Guimaraesiella (Malardifax), 2022
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5165.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:A03F9711-19D7-4D7A-B30E-842DA141B2A0 |
|
DOI |
https://doi.org/10.5281/zenodo.6836489 |
|
persistent identifier |
https://treatment.plazi.org/id/03B15059-B37D-FFEC-FF41-FC18FC73F82C |
|
treatment provided by |
Plazi |
|
scientific name |
Guimaraesiella (Malardifax) |
| status |
subgen. nov. |
Malardifax Gustafsson & Bush , new subgenus
Type species: Guimaraesiella pandolura pandolura Gustafsson & Bush, 2017: 231 .
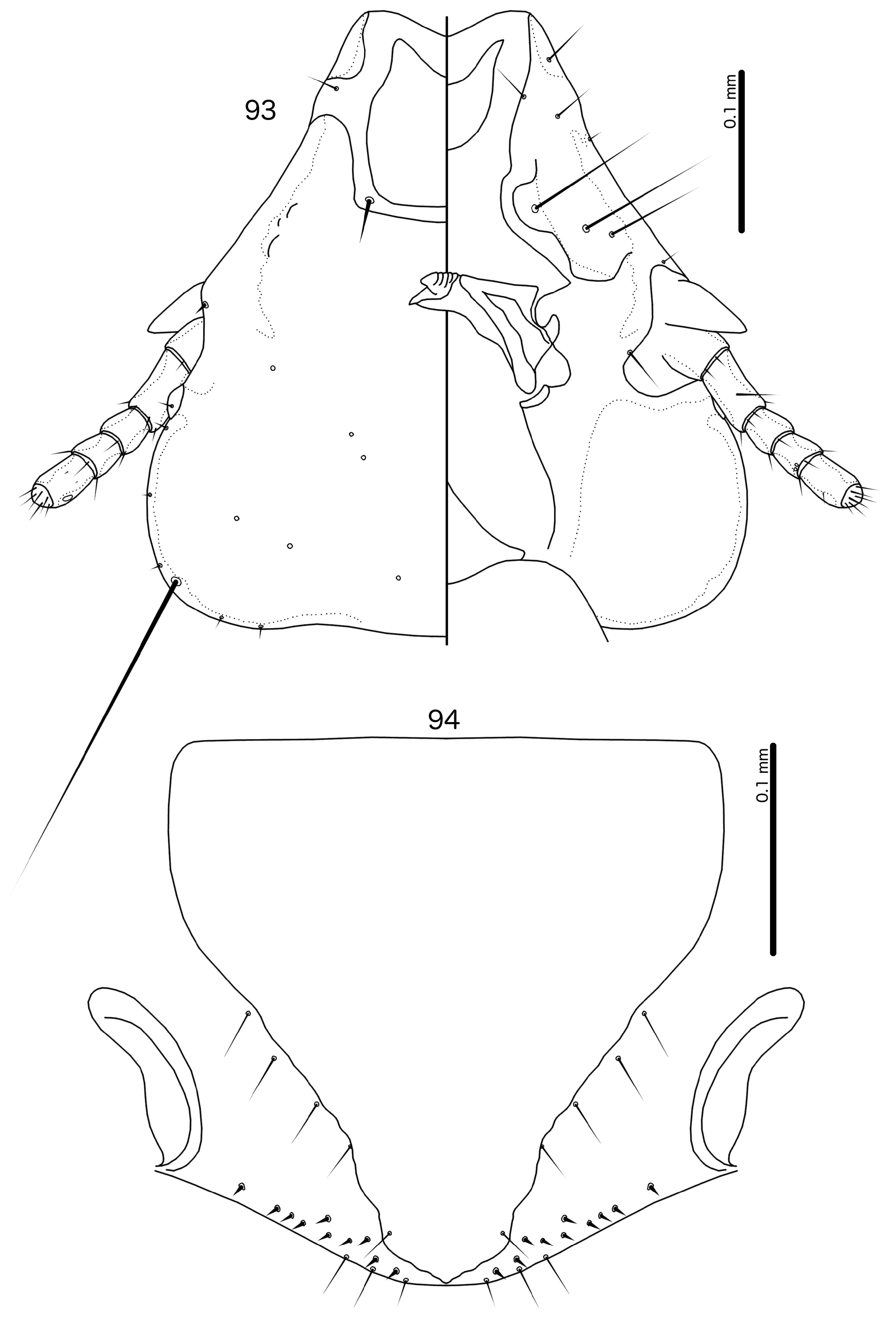
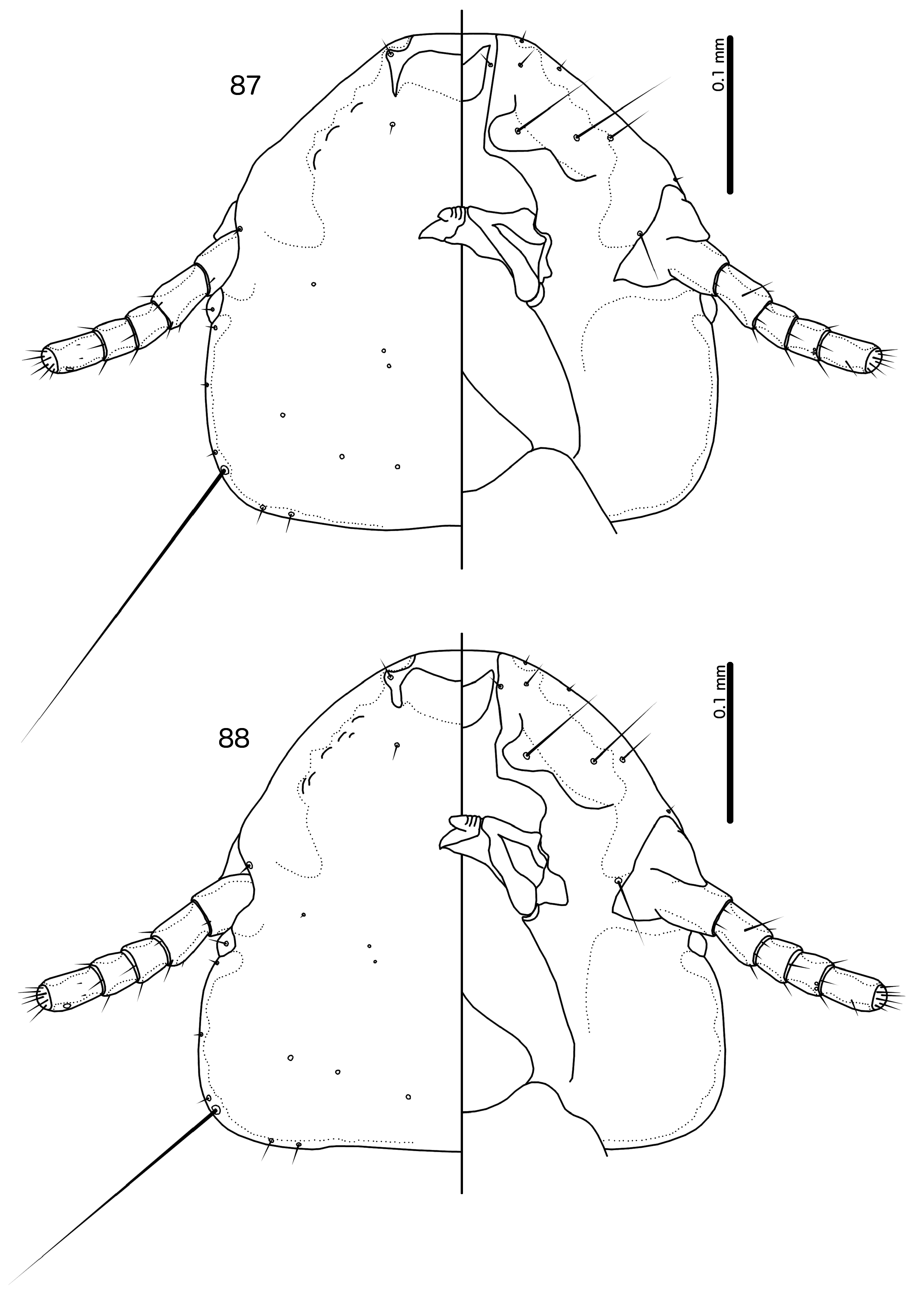
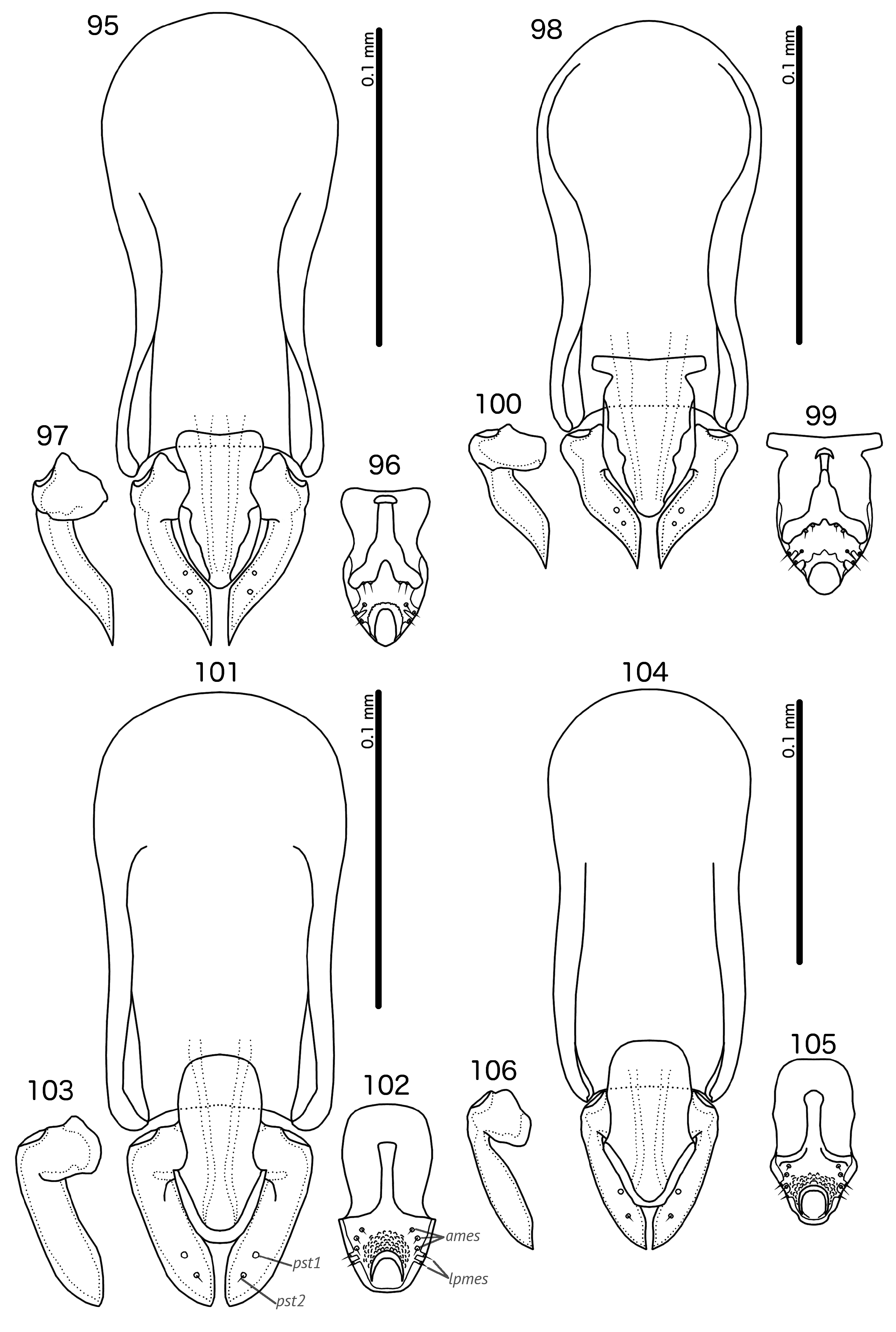
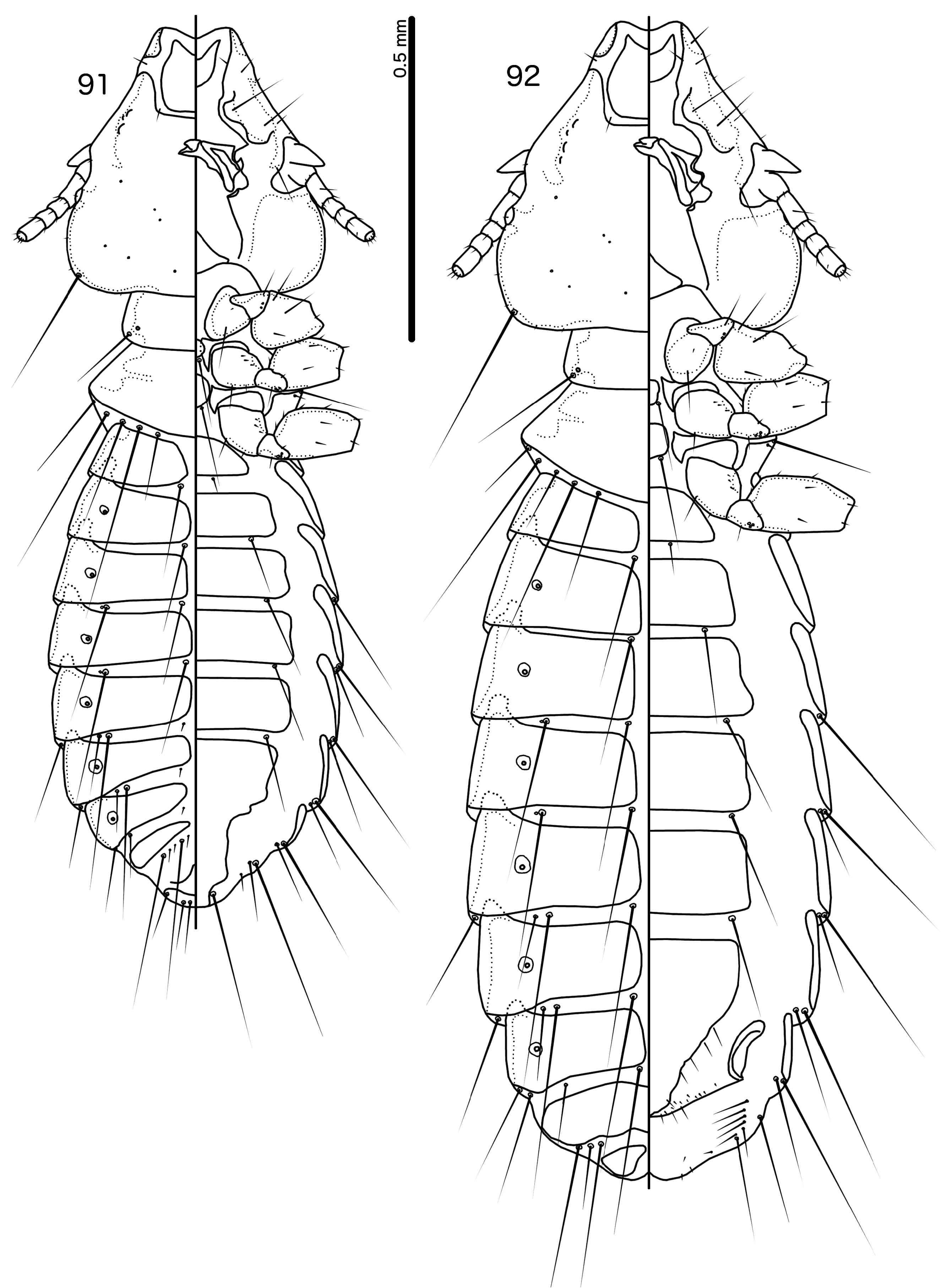
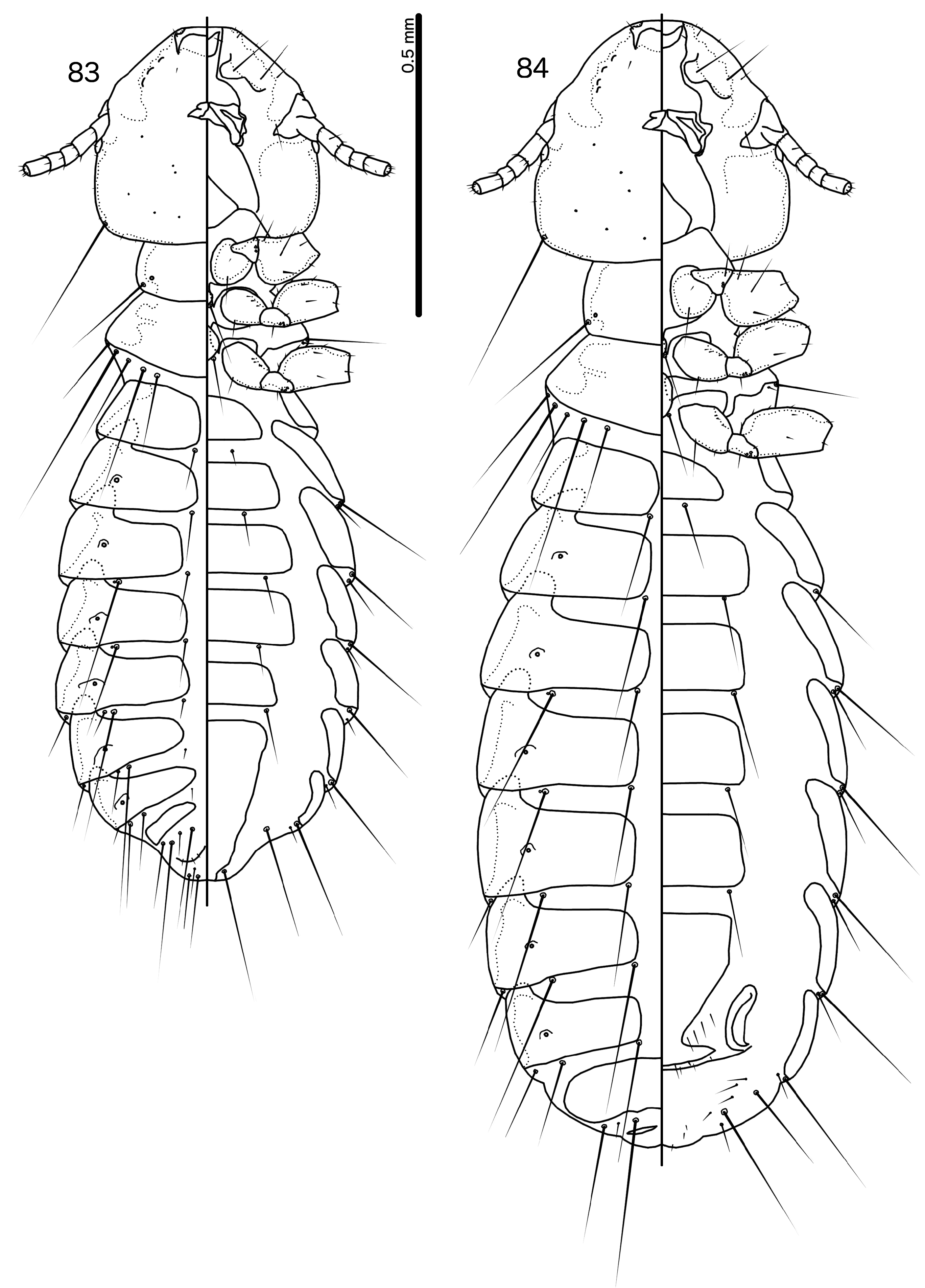
Diagnosis. Species in the subgenus Gu. ( Malardifax ) can be separated from species in the nominate subgenus by the following combination of characters: dorsal preantennal suture transversally continuous median to ads in Gu. ( Malardifax ) ( Fig. 93 View FIGURES 93–94 ), but not medianly continuous in Gu. ( Guimaraesiella ) [ Fig. 87 View FIGURES 87–88 ; in many species the suture reaches ads but does not extend medianly to this seta (e.g., Gustafsson et al. 2019b)]; distal mesosome with continuous dorsal thickening in Gu. ( Malardifax ) ( Fig. 101 View FIGURES 95–106 ), but with dorsal thickenings interrupted medianly in Gu. ( Guimaraesiella ) ( Fig. 95 View FIGURES 95–106 ); area around gonopore rugose in Gu. ( Malardifax ) ( Fig. 102 View FIGURES 95–106 ), but without any rugose area in Gu. ( Guimaraesiella ) ( Fig. 96 View FIGURES 95–106 ); aps present on female tergopleurites VI–VII in Gu. ( Malardifax ) ( Fig. 92 View FIGURES 91–92 ), but absent on all female tergopleurites in Gu. ( Guimaraesiella ) [ Fig. 84 View FIGURES 83–84 ; except possibly in Gu. haftorni ( Balát, 1958) , but the abdominal chaetotaxy in the single female known of this species may be aberrant ( Gustafsson et al. (2019c)]; parameres broadly rounded distally in Gu. ( Malardifax ) ( Fig. 103 View FIGURES 95–106 ), but narrowing to distal point in Gu. ( Guimaraesiella ) ( Fig. 97 View FIGURES 95–106 ).
In Gu. ( Malardifax ), the ventral sclerite of the mesosome is continuous with the ventral surface of the distal mesosome, including the area around the gonopore, without any visible interruption (e.g., Fig. 102 View FIGURES 95–106 ); in most Gu. ( Guimaraesiella ), there is a clear distal margin of the ventral sclerite that separates it from the gonopore (e.g., Fig. 96 View FIGURES 95–106 ), but this character is not consistent throughout the nominate subgenus, and is for instance not found in the type species Gu. (Gu.) papuana ( Giebel, 1879) (see Gustafsson & Bush 2017: fig. 358) and Gu. (Gu.) forcipata Gustafsson et al., 2019b .
Some species of Guimaraesiella have a dorsal preantennal suture similar to that of species in the subgenus Gu. ( Malardifax ). For instance, this character is found in Guimaraesiella myiophoneae ( Clay, 1936) and many of the species known from Neotropical hosts (e.g., Cicchino 1983), but they can all be separated from species of Gu. ( Guimaraesiella ) by the structure of the male genitalia and other characters, and are likely not part of the nominate subgenus. However, most of these species are in need of redescription before their subgeneric placement within Guimaraesiella can be established, and should presently be considered incerta sedis within Guimaraesiella .
A similar preantennal structure is also found in the subgenus Gu. (Mohoaticus) Mey, 2017, and in the tenella species-group of subgenus Gu. ( Cicchinella ) Gustafsson et al., 2019a. These groups can be separated from Gu. ( Malardifax ) by the structure of the male genitalia. Compare Figs 101–103 View FIGURES 95–106 with those in Gustafsson & Bush (2017: fig. 369), Mey (2017: figs 98–99) and Gustafsson et al. (2019a: figs 100–102).
Description. Head rounded trapezoidal, frons broadly concave, lateral margins of preantennal head concave to straight ( Fig. 93 View FIGURES 93–94 ). Frons hyaline, continuous with dorsal preantennal suture that reaches dsms, ads and lateral margin of head, as well as completely surrounds the dorsal preantennal plate. Marginal carina interrupted laterally and submarginally. Head chaetotaxy as in Fig. 93 View FIGURES 93–94 ; as2 absent; mts3 only temporal macroseta. Prothorax rectangular, psps on postero-lateral corner ( Fig. 91 View FIGURES 91–92 ). Pterothorax roughly pentagonal, with lateral margins divergent and posterior margin rounded; mms moderately separated medianly. Meso- and metasterna not fused, each with 1 seta on each side on postero-lateral corners. Male tergopleurites II–IX+X and female tergopleurites II–VIII medianly divided ( Figs 91–92 View FIGURES 91–92 ); sternal plates as large central plates; accessory sternal plates absent.
Male. Abdominal chaetotaxy sparse ( Fig. 91 View FIGURES 91–92 ). Subgenital plate trapezoidal, indented or convex laterally. Genitalia: basal apodeme rectangular, with anterior end rounded and lateral margins slightly concave ( Fig. 101 View FIGURES 95–106 ). Proximal mesosome roughly rectangular ( Fig. 102 View FIGURES 95–106 ). Ventral sclerite with elongated proximal extension approaching proximal margin of mesosome; proximal thickening present [in nominate subspecies of Gu. pandolura ; see Gustafsson & Bush 2017; fig. 374) or absent ( Fig. 102 View FIGURES 95–106 ). Distally, ventral sclerite is not interrupted, but appears to be continuous to distal margin of mesosome ( Fig. 102 View FIGURES 95–106 ). Gonopore subterminal, open distally; area around gonopore densely rugose. Mesosomal chaetotaxy: 3 ames sensilla on each side antero-lateral to gonopore; 2 lpmes sensilla on each side on lateral margin, lateral or antero-lateral to gonopore. Dorsal thickening of mesosome medianly continuous around distal margin. Parameres with medianly folded heads ( Fig. 103 View FIGURES 95–106 ); parameral blades stout, blunt distally, not elongated; pst1 sensilla, pst2 microseta.
Female. Abdominal chaetotaxy sparse ( Fig. 92 View FIGURES 91–92 ); aps present on tergopleurites VI–VII. Subgenital plate pentagonal, reaching to or near vulval margin, but without bulges or cross-pieces ( Fig. 94 View FIGURES 93–94 ). Vulval margin generally convergent or bulging medianly; few vms and many vss on each side; vos follow lateral margins of subgenital plate, with at least 1 distal vos separated from others by a distinct gap, and located near the vss.
Host distribution. Members of the Campephagidae . In addition, an undescribed species has been examined from a vangid host (Gustafsson & Bush, in prep.).
Geographical range: Southeast Asia.
Etymology. The name of the new subgenus is derived from “ malo ”, Latin for “I prefer”, “ ardens”, Latin for “fiery”, and “ fax ”, Latin for “fireball, comet”, referring to the coloration of minivets, the hosts of most of the specimens of this group we have examined.
Remarks. Gustafsson & Bush (2017: 234) listed records of lice morphologically similar to Gu. (Malardifax) pandolura from five host species besides the type host, but stated that the available specimens from these hosts were not suitable to establish whether they belonged to different species. Further examination of some of these specimens has revealed some variation in abdominal chaetotaxy and male genitalia, which may indicate that more than one taxon are involved. Here, we illustrate and briefly describe specimens of Gu. ( Malardifax ) from Pericrocotus ethologus laetus Mayr, 1940 , and Pericrocotus roseus stanfordi Vaughan & Jones, 1913 , to indicate some of the characters that differ among populations from different hosts. However, due to their close similarity, and the small number of specimens examined, we consider specimens from P. e. laetus and P. r. stanfordi as conspecific with specimens from the type host of Gu. (Ma.) pandolura .
Included taxon
Guimaraesiella (Malardifax) pandolura Gustafsson & Bush, 2017: 231 .
Type host: Pericrocotus speciosus semiruber Whistler & Kinnear, 1933 .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |