Daphnia tanakai, Ishida, S., Kotov, A. A. & Taylor, D. J., 2006
|
publication ID |
https://doi.org/ 10.1111/j.1096-3642.2006.00214.x |
|
DOI |
https://doi.org/10.5281/zenodo.5736167 |
|
persistent identifier |
https://treatment.plazi.org/id/03B087D7-0844-FF99-FEDD-22D9FF84723E |
|
treatment provided by |
Carolina |
|
scientific name |
Daphnia tanakai |
| status |
sp. nov. |
(2) DAPHNIA TANAKAI View in CoL SP. NOV.
Daphnia ambigua Scourfield View in CoL in Uèno & Tanaka, 1960: 296, figs 1, 2.
Daphnia curvirostris Eylmann View in CoL in Tanaka & Tominaga, 1986: 35–42, figs 2–7; Tanaka, 1997: 57–58, fig. 3; Tanaka, 1998: 30, fig. 1(A), (B).
Not Daphnia whitmanni Ishikawa, 1895: 147–153 , plate 22, figs 1–5.
Etymology
This species is dedicated to Dr S. Tanaka, renowned Japanese cladocerologist, who found this species and supplied us with a part of material.
Type locality
Lake Midori-ga-ike, Hida Mountain Range , Honshu Island, Japan. This is a medium-sized (maximum length 157 m), shallow (maximum depth 1.65 m) mountain lake located at 2430 m above sea level (36°34′39″N, 137°36′1″Ε). The type series was collected on 30 viii.2004 by S. Tanaka .
Holotype
One female (1.5 mm in body length), deposited at the National Science Museum, Tokyo, Japan, catalogue number NSMT-Cr 16117.
Allotype
One male, deposited at the National Science Museum, Tokyo, Japan, catalogue number NSMT-Cr 16118.
Paratypes
Thirty females and males, deposited at the National Science Museum, Tokyo, Japan, catalogue number NSMT-Cr 16119; 30 females and males, deposited at the Zoological Museum of the Moscow State University, Moscow, catalogue number MGU Ml 34; about 30 females and males in the personal collection of AAK, catalogue number AAK 2004–056 .
Other material examined
Japan, Honshu Island, Hida Mountain Range: Lake Midori-ga-ike, coll. 09.ix.1979 by S. Tanaka. Lake Kagami-ike, coll. 01.ix.2004 by S. Tanaka.
Short diagnosis
Female. Body subovoid, caudal spine completely absent or short. Rostrum relatively short. Spinules on ventral and dorsal margin normally completely reduced, or present only in region of postero-dorsal angle. First abdominal process relatively long, slightly bent, second process short, the third small and rounded. Postabdominal claw long, size of teeth in basal and, especially, medial pecten varies significantly between populations from longispina to pulex type. Antenna I with almost completely reduced body. Limb I anterior setae 3 and 4 larger than similar setae in D. curvirostris . Limb II with anterior seta 1 about 3/4 length of posterior seta.
Ephippium with axes of eggs perpendicular to its dorsal margin, postero-dorsal portion of valves not incorporated into ephippium.
Adult male. Head with reduced rostrum. Abdomen with a process on second (from distal end) segment. Postabdomen with convex ventral margin, gonopore opens subdistally, without a genital papilla. Antenna I short, antennular sensory seta short and thin, flagellum with slightly curved, hooked tip. Limb I with stiff setae 2–3 times larger than in D. curvirostris . Limb II: on inner-distal portion, the anterior seta 1 slightly bent.
Size. Females up to 1.79 mm, males 0.95–1.13 mm.
Description
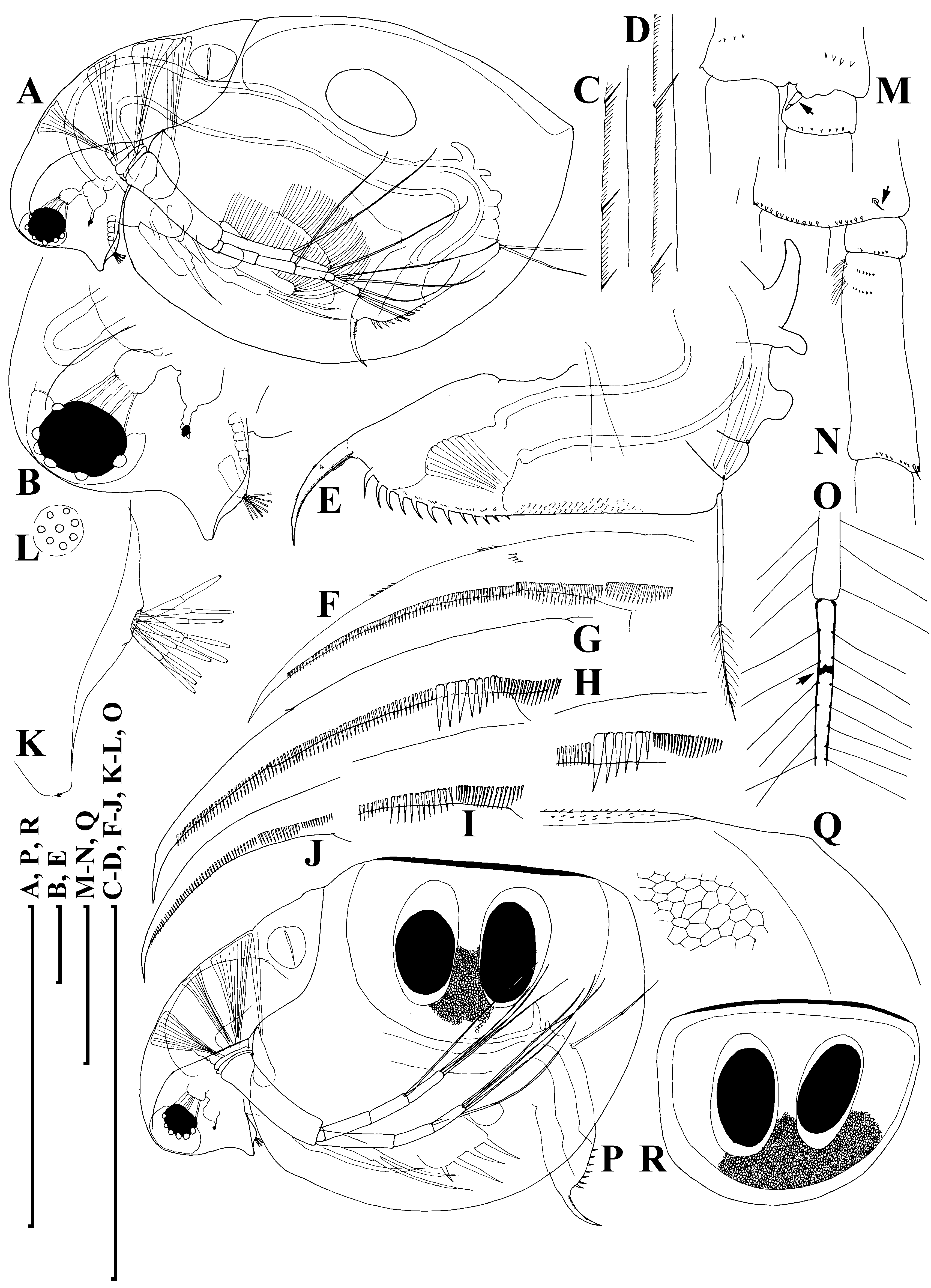
Adult parthenogenetic female. Body subovoid in lateral view, maximum height in middle ( Fig. 6A View Figure 6 ). Dorsal margin of valves slightly elevated above head, slightly convex, a shallow depression between head and rest of body. Postero-dorsal angle well expressed, but caudal spine completely absent or very short. Head with rostrum well developed, but significantly shorter than that in D. curvirostris , in lateral view, its tip projected posteriorly, not subdividing into two lobes; posterior margin of head convex. Compound eye large, ocellus small and located far from base of antenna I.
Carapace subovoid, in large females spinules on ventral and dorsal margin normally completely reduced, but if caudal spine expressed, these spinules present on it and in region of postero-dorsal angle. In postero-ventral portion of valve, on inner face of valve, a row of setules, organized in short series ( Fig. 6C View Figure 6 ); at posterior portion of valve, each series terminating in a setulated spine ( Fig. 6D View Figure 6 ), finer than that in D. curvirostris .
Abdomen relatively short, consisting of four segments. The first (basalmost) abdominal process relatively long, but shorter than that in D. curvirostris , slightly bent anteriorly, the second (middle) process short, the third (distalmost) small and rounded; the fourth segment lacking a process ( Fig. 6E View Figure 6 ). Postabdomen with preanal margin long, almost straight, covered with series of minute setules. Preanal and postanal angle not expressed. Paired spines on postanal and anal portion, their size continuously increasing distally. Postabdominal seta approximately as long as preanal margin, its distal segment shorter than basal one. Postabdominal claw long, regularly bent, with a pointed tip. On outer side, three successive pectens along the dorsal margin, but size of teeth in basal and, especially, medial pecten varies significantly between populations, and even within a single population, from longispina type to pulex type. All females from Lake Midori-ga-ike have postabdominal claws of longispina type ( Fig. 6F View Figure 6 ), in contrast, majority of females from Lake Kagami-ike had pulex type claws ( Fig. 6G, H View Figure 6 ), while small part has intermediate claws ( Fig. 6I View Figure 6 ); finally, some juveniles were of longispina type ( Fig. 6J View Figure 6 ). All females have fine rows of denticles at middle of ventral margin, and at distal end of medial pecten.
Antenna I with almost completely reduced body, nine aesthetascs of different size arising immediately from head surface, antennular sensory seta not found ( Fig. 6K, L View Figure 6 ). Antenna II in general as in previous species. A small sensory seta posteriorly at distal margin of basal segment ( Fig. 6M View Figure 6 , arrow), a small distal seta was found at its anterior face ( Fig. 6N View Figure 6 , arrow). A weakly pigmented insertion within distal segment of swimming seta ( Fig. 6O View Figure 6 , arrow) located further from joint with basal segment compared with D. curvirostris . Spine on the second segment of exopod also rudimentary.
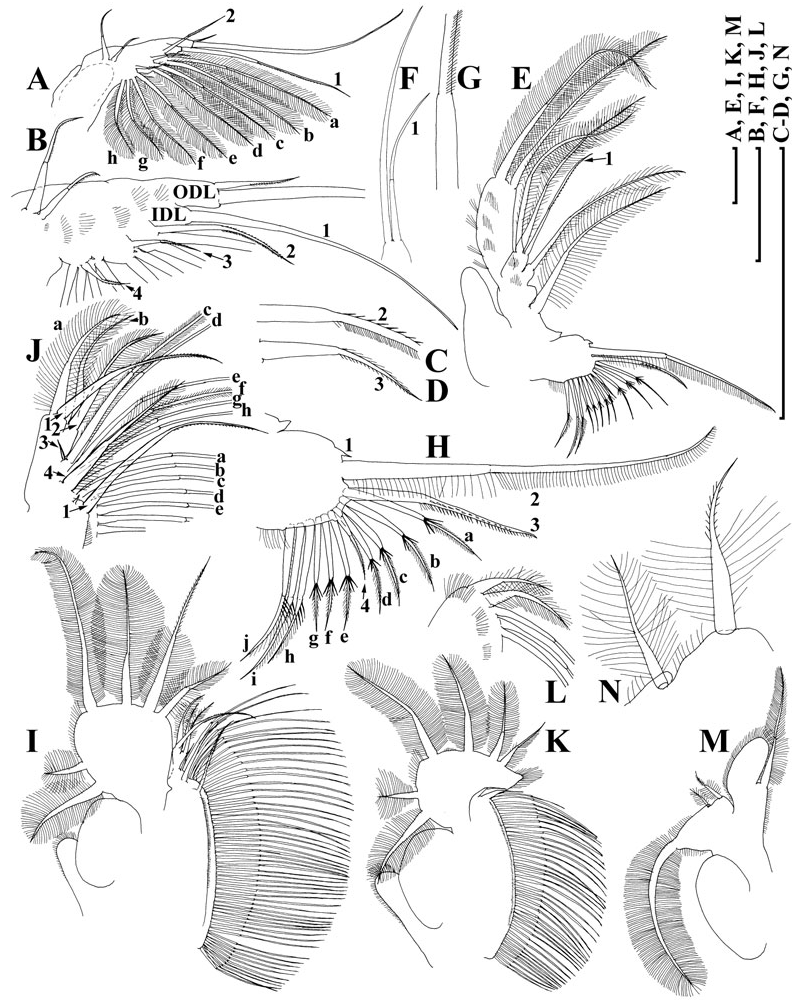
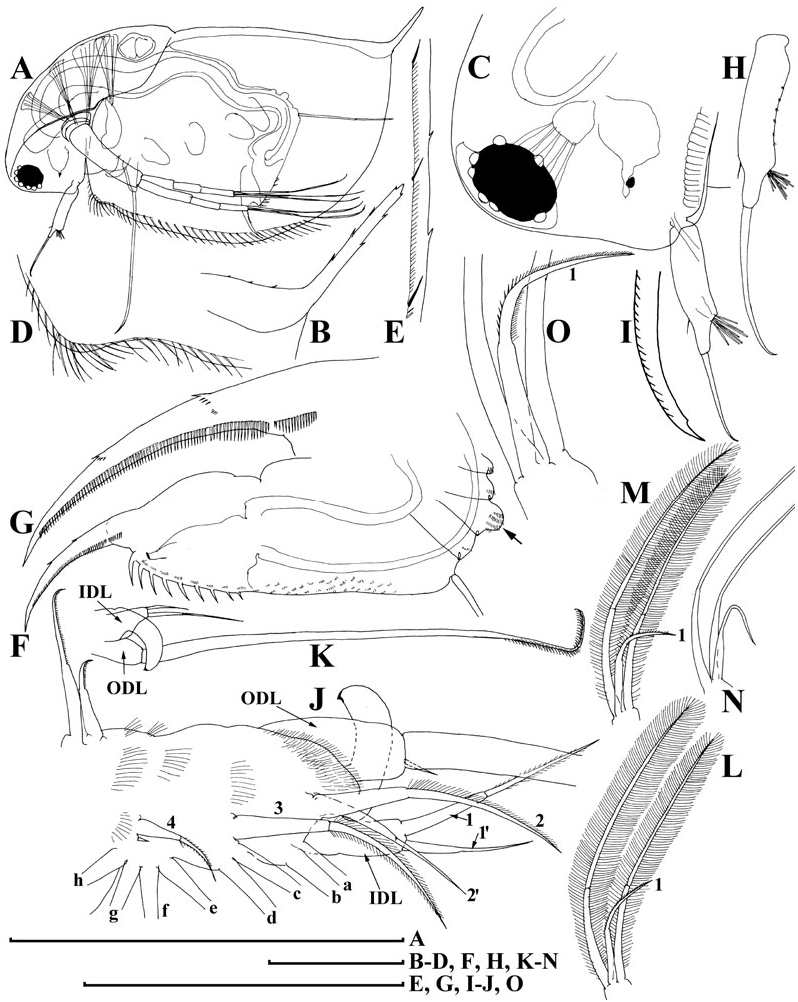
Limb I ( Fig. 7A, B View Figure 7 ) with outer distal lobe had the second (smaller) seta larger, anterior seta 2 asymmetrically armed ( Fig. 7C View Figure 7 ). Anterior setae 3–4 ( Fig. 7D View Figure 7 ) larger than similar setae in D. curvirostris , each accompanied by a minute sensillum. Limb II ( Fig. 7E View Figure 7 ) with anterior seta 1 ( Fig. 7F View Figure 7 ) shorter than that in D. curvirostris , and armed with shorter setules ( Fig. 7G View Figure 7 ). Gnathobase II with shorter setules on distal segment of seta 2, and small denticles on seta 3, 10 posterior setae of gnathobasic ‘filter plate’ ( Fig. 7H View Figure 7 : a–j). Limb III ( Fig. 7I View Figure 7 ) very similar to that of D. curvirostris , but in its inner portion ( Fig. 7J View Figure 7 ) posterior seta 1 armed in different way, seta 3 rudimentary, seta 4 relatively short; gnathobasic seta 1 with naked basal segment and short setules distally, a small sensillum near it, 44–49 posterior soft setae in filter plate III. Limb IV as in previous species, 36–41 posterior setae in filter plate IV ( Fig. 7K, L View Figure 7 ). Limb V as in previous species, but distalmost seta of exopodite armed distally with short setules ( Fig. 7M, N View Figure 7 ).
Ephippial female. Dorsal margin of valves almost straight. Dorsal wall of carapace was additionally chitinized, formed a dorsal plate, had fine spinules ( Fig. 6P, Q View Figure 6 ). Ephippium with two resting eggs, axes of which perpendicular to its dorsal margin ( Fig. 6R View Figure 6 ), postero-dorsal portion of valves not incorporated into ephippium.
Adult male. Body low, subquadrangular, dorsal margin of valves straight, not elevated above head, shallow depression between head and valves ( Fig. 8A View Figure 8 ), postero-dorsal angle distinct, with a distinct caudal spine protruding postero-dorsally, minute spinules on the spine and dorsal margin of carapace ( Fig. 8B View Figure 8 ). Head with reduced rostrum ( Fig. 8C View Figure 8 ).
Valve with antero-ventral angle slightly prominent ventrally, whole ventral margin with long, numerous setae submarginally on inner face of valve ( Fig. 8D View Figure 8 ), rows of fine setules at posterior margin of valve on its inner face, organized in series ( Fig. 8E View Figure 8 ).
Abdomen with a process on second (from distal end) segment ( Fig. 8F View Figure 8 , arrow). Postabdomen with convex ventral margin, preanal angle smoothed, paired teeth small. Gonopore opens subdistally, without a genital papilla. Only males with postabdominal claws of longispina and intermediate types were found ( Fig. 8G View Figure 8 ).
Antenna I short for a Daphnia male, almost straight, antennular sensory seta short and thin, not reaching base of male seta (flagellum) ( Fig. 8C, H View Figure 8 ), which is as long as body of antenna I, with slightly curved distal portion, supplied with minute setules and a spinule at its tip ( Fig. 8I View Figure 8 ).
Limb I with a wider copulatory hook ( Fig. 8J, K View Figure 8 ), both setae of IDL and anterior setae 2–3 significantly larger than those in D. curvirostris . On inner-distal portion of limb II, the anterior seta 1 asymmetrically setulated distally, slightly ( Fig. 8L View Figure 8 ) moderately ( Figs 8M, O View Figure 8 ) or significantly ( Fig. 8N View Figure 8 ) bent.
Size. Juvenile and adult females from Lake Midori-ga-ike 0.64–1.79 mm, ephippial females 1.35–1.75 mm, ephippium 0.70–0.78 mm, adult males 0.95–1.13 mm according to our measurements; adult parthenogenetic females from the same lake 1.64 ± 0.11 mm according to Tanaka & Tominaga, 1986).
Differential diagnosis
Daphnia tanakai sp. nov. and D. curvirostris Eylmann, 1887 are superficially similar in morphology, but D. tanakai sp. nov. is unique in the following characteristics: (1) short rostrum, not subdivided into two lobes by fornix line in lateral view; (2) no denticles on posterior portion of valves; (3) all postabdominal processes shorter; (4) size of teeth in two basal pectens on postabdominal claw varies significantly from longispina to pulex type; (5) on limb I, anterior seta 3 normally developed; (6) anterior seta 1 on distalmost endite of limb II short; (7) on limb III, seta 3 rudimentary. In addition, the male of D. tanakai sp. nov. has: (8) a reduced rostrum; (9) a postabdomen with inflated ventral margin; (10) an abdomen with a process on second segment (from basal side); (11) a sensory seta on antenna I very short, and not reaching bases of male seta (flagellum); (12) on limb I, large anterior setae 2–3; (13) anterior seta 1 on distal-most endite of limb II only slightly curved not hook-like.
Uèno & Tanaka (1960) assigned D. tanakai sp. nov. specimens to Daphnia ambigua Scourfield, 1947 , but unlike D. tanakai sp. nov., the female of D. ambigua has aesthetascs reaching the tip of the rostrum, well developed abdominal processes, two abdominal processes in the male, and a male flagellum possessing a spoon-like widening at the tip.
Daphnia dentifera Forbes, 1893 is a morphologically similar species to D. tanakai sp. nov. However, unlike D. tanakai sp. nov., D. dentifera adults in the late stage moult have a dark insertion within the distal segment of the swimming setae (see Benzie, 2005). Also, D. dentifera males have a reduced flagellum on antenna I that is subequal to the aesthetascs (see Brooks, 1957; Benzie, 2005).
Daphnia pulex Leydig, 1860 can be distinguished from D. tanakai sp. nov. by antenna I in the female. Daphnia pulex possesses a distinct tubular extension from the anntennular mound.
Daphnia parvula Fordyce, 1901 is another morphologically similar species to D. tanakai sp. nov. However, unlike D. tanakai sp. nov., D. parvula males have a reduced flagellum on antenna I that is only slightly longer than the aesthetascs (see Brooks, 1957; Alonso, 1996). Also, D. parvula lacks the ocellus pigmentation that is present in D. tanakai sp. nov. Finally, Penton & Crease (2004) presented robust phylogenetic evidence that D. parvula is a member of pulex clade, whereas we have shown here that D. tanakai sp. nov. is a member of the D. longispina clade.
Taxonomic comments
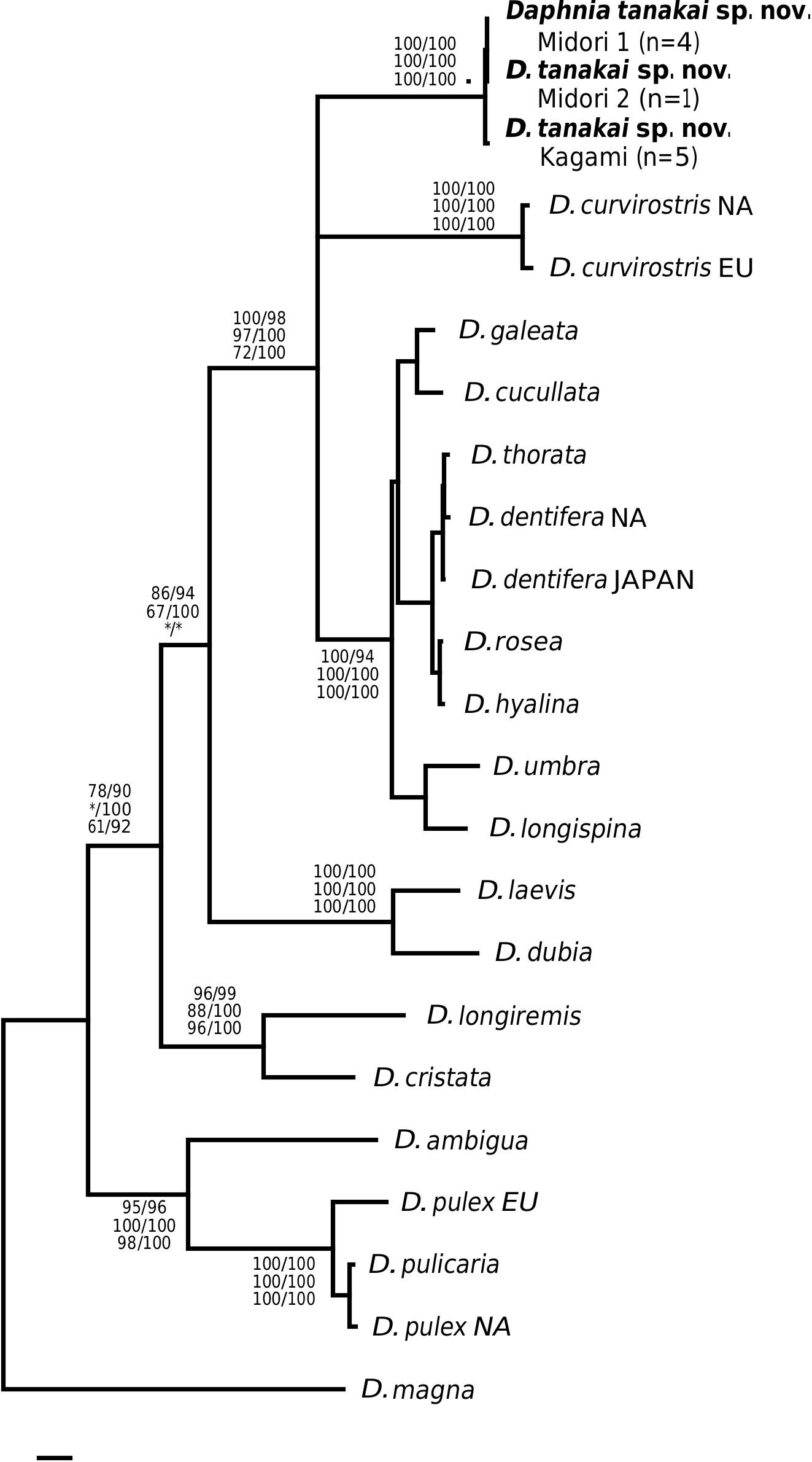
This taxon was first determined as D. ambigua and then as D. curvirostris ( Uèno & Tanaka, 1960; Tanaka & Tominaga, 1986). We found that the specimens examined here represent a separate species, differing from D. ambigua and D. curvirostris morphologically (see Differential diagnosis), chromosomally ( Tanaka & Tominaga, 1986; Beaton & Hebert, 1994) and genetically (see Fig. 2 View Figure 2 ).
Daphnia whitmani Ishikawa, 1895 was described from the vicinities of Tokyo. According to Ishikawa’s realistic pictures ( Ishikawa, 1895: plate 21), this animal seems to be a species similar to D. curvirostris but has a shorter rostrum. In contrast to D. tanakai sp. nov. (also found in Japan), the female of D. whitmani has long postabdominal processes, its male has a well developed rostrum, long flagellum and sensory seta on antenna 1, and relatively short stiff setae on endites 2 and 3 of the limb I, like D. curvirostris . Perhaps, D. whitmani is an ecological morph of D. curvirostris with a shorter rostrum. No other curvirostris -like species have been described from Asia.
Distribution
At present, D. tanakai sp. nov. is known only from several fishless pools and ponds in the Hida Mountain Range (2070–2550 m above sea level), Honshu Island, Japan.
DISCUSSION
The validity and relations of the subgenera of Daphnia have been controversial throughout the history of daphniid systematic biology. For example, species of the genus Daphniopsis Sars, 1903 , group with the subgenus Daphnia (Ctenodaphnia) Dybowski & Grochowski, 1895 in phylogenetic analyses ( Colbourne & Hebert, 1996; Omilian & Taylor, 2001; Hebert et al., 2002). Also, the subgenus name Daphnia (Hyalodaphnia) Schödler, 1866 has been misapplied in recent studies (e.g. Colbourne & Hebert, 1996; Schwenk et al., 2000; Penton & Crease, 2004). The recent applications of the ‘subgenus Hyalodaphnia ’ contain the type species of the genus Daphnia , namely D. longispina O. F. Müller, 1785 . However, the type species of a genus is, at the same time, a type of nominotypical subgenus (Article 44.1 of the ICZN, 2000), so D. longispina must belong to the subgenus Daphnia s. s., not to any other subgenus (see Johnson, 1952; Brooks, 1957; Flössner, 1972, 2000). Importantly, Schödler (1866), although aware of the existence D. longispina , established his genus Hyalodaphnia without including D. longispina as a member. His Hyalodaphnia lacked an ocellus, a feature that is prominent in D. longispina . If the pulex group does warrant a higher taxonomic ranking, then a new name must be suggested because the subgenus Daphnia is reserved for the group containing D. longispina .
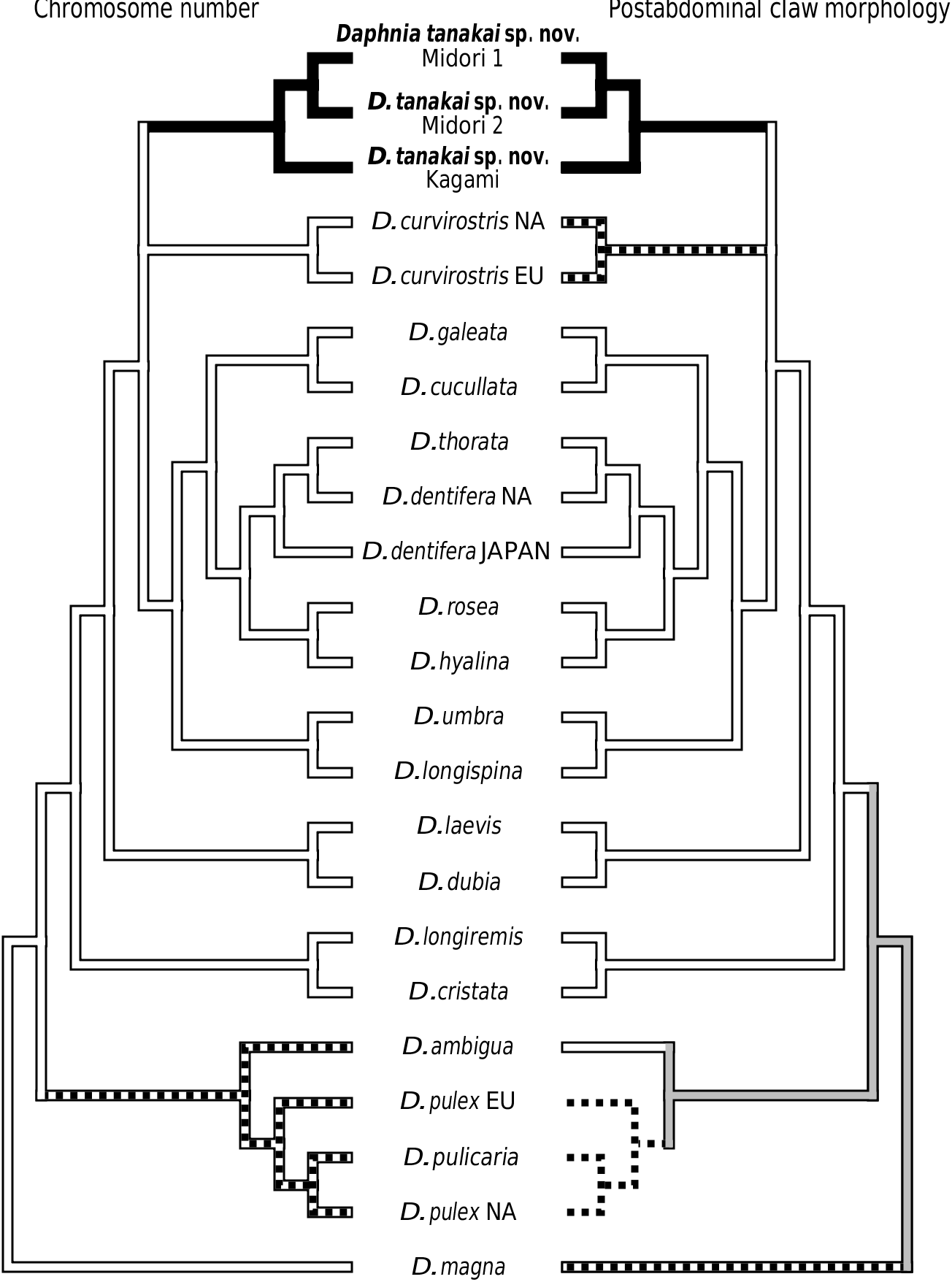
Nevertheless, if the gene tree presented in Figure 1 View Figure 1 is correct, then we find little objective basis upon which to erect additional subgenera to Daphnia sensu Johnson, 1952 . For example, our tree provides the first strong evidence that the claw character can rapidly evolve. Some species such as D. parvula show claw pecten length variation, but not of the magnitude seen in D. tanakai sp. nov. (i.e. from D. pulex type to D. longispina type). Daphnia tanakai sp. nov. possessed a variable claw morphology and the habitat correlation described by Tanaka & Tominaga (1986), in which the pronounced pectens are found in shallow pools and the reduced pectens are found in deeper ponds; this is supported in our study. Interestingly, this habitat–claw morphology association is present throughout the subgenus Daphnia , with ephemeral shallow pond dwellers possessing pronounced claw pectens ( D. curvirostris , D. tanakai sp. nov. and many D. pulex group species), and deeper pond and lake species possessing reduced claw pectens (most of the D. longispina group plus D. ambigua , D. parvula and D. retrocurva ) ( Brooks, 1957; Colbourne et al., 1997). The exceptions to the pattern are the putative recent colonists of large lakes, Daphnia pulicaria and Daphnia catawba , which have pronounced pectens. The prominence of the pecten varies in other daphniid genera, such as Moina , Ceriodaphnia and Simocephalus , but less is known of the phylogeny of these groups ( Goulden, 1968; Flössner, 2000; Orlova-Bienkowskaya, 2001). Clearly, D. tanakai sp. nov. would be a very informative group for studying the functional morphology and evolution of the postabdominal claw.
Other morphological characters suggested for discrimination of the pulex and longispina groups ( Alonso, 1996) are also diminished by our results. In D. tanakai sp. nov., the curvature of the anterior seta on the second limb of the male is variable, rendering this character polymorphic. Other potential sources of new morphological characters, i.e. female and male thoracic limbs, have not been well studied at present.
Our results also suggest that chromosome evolution has been less conserved than proposed for daphniids. Although we did not independently count the chromosomes of D. tanakai sp. nov. from Tanaka & Tominaga (1986), it is unlikely that the 2 n = 22 conclusion is a counting error. Counting errors from chromosome squashes usually result when some chromosomes are overlapping in the preparations, yielding a reduced count of the actual number, but 2 n = 22 is an increased count over D. curvirostris , the D. longispina group and the ancestral condition of 2 n = 20. The chromosomal justification for subgenera is diminished by this new character state in Daphnia . The pulex group cannot be justified as a subgenus solely on its possession of a derived chromosome number without elevating D. tanakai sp. nov. to a new subgenus.
Finally, the genetic divergences of clades have been used to justify subgenera ( Colbourne & Hebert, 1996). Here again we find little justification for elevating the pulex group to a new subgenus. We did find that the pulex group was basal to the other Daphnia taxa that we sampled, but there are also at least two other very divergent clades in the longispina group (the D. laevis clade and the D. longiremis clade). Also, the most divergent pulex group species were sampled in our study, and the within clade divergence of the pulex subgenus is less than the divergence found within at least three of the longispina group lineages. The ND2 tree based on divergence is more of a grade than two discrete subgeneric clades.
We have identified and described a new divergent species of Daphnia from Japan and presented the first gene tree for the genus Daphnia based on the ND2 gene. The tree is largely consistent with estimates based on other genes (12S rDNA, COI, 28S rDNA), but the ND2 gene has more robust support for the clades examined. The ND2 tree reveals that D. tanakai sp. nov. represents one of the most genetically divergent lineages in the subgenus Daphnia . Although the ND2 gene seems to possess clock-like properties (based on branch length evenness), further genetic analyses are needed to confirm that the divergence of D. tanakai sp. nov. is not a gene-specific rate acceleration. Japan may represent an important area for cladoceran diversity because it probably lacked permafrost during the Pleistocene and, unlike much of Beringia, represented a refugium for temperate species. Other endemic Japanese cladoceran species have recently been described (e.g. Kotov & Tanaka, 2004) and there are likely several more undescribed Japanese species.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Daphnia tanakai
| Ishida, S., Kotov, A. A. & Taylor, D. J. 2006 |
Daphnia ambigua
| Scourfield 1947 |
Daphnia whitmanni
| Ishikawa 1895 |
Daphnia curvirostris
| Eylmann 1887 |