Daphnia similis Claus, 1876
|
publication ID |
https://doi.org/10.11646/zootaxa.4161.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:C2A54ABA-7299-4601-A852-D9B1635443DC |
|
DOI |
https://doi.org/10.5281/zenodo.5624712 |
|
persistent identifier |
https://treatment.plazi.org/id/03B087AC-512A-FF82-FF02-4FBCFDE7F9C7 |
|
treatment provided by |
Plazi |
|
scientific name |
Daphnia similis Claus, 1876 |
| status |
|
Daphnia similis Claus, 1876 View in CoL
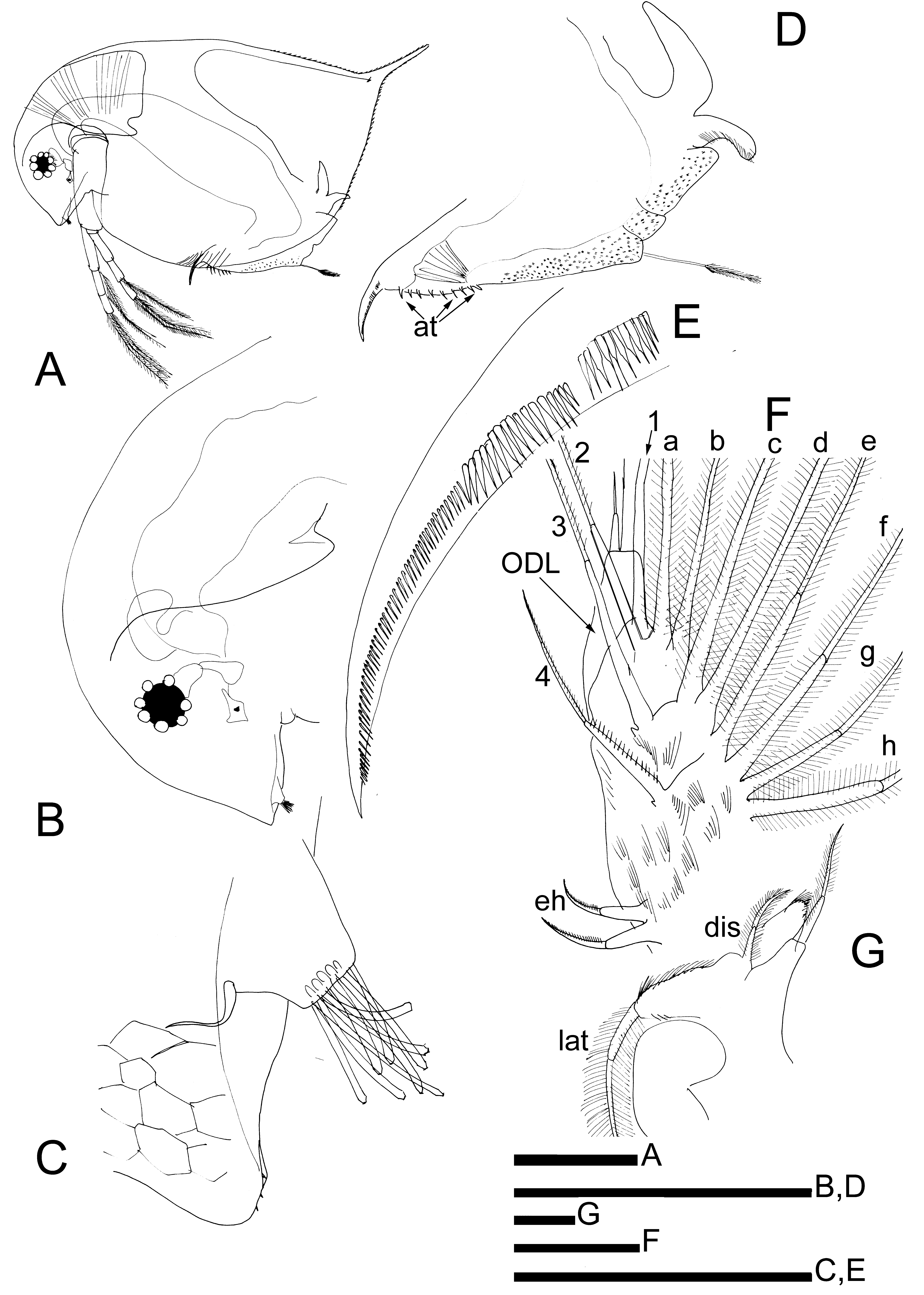
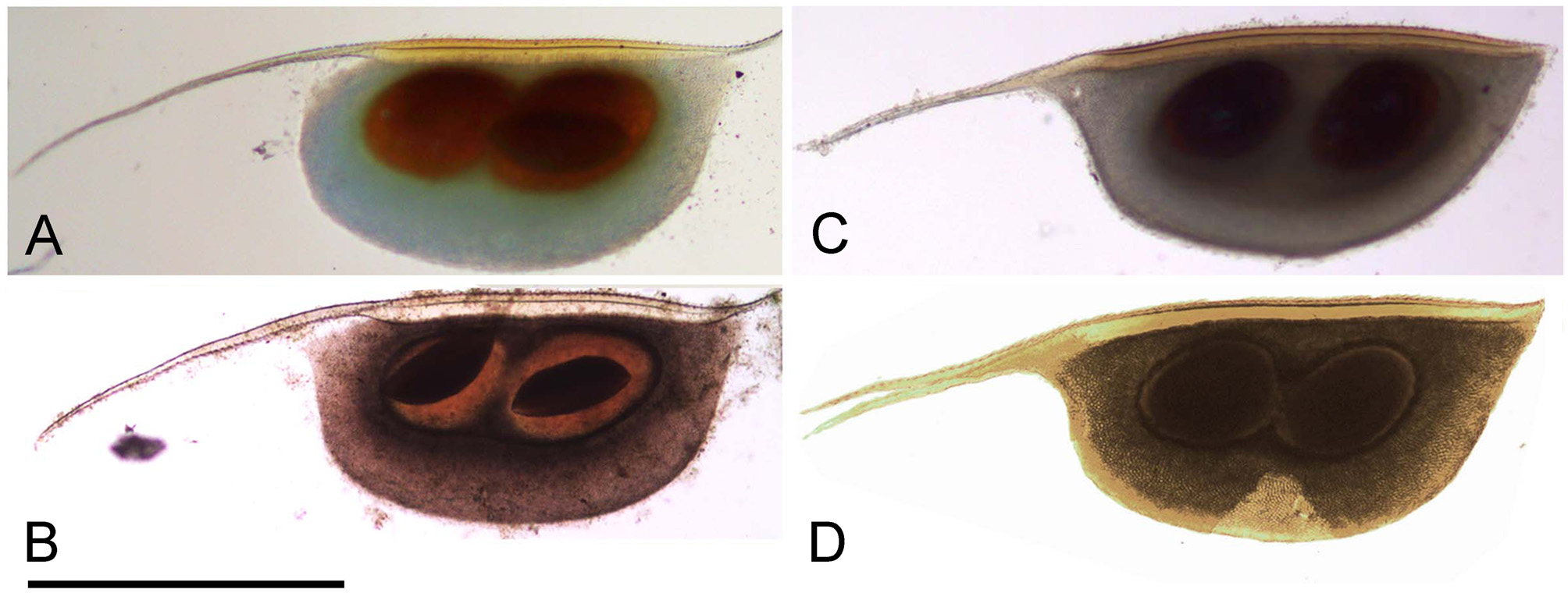
( Figs 2–3 View FIGURE 2 View FIGURE 3 , 4 View FIGURE 4 A–B)
Daphnia similis Claus 1876 View in CoL : p. 364; Flössner 1972: p. 111–113, figs 49–50; Margaritora 1985: p. 114–116, fig. 48; Flössner 2000: p. 138–140, fig. 52; Alonso 1996: p. 165–166, fig. 73; Hudec 2010: p. 84–87, fig. 8.
Type locality. Pool of Gihon (presently known as Birket Mamilla or Mamilla Pool, N31.7781°; E35.2206°), Jerusalem, Israel. GoogleMaps
Claus (1876) specifically writes at the beginning of his paper that the mud came from the same place as D. atkinsoni described by Baird (1859) who writes about mud from “pool of Gihon in Jerusalem ”. Bromley (1993) matches the old and present names: “It should be pointed out that Birket Mamilla, Jerusalem (also known as the Gihon Pool) is the type locality of several Entomostracan species, including two cladocerans, namely, Daphnia atkinsoni ( Baird, 1859) and Daphnia similis ( Claus, 1876) . In recent years, however, D. similis seems to have disappeared from this pool”. Birket Mamilla is a well identifiable place, a large ancient cistern, now in the city centre but in the 19th century several hundred meters away from the city walls. AP sampled this locality couple of times, but no Daphnia was collected.
Type material. Lost. It was initially deposited to the Naturhistorisches Museum, Vienna University ( Richard 1896; Hudec 1991), but any samples of C. Claus are absent in this collection now (P.C. Dworschak, personal communication).
Material studied here. Israel. Flooded field near Tel Ashdod, coll. in 29.01.2004 by A. Petrusek. Mashkena pool, 200 m N. of Golani crossing, W. of Tiberias, coll. in 28.01.2008 by A. Petrusek. Populations 3–4 in Table 1 View TABLE 1 . Spain. Population 7 in Table 1 View TABLE 1 . Hungary. Population 5 in Table 1 View TABLE 1 . Slovakia. Shallow ditch, Šamorín, coll. in 10.10.1956 by M. Vranovský . Russia (European). Populations 8–10 in Table 1 View TABLE 1 .
Short diagnosis. Parthenogenetic female. Body subovoid, body depth/length (without shell spine) = 0.61– 0.71, caudal spine relatively short, no distinct depression between head and rest of body ( Fig. 2 View FIGURE 2 A). Rostrum short and rounded, posterior margin of head slightly convex, a small pre-rostral fold near base of labrum; ventral margin of head without pre-ocular and post-ocular depressions, eye capsule located below the level of anteriormost point of head; ocellus present ( Fig. 2 View FIGURE 2 B–C). Headshield with slightly projected, rounded fornices, a projection from valves penetrates to about 1/2 of headshield length. First abdominal segment with a relatively long (longer than postabdominal claw) process, slightly bent anterior; second segment with a smaller process (shorter than postabdominal claw), third segment with a low, mound-like process; fourth segment lacking a process ( Fig. 2 View FIGURE 2 D). Preanal angle of postabdomen not expressed, postanal angle not expressed. On outer side of postabdominal claw, first and second (proximal) pectens consisting of relatively strong teeth, each approximately two times shorter than claw diameter; third pecten consisting of numerous fine setules not reaching the tip of claw ( Fig. 2 View FIGURE 2 E). Body of antenna I well-developed, tips of aesthetascs not projected beyond tip of rostrum, antennular sensory seta arise from base of mound of the antenna I and reaching tip of mound ( Fig. 2 View FIGURE 2 C). On limb I setae 1–3 long ( Fig. 2 View FIGURE 2 F: 1–3), bear short setules, seta 4 shorter than the former, with short setules ( Fig. 2 View FIGURE 2 F: 4). Limb II–IV as in other species of this group. Limb V with exopodite supplied with single small distal setae ( Fig. 2 View FIGURE 2 G: dis) and a long, curved lateral seta ( Fig. 2 View FIGURE 2 G: lat).
Ephippium. Old ephippium "O"-shaped ( Fig. 4 View FIGURE 4 A), because its anterior margin is regularly convex. Initially (in a very fresh moulting exuvium of the ephippial female), the whole bivalved carapace integument is kept, then the ventral portion of valve rejected, but a delicate anterior portion kept, and imparting a “D” shape to the ephippium ( Fig. 4 View FIGURE 4 B). Note that in non-fresh ephippia this anterior portion is normally lost. Ephippium contains two eggs with axis located at a very acute angle to dorsal margin, anterior process relatively short, postero-dorsal portion of valves (with shell spine) incorporated into ephippium.
Adult male. Body elongated, body depth/length (without shell spine) = 0.48–0.59, rectangular-rounded, dorsal margin of valves almost straight, very slightly elevated above head, no distinct depression between head and valves, postero-dorsal angle distinct, with a long caudal spine. Anterior part of the ventral margin densely covered by relatively long setae with a projection and an adjacent depression (slight) ( Fig. 3 View FIGURE 3 A). Head with a very short, rounded rostrum, lacking both pre-ocular and post-ocular depression. Compound eye modest in diameter, does not occupy all anterior part of head ( Fig. 3 View FIGURE 3 B). Abdomen without processes on each of its segments. Postabdomen with distal portion as a short tube, dorsal margin depressed in preanal region, gonopore ( Fig. 3 View FIGURE 3 E–G: gnp) opens subdistally, on a reduced genital papilla. Anal teeth ( Fig. 3 View FIGURE 3 E–F: at) not reduced, as in female. On outer side of postabdominal claw, the first and second (proximal) pectens consisting of relatively strong teeth, longest teeth only somewhat shorter than claw diameter; third pecten consisting of numerous fine setules not reaching tip of claw ( Figs 3 View FIGURE 3 E–G). Antenna I long, with a bunch of small denticles distally, antennular sensory seta thin, located distally on end of antenna I body; flagellum long, bisegmented, its distal segment setulated ( Fig. 3 View FIGURE 3 B–D). Outer distal lobe ( Fig. 3 View FIGURE 3 H: ODL) with a long seta and a rudimentary seta, inner distal lobe ( Fig. 3 View FIGURE 3 H: IDL) of limb I with a bent copulatory hook having a narrowing tip, and two setae of very different size ( Fig. 3 View FIGURE 3 H: 1 and 1'); additional male seta ( Fig. 3 View FIGURE 3 H: 2') on endite 4; anterior setae 2, 3 and 4 smaller that these in female and supplied with longer setules ( Fig. 3 View FIGURE 3 H: 2–4); posterior setae on endites 4–3 ( Fig. 3 View FIGURE 3 H: a–d) as in female, those on endite 2 somewhat shorther than in female ( Fig. 3 View FIGURE 3 H: e–h). On distalmost endite of limb II, anterior seta straight and asymmetrically setulated ( Fig. 3 View FIGURE 3 I: 1). Limb V without additional small seta on distal portion of exopodite.
Size. Females up to 2.57 mm in our material (up to 2.8 mm according to Flössner 1972, up to 3 mm according to Hudec 2010), adult males 1.27–1.57 mm in our material (up to 1.8 mm according to Flössner 1972 and Hudec 2010).
Distribution. D. similis , as revealed by genetic markers, is present in Israel, Spain, Hungary, Turkey and European Russia. We also identified this species based on male characters in the populations from Slovakia. In Europe D. similis is limited to warmer areas, it was reported to be common for example in the Balkan Peninsula, Hungary or Slovakia ( Petrusek 2003). There were a few old records of daphniids which could belong to D. similis from Germany in the 19th century, but during whole 20th century no investigator reported D. similis from Germany ( Petrusek 2003). A recently discovered population in the vicinity of Munich belongs to another species, D. (C.) inopinata sp. nov., see below. In European Russia, D. similis is present up to 51ºN (Saratov Area of Russia ( Yevdokimov & Yermokhin 2009; this study). A record of D. similis from the Leningrad Area ( Hudec 1991) was apparently mistaken.
The eastern range limit of D. similis s.str. in Eurasia is poorly studied. Glagolev (1995) confirmed the presence of D. similis s.lat. in Siberia up to the vicinity of Krasnoyarsk in the North, up to 56 ºN, but according to our data, northernmost limit of D. similis s.lat. distribution in Asia is about 54°N: Novosibirsk Area in Western Siberia and Baikal region in Eastern Siberia, see Sheveleva (2007), Yermolaeva (2010), Semenova & Aleksjuk (2010), in the latter paper it was misidentified as D. carinata . But populations from Western Siberia need to be verified i.e. by morphological examination of the males as they could belong to D. sinensis . Populations from Eastern Siberia most probably belong to the latter taxon. D. similis s.str. seems absent in the Far East of Asia and in eastern and southern China (see Gu et al. 2013, Ma et al. 2016).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Daphnia similis Claus, 1876
| Popova, Ekaterina V., Petrusek, Adam, Kořínek, Vladimír, Mergeay, Joachim, Bekker, Eugeniya I., Karabanov, Dmitry P., Galimov, Yan R., Neretina, Tatyana V., Taylor, Derek J. & Kotov, Alexey A. 2016 |
Daphnia similis
| Claus 1876 |