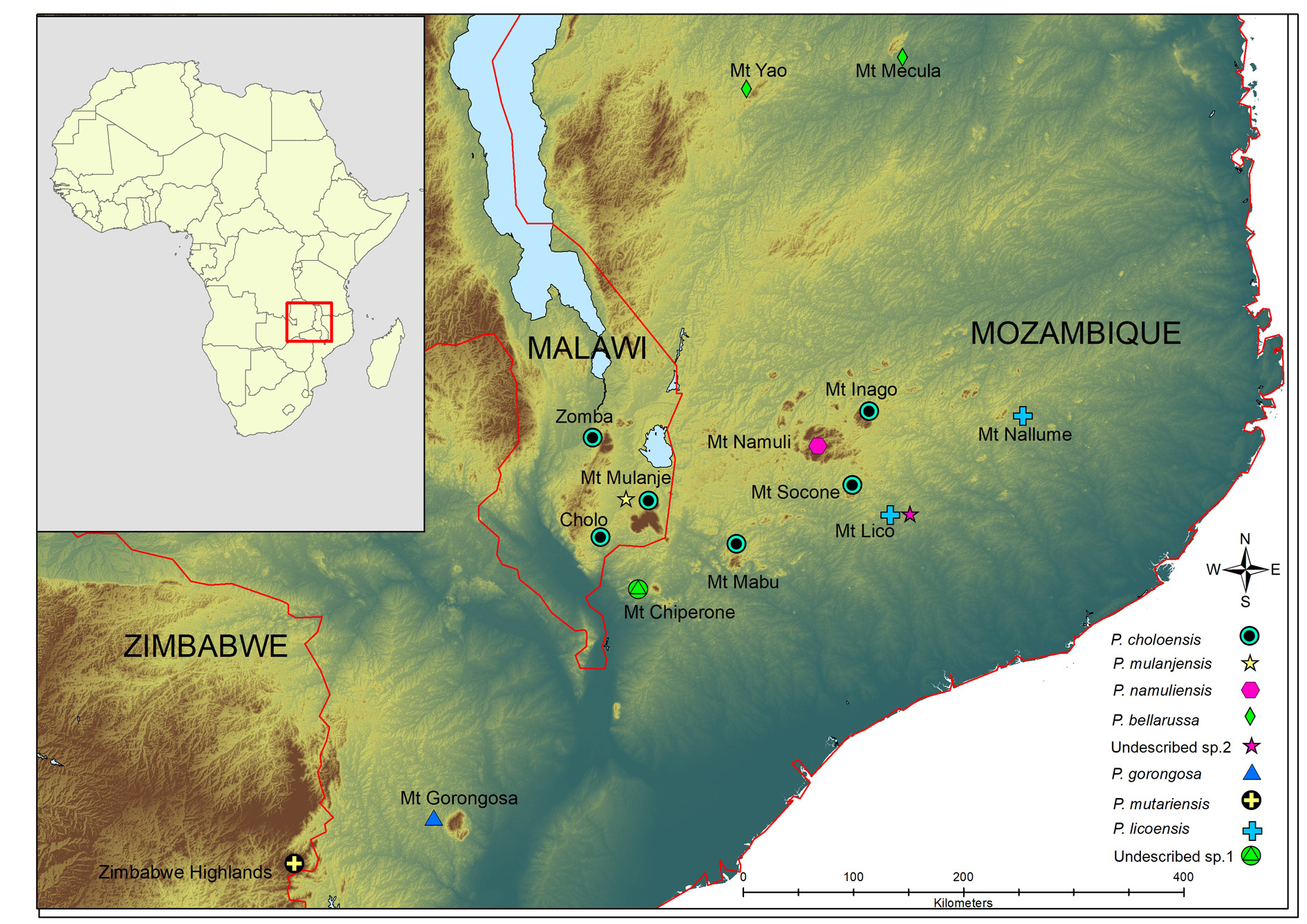
Known from Mount Lico, Zambezia Province, Mozambique. More recently, the species has also been collected from Mount Nallume (Daniels pers. obs.).
Remarks
Phylogenetically, P. licoensis sp. nov. was retrieved as sister to P. choloensis ( Fig. 1 View Fig ). Both species are characterized by a small but prominent epibranchial tooth on the anterolateral margins of the carapace and a arched right dactylus. However, the two species can easily be distinguished morphologically. Potamonautes choloensis is a large-bodied species (CL> 42.00 mm) ( Fig. 6 View Fig A–C; Table 2 View Table 2 ), with a swollen carapace ( CH /CL = 0.50), while P. licoensis sp. nov. is small-bodied (CL> 20.00 mm) and has a more flat carapace ( CH /CL = 0.44). Potamonautes licoensis sp. nov. shows adaptations to living under stones in fast flowing mountain streams. Gonopods 1 and 2 of P. choloensis and P. licoensis sp. nov. are different in appearance. Gonopod 1 is nearly straight ( Fig. 7 View Fig A–B) in P. choloensis , while arching in P. licoensis sp. nov. ( Fig. 3 View Fig A–B). The terminal segment of gonopod 2 is filamentous in both species, however, in P. choloensis it is less curved ( Fig. 7C View Fig ) while it exhibits a significant curvature in P. licoensis sp. nov. ( Fig. 3 View Fig C–D). Furthermore, the two species are characterized by an uncorrected ‘p’ distance for the COI locus similar to what has been recorded in other sister species pairs ( Daniels & Bayliss, 2012; Daniels 2017; Daniels et al. 2019). Similarly, Gouws et al. (2015) found deep genetic divergence, ranging from 9.20 to 11.80% between lineages within P. sidneyi , leading to the recognition of P. danielsi . In addition, P. choloensis and P. licoensis sp. nov. are ecologically distinct. While both species are associated with forest streams at high altitude, Potamonautes choloensis is known to occur between 1000–2000 m a.s.l. and has been collected from Mounts Inago, Mulanje, Mabu, Cholo, the Zomba Plateau and more recently from Mount Socone ( Fig. 8 View Fig ). The species is present in both Malawi and Mozambique (Daniels unpubl. data; Bayliss pers. com.; Chace 1953; Daniels & Bayliss 2012). Potamonautes licoensis sp. nov. is known from a maximum of 700–900 m a.s.l. and is present on Mounts Lico and Nallume, and is endemic to Mozambique. More extensive sampling is required to confirm the distribution of P. licoensis sp. nov.
Superficially, P. licoensis sp. nov. resembles two other freshwater crab species that are also present in Mozambique, to whom it is phylogenetically related ( Fig. 1 View Fig ; clade 1). Potamonautes obsesus and P. calcaratus both have a single small, near spine-like tooth on the anterolateral margin of the carapace. However, both of the latter species are large-bodied and characterized by near swollen carapaces, chelipeds and gonopods, morphologically distinct from P. licoensis sp. nov. ( Reed & Cumberlidge 2004). In addition, P. obesus and P. calcaratus are both burrowing, semi-terrestrial species, and live in ephemeral pans in low-lying areas, where they dig into soft soil to locate the water table during the dry season ( Daniels et al. 2002a; Reed & Cumberlidge 2004). Both species are widespread; P. calcaratus is present in northeastern South Africa and into Mozambique, while P. obsesus is present in Somalia, Kenya, Tanzania, Malawi, Mozambique and Zimbabwe ( Reed & Cumberlidge 2004). Both species show deep genetic differentiation, a pattern typical of burrowing species, and both might potentially represent a species complex ( Daniels & Bayliss 2012). Potamonautes licoensis sp. nov., in contrast, lives at high altitude in rain forest streams. Potamonautes namuliensis , endemic to Mount Namuli in Mozambique, has a smooth anterolateral margin with an arched dactylus of the major pereopod and can easily be differentiated from P. licoensis sp. nov. Potamonautes montivagus ( Chace 1953) and P. bellarussus are both large bodied species with CL> 43.8 mm and 39.29 mm, respectively, and lack dentition on the anterolateral carapace margins ( Chace 1953; Daniels et al. 2014). In P. montivagus the terminal segment of gonopod 1 is at a 90 o angle, very distinct from both P. bellarussus and P. licoensis sp. nov. Potamonautes montivagus is distributed in Malawi where it is present on Mounts Cholo, Mulanje (formerly spelled Mlanje), Chiradzulu, Ntchisi, Zomba Plateau and Dedza ( Chace 1953). Potamonautes bellarussus is only known from two localities, Mounts Mecula and Yao in the Niassa Province of Mozambique, although specimens have recently been collected from neighbouring Malawi and Tanzania ( Daniels et al. 2014; N. Cumberlidge and M. Genner pers. com.). Potamonautes sidneyi is present in Mozambique and widespread in southern Africa. It is a large bodied species mainly found in rivers and lacks dentition on the anterolateral carapace margin ( Peer et al. 2017). Phylogenetically, P. sidneyi is not closely related to P. licoensis sp. nov., ( Fig. 1 View Fig ; clade 2). Potamonautes sidneyi is a species complex and comprises several genetically distinct lineages and is in need of a taxonomic revision ( Gouws et al. 2015). Potamonautes bayonianus is present in Mozambique, however, it is a large-bodied riverine species, with a sharp and prominent tooth on the anterolateral carapace margin and distantly related to P. licoensis sp. nov. Finally, P. gorongosa , endemic to Mozambique, is phylogenetically and morphologically distinct from P. licoensis sp. nov. The former species lacks a dentition on the anterolateral carapace margin and does not have the highly-arched dactylus ( Fig. 1 View Fig ; clade 2) of P. licoensis sp. nov. ( Fig. 1 View Fig ; clade 1). Potamonautes licoensis sp. nov. bears a superficial resemblance to P. parvispina ( Stewart 1997) . The latter species is also mountain-living, has a small tooth on the anterolateral carapace margins and a highly-arched right dactylus. However, P. parvispina is endemic to the Cederberg Mountains in the Western Cape Province, South Africa, and is phylogenetically distantly related to P. licoensis sp. nov. ( Fig. 1 View Fig ; clade 2). All the remaining South African mountain-living freshwater crab species ( P. clarus , P. depressus , P. brincki , P. parvicorpus and P. tuerkayi ) lack dentition on the anterolateral carapace margin and are phylogenetically distinct from P. licoensis sp. nov.
Table 1. Potamonautes licoensis sp. nov., measurements (in mm) of the holotype and of additional male and female specimens examined, presented as a range.
| Variable | Abbreviation | Holotype | Males | Females |
|---|---|---|---|---|
| carapace length | CL | 17.55 | 19.91–13.13 | 16.62–12.29 |
| carapace width at widest point | CWW | 22.89 | 20.91–16.56 | 20.01–15.76 |
| carapace posterior margin | CWP | 10.66 | 11.01–8.34 | 10.71–7.98 |
| frontal width | FW | 8.60 | 8.10–8.33 | 8.17–5.44 |
| distance between postfrontal crest and anterior margin | PFCD | 2.74 | 2.48–2.34 | 2.64–2.06 |
| carapace height | CH | 7.75 | 7.66–6.05 | 7.61–5.84 |
| major cheliped propodus length | MCPL | 22.59 | 11.55–8.72 | 11.79–8.72 |
| pereiopod 2, merus length | P2ML | 9.88 | 8.53–7.06 | 8.15–7.14 |
| pereiopod 2, merus width | P2MW | 3.61 | 3.41–2.74 | 2.67–2.56 |
| pereiopod 5, merus length | P5ML | 9.36 | 9.01–7.79 | 10.09–7.29 |
| pereiopod 5, merus width | P5MW | 3.07 | 3.81–3.01 | 2.79–3.10 |
| SAM |
South African Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |