Cladonia rangiferina (L.) F. H. Wigg
|
publication ID |
https://doi.org/10.1016/j.phytochem.2018.09.011 |
|
DOI |
https://doi.org/10.5281/zenodo.10514905 |
|
persistent identifier |
https://treatment.plazi.org/id/03B10B21-FFAE-FFB0-FCC8-8720E2B1F950 |
|
treatment provided by |
Felipe |
|
scientific name |
Cladonia rangiferina |
| status |
|
3.3. Proposed polyketide synthesis in Cladonia rangiferina View in CoL
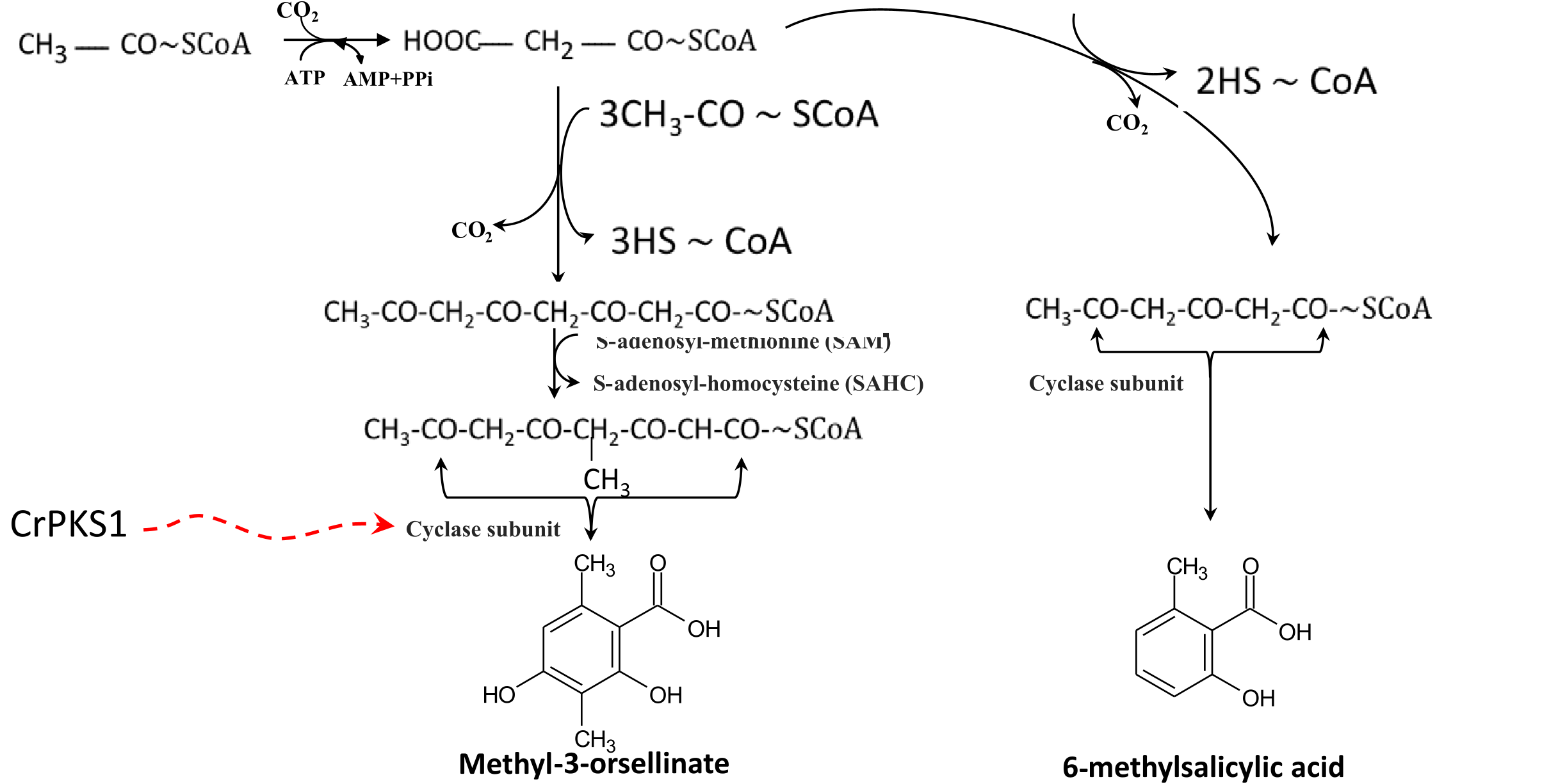
A pervious study ( Elshobary et al., 2016) showed that CrPKS1 and CrPKS16 may be genes that encode non-reducing enzymes and CrPKS3 may encode a reducing enzyme. Furthermore, CrPKS1 was most closely related to the putative PKS from Pyrenophora tritici-repentis (Diedicke) Drechsler and Macrophomina phaseolina (Tassi) Goidanich (both with maximum identity of 78 and 79%, respectively), which were responsible for production of 6-methylsalicylic acid synthase. The 6-methylsalicylic acid is considered the first cyclic compound in the polyketide pathway and a common precursor for the cyclic polyketide compounds ( Legaz et al., 2011). Alternatively, the C. grayi PKS1 ( CgPKS1) (similarity with CrPKS1 was 99% identity) was shown to fall within a phylogenetic clade that had a methyltransferase domain ( Armaleo et al., 2011) suggesting it may produce the first cyclic compound (methyl-3-orsellinate) in the atranorin and fumarprotocetraric acid pathway ( Fig. 4 View Fig ). Accordingly, CrPKS1 is expected to be highly expressed in the thallus outer layer where the acetate/malonate and cyclisation presumably occur after transportation of algal sugars.
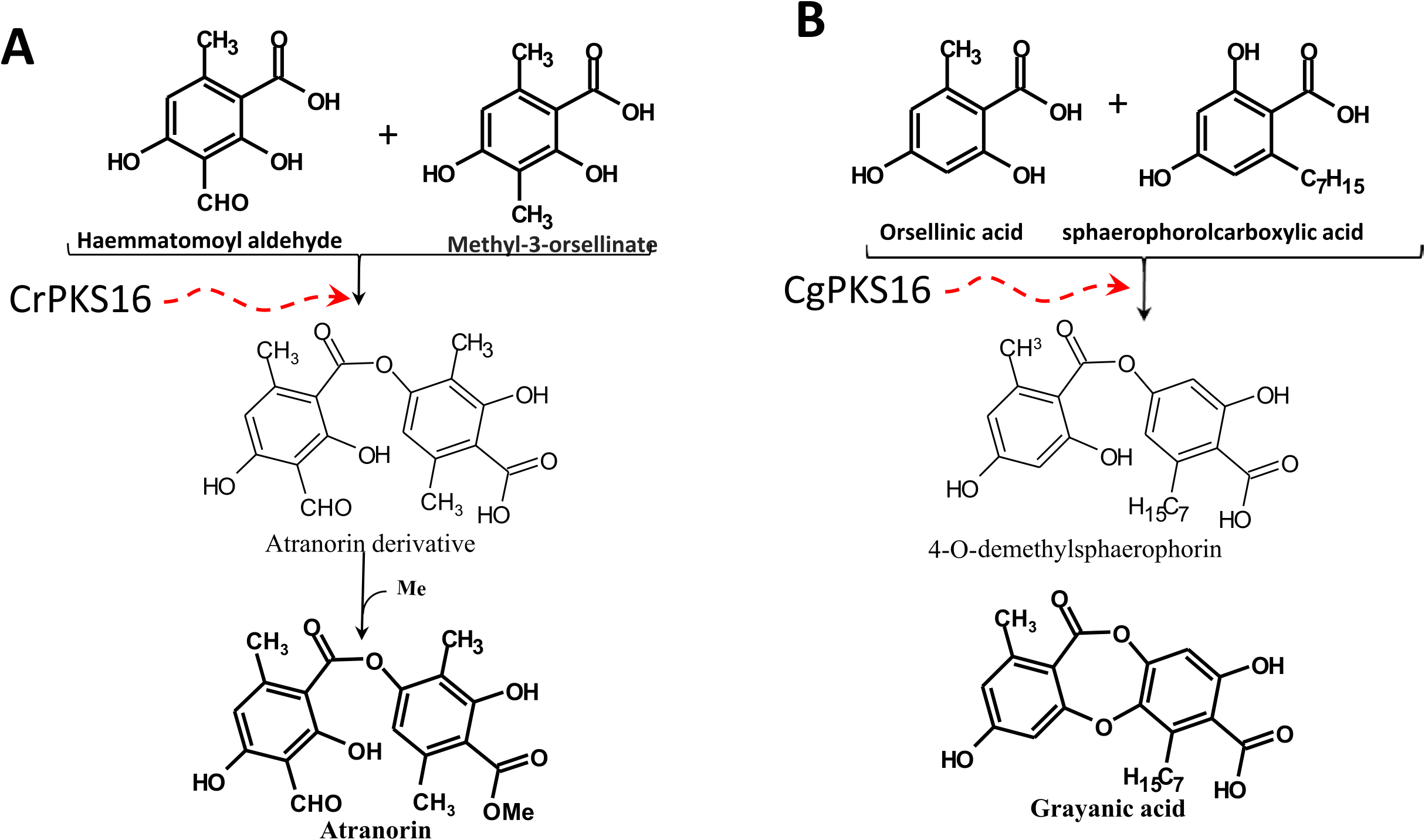
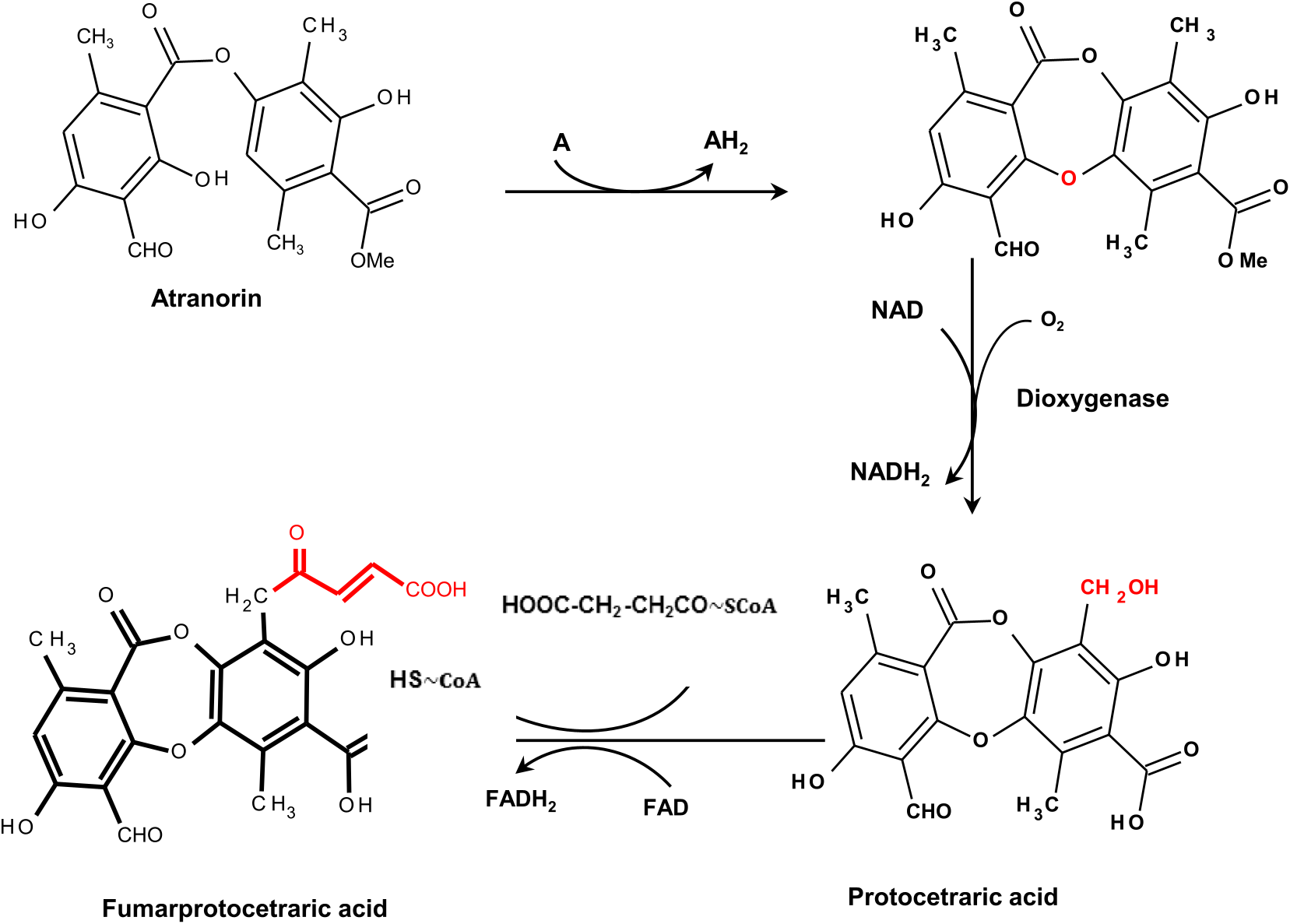
CrPKS16 was most closely related to the putative PKS from C. grayi ( CgPKS16; maximum identity of 100%) which was hypothesized to be responsible for the synthesis and linking of two cyclic compounds ( Methyl-3-orsellinate and sphaerophorolcarboxylic acid) to produce the grayanic acid precursor (4-O-demethylsphaerophorin; Fig. 5A View Fig ) ( Armaleo et al., 2011). Both 4-O-demethylsphaerophorin and atranorin are similar depsides except in the side chain at C 16 and the methylated carboxyl group ( Fig. 5 View Fig ). Accordingly, CrPKS16 may be involved in the linkage of two cyclic compounds (Methyl-3-orsellinate and Haemmatomoyl alcohol) to form atranorin ( Fig. 5B View Fig ). CrPKS16 was expressed in both the outer and inner thallus tissue, which was consistent with the TLC data showing atranorin in both layers. However, the transformation of depsides to depsidones requires cytochrome P450 to form grayanic acid from depside precursors ( Armaleo et al., 2011). In this context, Elix and Stocker-Wörgötter (2008) and Millot et al. (2009) suggested that a depsidone could be formed from the oxidation of a para-depside by dioxygenase. If depsides can be converted to depsidones ( Seshadri, 1944; Culberson, 1964), the production of fumarprotocetraric acid in C. rangiferina may initially require the production of atranorin ( Fig. 6 View Fig ) ( de Armas et al., 2016). In this study, grayanic acid was not produced by C. rangiferina , so CrPKS16 likely does not have a role in grayanic acid production. It may, instead, contribute to the biosynthesis of the depside, atranorin. This agreed, in part, with our TLC results which showed fumarprotocetraric acid present in the inner thallus layer with atranorin. If atranorin was formed in the outer layer and then transformed to fumarprotocetraric acid in the inner layer by dioxygenase (YQE1), which was upregulated in this layer, atranorin would appear to be present in both layers, and only fumarprotocetraric acid would appear to be present in the inner layer. However, the absence of YQE1 expression in the apical inner layer does not support this hypothesis. CrPKS3 was closely related to a reducing PKS gene from Usnea longissima Ach. (maximum identity of 74%) which may be responsible for the biosynthesis of depside side chains ( Wang et al., 2011). This agreed with our results which showed that CrPKS3 was more highly expressed in the outer than inner thallus layers where depside synthesis occurred.
The Mass Spectrum analysis of atranorin (pure and in the extract) was consistent with previous reports ( Musharraf et al., 2015) displaying the deprotonated molecular ion [M− H]− at m/z 373 with daughter ions observed at m/z 195 and 177 (Supplementary Fig. 1A View Fig , insert). Similarly, fumarprotocetraric acid had a [M− H]− precursor m/z 471 (both pure and in extract) in agreement with MoNA ( MoNA ID: NP_C1_297_p3_F03_NEG_iTree_11), with the daughter ion observed at m/z 355 (Splash: splash10-0a4i-0009000000-6d53e7820a534e1cad6a) ( Fig. 1B View Fig , insert). The analysis of both standards and published data strongly suggest the presence of atranorin and fumarprotocetraric acid as the two major compounds produced by Cladonia rangiferina .
4. Conclusion
In conclusion, the three PKS genes ( CrPKS1, CrPKS3, CrPKS16), MFSUG2, and C 2 H 2 transcription factors were upregulated in the outer apical portion more than the other thallus portions. These findings are consistent with more metabolic activity where the ribitol sugar from the alga ( Asterochloris sp. ) is transferred to the fungus for polyketide production. However, the C 2 H 2 transcription factor was upregulated in both apical portions where polyketides were synthesized. In contrast, PacC was upregulated in the basal portion distal from polyketide synthesis. YQE1 was upregulated in the basal inner layer where fumarprotocetraric acid biosynthesis may occur by oxidation of depsides. CAT was expressed in the outer layers of the thallus where polyketide biosynthesis initiated, which was thought to reduce the oxidative stress from polyketide biosynthesis. In contrast, the apothecia showed low expression levels of all genes. The results in this study are validated by current knowledge of sugar transport in lichens and the location of polyketide production consistent with known function. The utility of performing the LMD technique on sections of C. rangiferina has implications for further tissue-specific expression studies such as nitrogen mobilization in cyanobacterial lichens and it illustrates a different approach for examining activity of hydrophobins or other proteins in the lichen thallus.
5. Materials and methods
5.1. Lichen material
The mat-forming lichen, C. rangiferina (L.) F. H. Wigg. ( KP001201 ) was collected June 2014 from Sandilands Provincial Forest, Manitoba, Canada (N49̊ 22 ′ 37 ″, W96̊ 6 ′ 31 ″), cleaned from debris, and stored in a plastic bag at 4 ̊C. The collection site was a Jack pine ( Pinus banksiana Lamb. ) dominated ridge underlain by sandy glacial till on the Precambrian Shield. Other species present include black spruce ( Picea mariana (Mill.) Britton, Sterns &Poggenb. ), Alnus sp. , Prunus pensylvanica L. in open areas, mosses ( Pleurozium schreberi (Brid.) Mitt. , Hylocomium splendens (Hedw.) Schimp. , Dicranum spp. ) in protected depressions, and other lichens ( Cladonia spp. , Peltigera spp. ). See Kotelko et al. (2008) for a more detailed list of the common lichens and bryophytes in the area. The area was moist to dry with moisture retention because of the forest cover. The upper apical and lower basal portions of the lichen thallus were cut in cross section and separated into two layers: the outer layer with loose fungal hyphae surrounding algal cells and the innermost layer with compact fungal hyphae with no algal cells. The apothecia, containing only fungal tissue, were separated from the thallus at the base of the apothecium.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |