Arcticotantulus kristenseni, Knudsen, Steen Wilhelm, Kirkegaard, Maja & Olesen, Jørgen, 2009
|
publication ID |
https://doi.org/ 10.5281/zenodo.186378 |
|
DOI |
https://doi.org/10.5281/zenodo.5612790 |
|
persistent identifier |
https://treatment.plazi.org/id/03B07F41-FFF2-FFE1-FF15-3AFEFAC70689 |
|
treatment provided by |
Plazi |
|
scientific name |
Arcticotantulus kristenseni |
| status |
sp. nov. |
Species. Arcticotantulus kristenseni sp. nov.
Material examined. Holotype: ZMUC CRU 4889, tantulus larva attached dorsally to the first abdominal somite of a copepodid IV stage of an unidentified harpacticoid copepod ( Bradya sp.) mounted on a stub for SEM; collected at Iqpik fishing ground, Disko Bay, Greenland (69°17.2'N, 53°14.4'W), depth 198 m, by R. M. Kristensen, 16 August 2001.
Paratypes: (n = 4) ZMUC CRU 4890, mounted on a stub for SEM, same locality information as for holotype. ZMUC CRU 4891 (n = 18) mounted on a stub for SEM; collected at Iqpik fishing field, Disko Bay, Greenland (69°18.0'N, 53°11.5'W), depth 166–170 m, by S. W. Knudsen, M. Kirkegaard, and R. M. Kristensen, 12 July 2002. ZMUC CRU 4872–4888 (n =15) as whole mounts in 100% glycogen solution for light microscopy; collected at Iqpik fishing ground, Disko Bay, Greenland (69°18.0'N, 53°11.5'W), depth 166–170 m, by S. W. Knudsen, M. Kirkegaard, and R. M. Kristensen, 12 July 2002.
Diagnosis. Tantulus larva comprising cephalon, thorax of six pedigerous somites and one limbless somite, and an undivided abdomen. First thoracic tergite largely concealed beneath posterior margin of dorsal cephalic shield. Cephalic shield triangular; ornamentation consisting of two anterior and four posterior pairs of pores, three pairs of which have an emergent sensillum; surface lamellae present. Cephalic stylet slightly curved in lateral aspect. Thoracopods 1 to 5 each with unsegmented protopod bearing a well developed medial endite. Exopod of thoracopods 1 to 5 two-segmented with 3 (leg 1) or 4 setae (legs 2–5). Of these, the two on the outer margin share a common base, and the one (or two) on the inward side has a second base – if two setae are present on the inward side, they also share a common base. Endopod indistinctly subdivided (terminal part as rigid spine bearing a spatulate process) with 1 seta (legs 1 and 2) or 2 setae (legs 3–5). The endopod setae originate medially from the first segment of the endopod. Thoracopod 6 with unsegmented protopod and one unsegmented ramus. Abdomen twice as long as wide with superficial ornamentation. Caudal rami small, each with three setae (two large and one small). Host is an undescribed species of the genus Bradya (Ectinosomatidae) (Copepoda: Harpacticoida ).
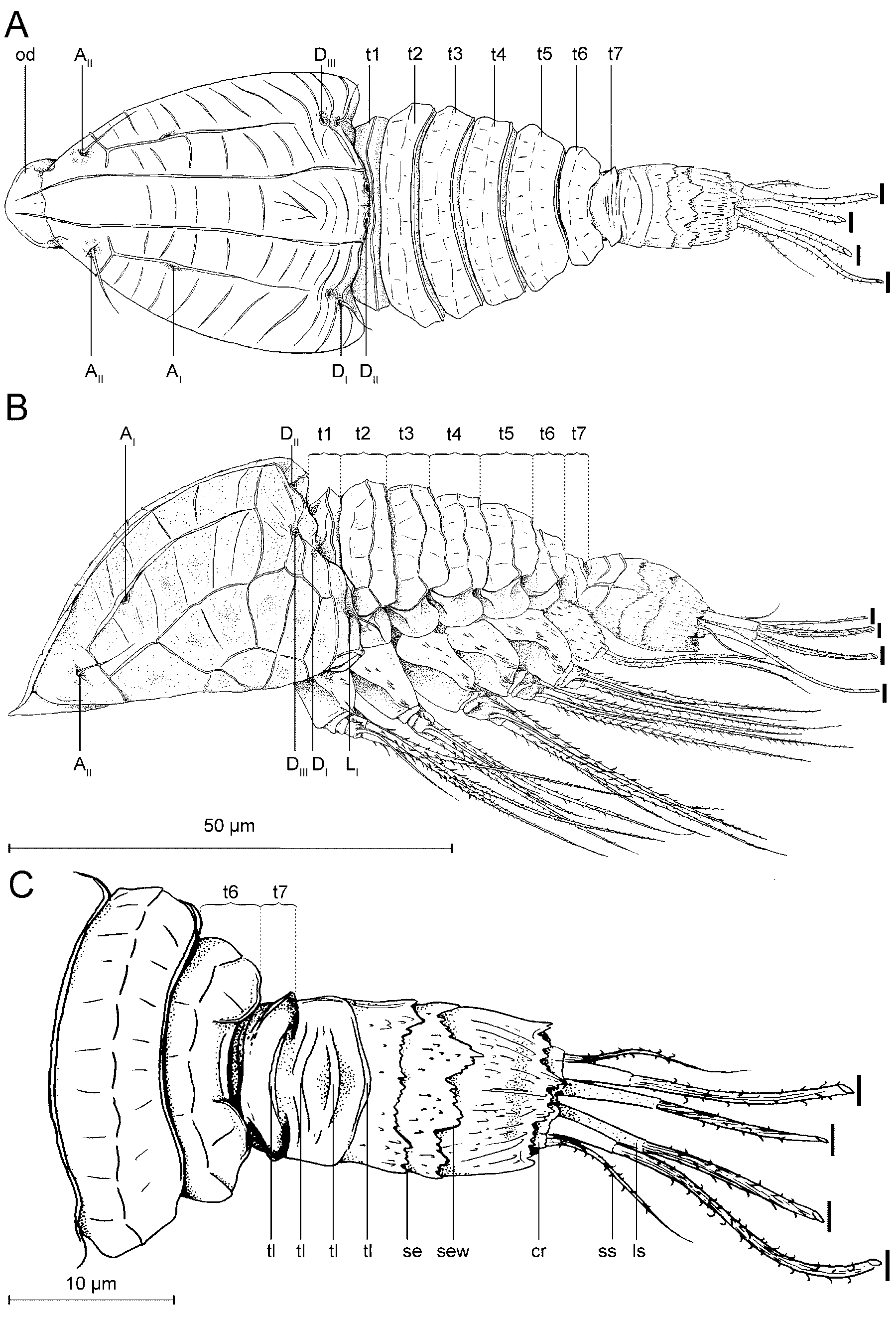
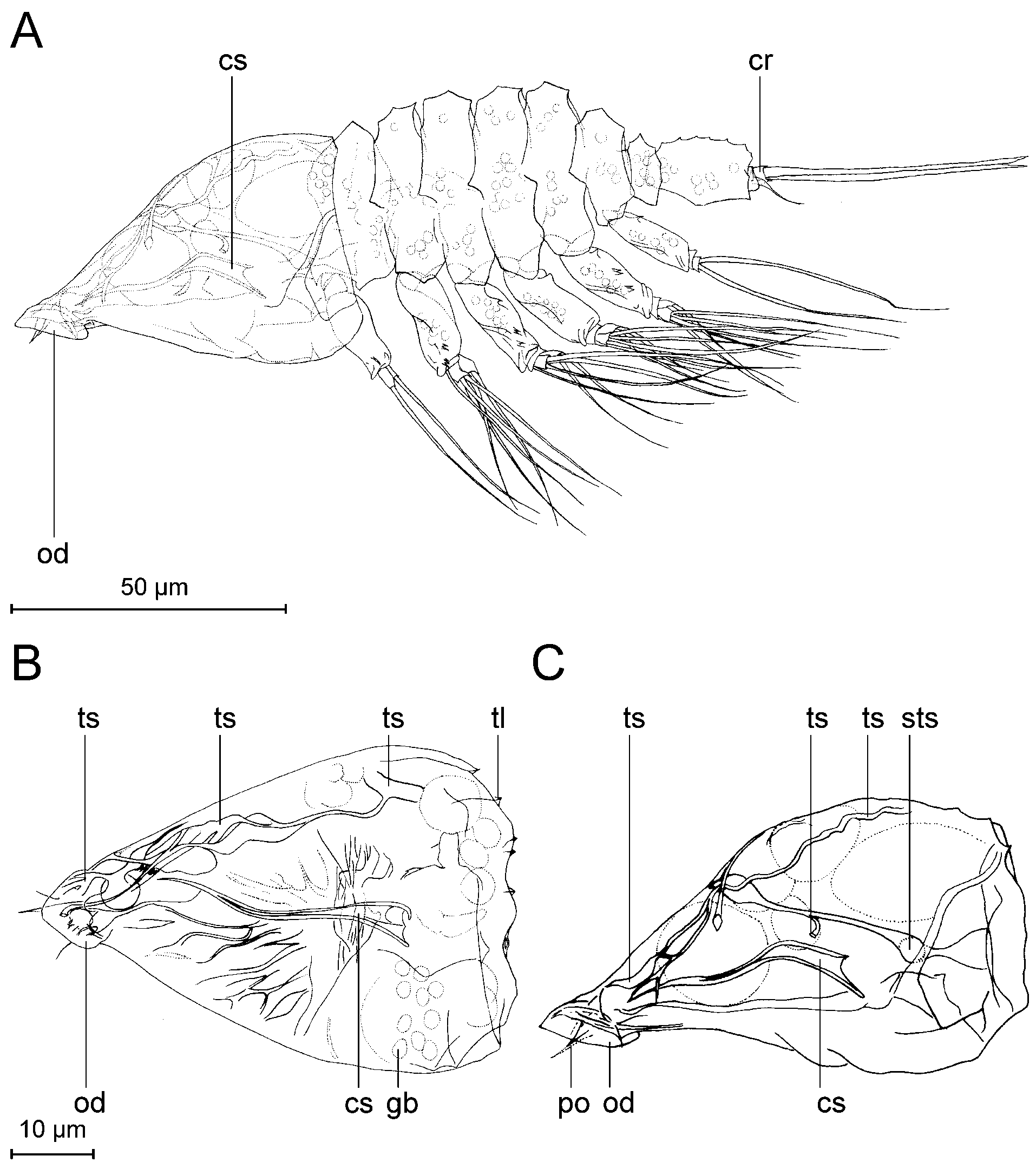
Description. Tantulus larva: The body consists of a prosome and a urosome ( Figs 3 View FIGURE 3 , 10) (terminology after Boxshall & Vader 1993): Fig. 10B, D clarifies the relation between the prosome/urosome and the thorax/ abdomen. The prosome consists of a cephalon and the six anterior pedigerous thoracic somites ( Fig. 3 View FIGURE 3 A, t1- t6). The urosome is two-segmented, consisting of the seventh, limbless, thoracic somite ( Fig. 3 View FIGURE 3 A, t7) and a free, rather long abdominal somite. Total body length varies from 147 µm to 192 µm, measured from the anterior margin of the cephalic shield to the posterior end of the caudal rami. The cephalic shield is 1.3 to 1.5 times longer than wide, varying in length from 43 µm to 48 µm, and in width from 29 µm to 37 µm. The cephalic shield tapers off towards the anteriorly located oral disc and is ornamented with longitudinal lamellae approximately 1 µm high. A rostrum is absent. The cephalon bears six pairs of pores in total (based on SEM) ( Figs 3 View FIGURE 3 , 12). The cephalic pore pairs follow the formula: A I-II, D I-III, L I, and no median or ventral pores were observed. Two pairs are located anterior on the cephalon ( Figs 3 View FIGURE 3 A, 12D) (A I- II); of them, A II has an emergent sensillum. Three pairs are located dorsally, more or less close to the posterior margin ( Figs 3 View FIGURE 3 A, 12) (D I-III); of them, D I has an emergent sensillum. Finally, one pair is located subdorsally at the posterior margin of the cephalon ( Figs 3 View FIGURE 3 A, 12) (L I), with an emergent sensillum. The oral disc ( Figs 3 View FIGURE 3 A, 3B) is approximately 10–12 µm in diameter. A protruding organ ( Fig. 4 View FIGURE 4 C, po) stretches from the oral disc into the host, but is barely visible.
Internal structures in the head were only weakly discernible in light microscopy. The cephalic stylet ( Fig. 4 View FIGURE 4 A, cs) is slightly curved, with a hollow base. Tubular structures (ts) are spread throughout the inside of the head with no apparent symmetry ( Fig. 4 View FIGURE 4 ). No surface openings connecting to the tubular structures were discernable. Globular bodies (gb) seem to be randomly distributed near the posterior rim of the head ( Fig. 4 View FIGURE 4 B).
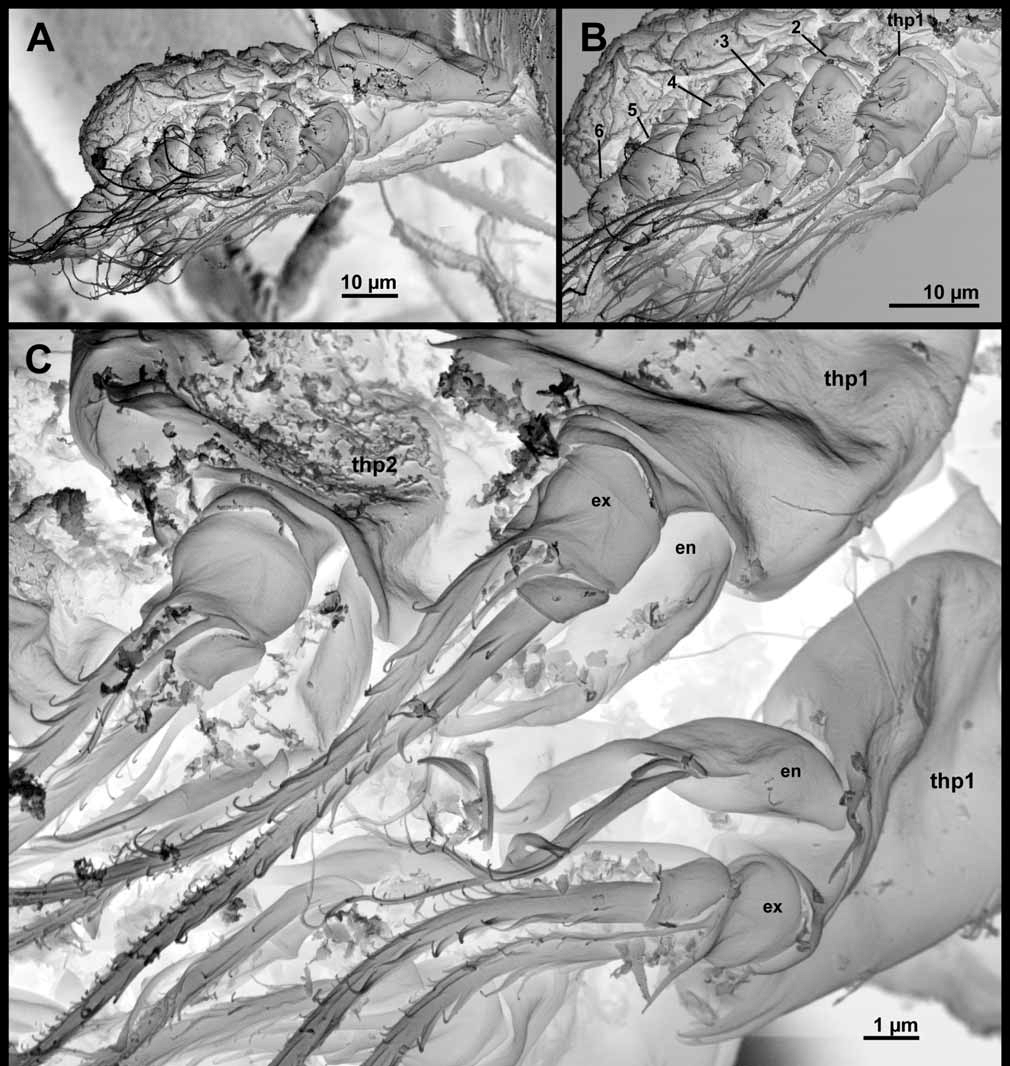
Six thoracopods are present ( Figs 5 View FIGURE 5 , 6 View FIGURE 6 , 13 View FIGURE 13 , 14 View FIGURE 14 ). Thoracopods 1–5 consists of an unsegmented protopod with small, superficial spines or small ‘hairs’, a median endite with one spine, an exopod, and an endopod.
Thoracopod 1 ( Figs 5 View FIGURE 5 A, 13C) has an endopod, which is indistinctly divided into two segments, and a twosegmented exopod. The exopod bears two long setae and one small, all with denticles (based on SEM). The endopod bears one seta with denticles and a long, slender process with a spatulate tip.
Thoracopod 2 ( Figs 5 View FIGURE 5 B, 13C, 14A–C) has a two-segmented exopod that bears two small spines, two large setae with denticles, and two small, slender setae (these appear naked in LM but show small denticles in SEM). The endopod ( Fig. 5 View FIGURE 5 B) is similar to that on thoracopod 1.
Thoracopods 3–4 ( Fig. 5 View FIGURE 5 C and 5D) have a two-segmented exopod and an unsegmented endopod that is produced into a long, slender process. The exopod bears a small spine on the distal segment ( Fig. 5 View FIGURE 5 C, 5D), two setae with long denticles, and two thin setae that appear naked in LM. The proximal portion of the endopod is thick relative to the long, slender distal portion, which bears two setae and has a spatulate process distally ( Fig. 5 View FIGURE 5 C, 5D).
Thoracopod 5 ( Fig. 6 View FIGURE 6 A) has a two-segmented exopod that bears two small spines ( Fig. 6 View FIGURE 6 A, sex), two setae with denticles, and two thin setae that appear naked in LM. The endopod is similar to those on thoracopods 3 and 4.
Thoracopod 6 ( Fig. 6 View FIGURE 6 B) consists of a protopod with small spines but no median endite. The protopod has a one-segmented exopod with setae that have denticles. We did not detect any coupling spines on the median endites of the protopods but cannot exclude that they are present.
The first tergite is largely concealed beneath the posterior rim of the cephalon ( Fig. 3 View FIGURE 3 , 10). The tergites on the first to sixth thoracic somites each have a distinct transverse lamella and are further subdivided into characteristic polygonal patterns ( Figs 3 View FIGURE 3 , 10). The seventh thoracic somite (urosome segment 1) is short – about twice as wide as long ( Figs 3 View FIGURE 3 C, 10D). The unsegmented abdomen (urosome segment 2) is relatively long – about twice its width – with sides that are parallel anteriorly but converge slightly posteriorly. Dorsally the abdomen is superficially divided into a segment-like pattern: the anterior part bears about three rows of curved, transverse lamellae, while the posterior part bears three transversely orientated and denticle-serrated lamellae, the middle one of which has a characteristic ‘w-shaped’ pattern (Fig. 10D, arrow). The posterior lamella partly covers a pair of small caudal rami, each with two large and one small setae all bearing denticles ( Figs 3 View FIGURE 3 , 10B).
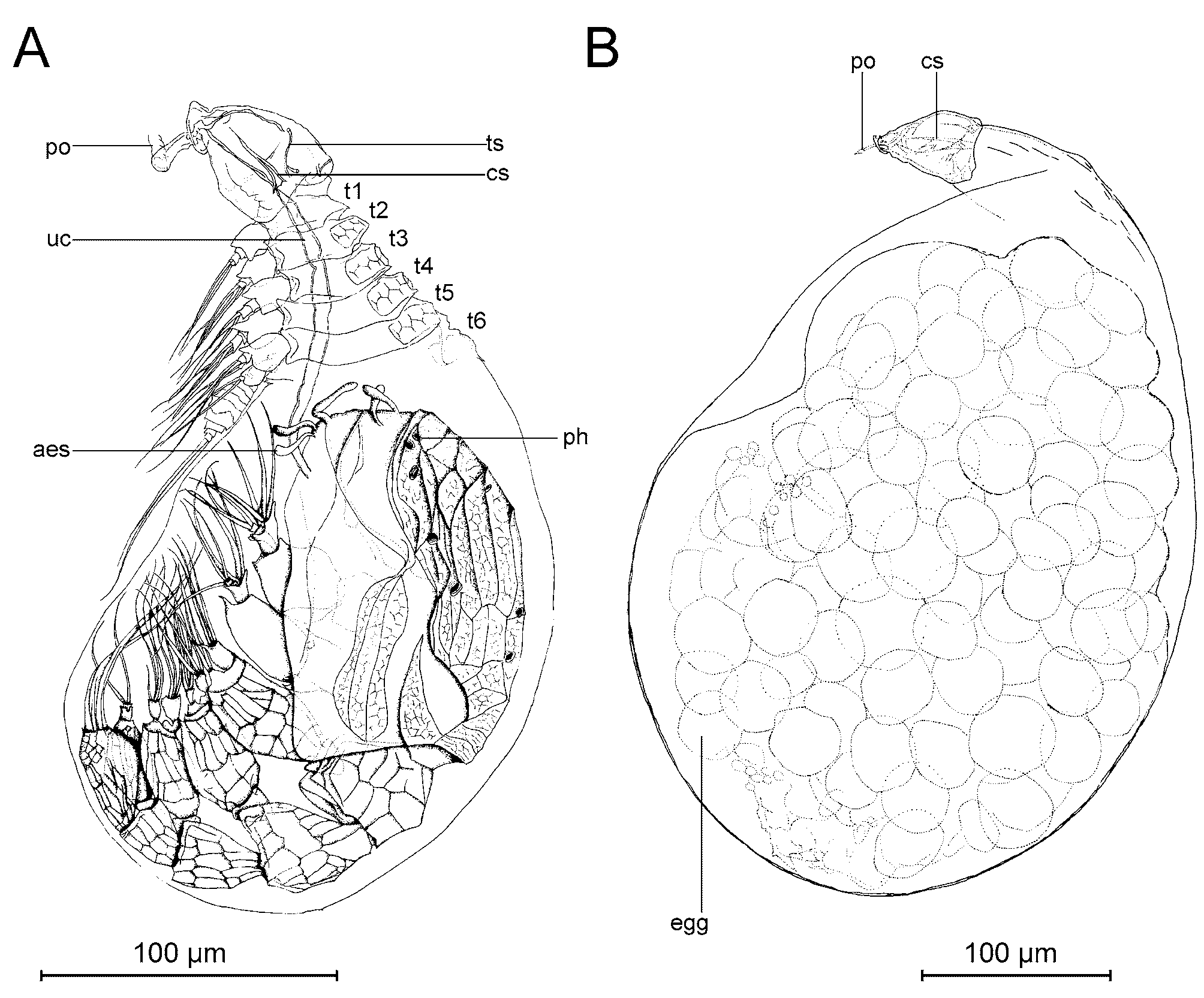
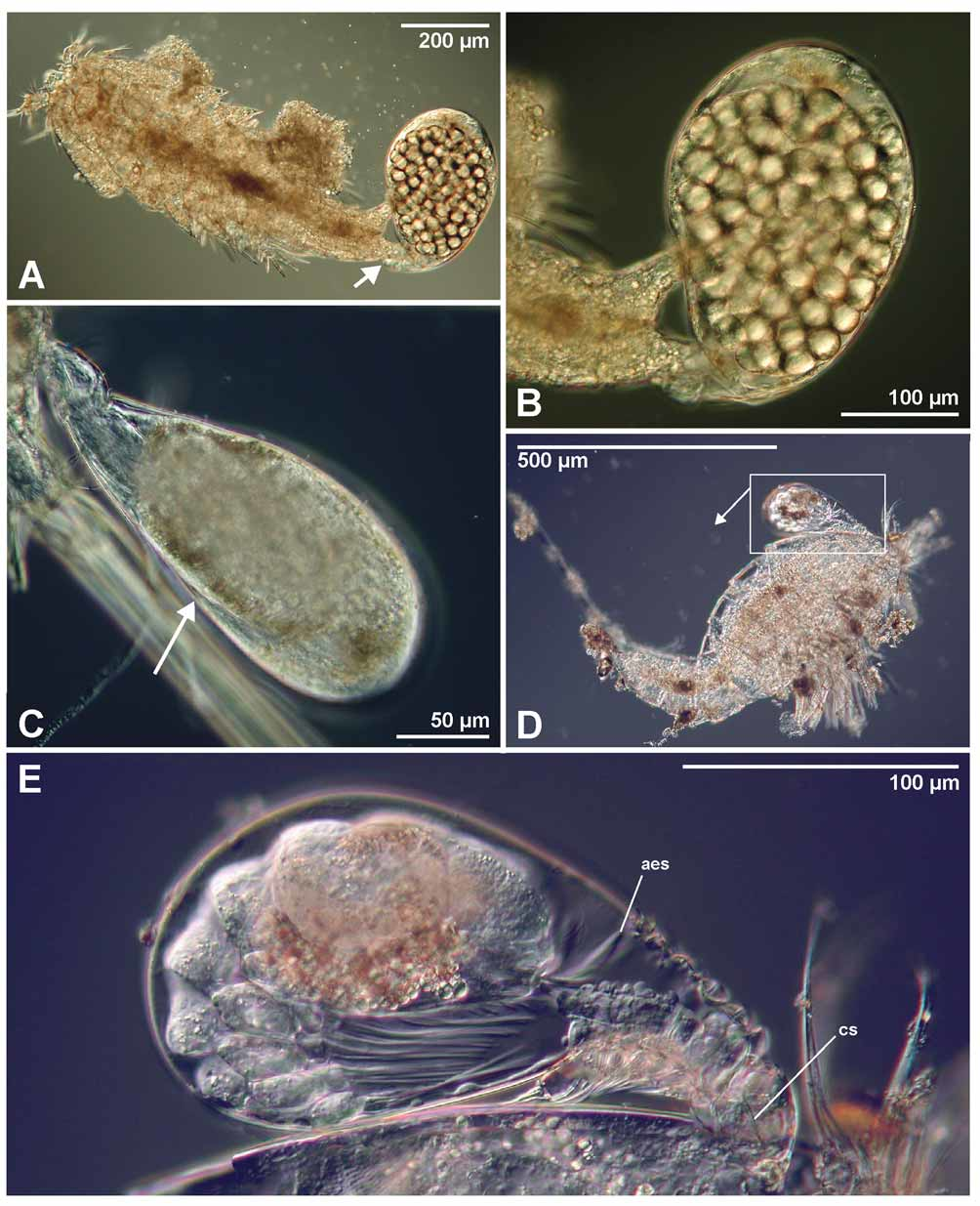
Male: In spite of several attempts, we were unable to free a male successfully from the sac of the tantulus larvae. A complete description of the male depends on the setation of the limbs, and we have been unable to discern these features with certainty in light microscopy alone. Instead, only a few remarks will be made to supplement the figures presented. The description is based on developing males not yet released from the surrounding tantulus trunk sac ( Figs 7 View FIGURE 7 A, 8, 9, 11B, 14, 17). The trunk sac is developed behind the sixth tergite of the attached larva and there is no additional swelling between the cephalic shield and the first tergite ( Figs 7 View FIGURE 7 A, 8, 11A). The developing male inside the trunk sac ( Fig. 7 View FIGURE 7 A) has six pairs of thoracopods with setae and a fine, honeycomb-like ornamentation on the head shield as well as on the six tergites and the urosome. On the head shield of the developing male large pores are visible in LM, but apparently no internal structures are connected to them at this stage of development. A long umbilical cord-like organ (term from Boxshall 1988) is visible through the trunk sac, connecting the male with the head of the attached larvae. No penis could be discerned in light microscopy but it could have been overlooked, since it is usually found behind the sixth pair of thoracopods (see Boxshall & Huys 1989; Huys et al. 1992b; Boxshall et al. 1989). Three pairs of aesthetascs are present on the head of the male ( Fig. 7 View FIGURE 7 A).
FIGURE 10. Tantulus larva of Arcticotantulus kristenseni sp. nov. (SEM), all photos of holotype (ZMUC CRU4889). A. Dorso-lateral view. B. Dorsal view, showing demarcations between prosome and urosome, and between thorax and abdomen. C. Close-up of anterior cephalic pores and oral disc, pore A I without emergent sensillum, pore A II with emergent sensillum. D. Dorsal view of urosome, showing demarcation between thorax and abdomen, With arrow pointing at characteristic ‘w-shaped’ serrated edge in cuticle of abdomen (sew).
Parthenogenetic female: The larval thoracic somites and urosome are sloughed early in development when the trunk sac is as yet still smaller than the cephalon ( Fig. 16 View FIGURE 16 A). The two least developed females found measured 67 µm and 82 µm in length, respectively ( Fig. 16 View FIGURE 16 A, 16B). The trunk sac grows ( Fig. 16 View FIGURE 16 ) until the complete length of the female is about 405 µm ( Figs 7 View FIGURE 7 B, 17A, 17B). The egg sac inside the trunk sac measured 345 µm in length. No gonopore or abscission scar of the larval trunk was discernible but it may have been overlooked. No long, slender neck was seen on any specimen, similar to the one reported from females of some other species ( Boxshall 1991; Boxshall & Vader 1993; Grygier & Sieg 1988). Three undeveloped females ( Fig. 16 View FIGURE 16 ) had what could be the beginning of a rootlet system penetrating the cuticle of the host through the oral disc ( Fig. 16 View FIGURE 16 D, arrow).
FIGURE 12. Cephalon of tantulus larva of Arcticotantulus kristenseni sp. nov. with well-developed male inside (SEM), all photos of same same specimen (ZMUC CRU4891).Pores on cephalon: A I-II, D I-III, L I. A. Lateral view. B. Close-up of anterior pores: A I-II. C. Close-up of posterior pores: D I-III, L I. D. Dorsal view.
Sexual female: One fully developed individual with the larval trunk sloughed – which is the external characteristic of a female – was observed ( Fig. 17 View FIGURE 17 C). Since the trunk sac of this individual did not contain developing eggs, but a large mass of apparently undifferentiated tissue, we interpret it as being a sexual female in the early course of its development. The contents of the trunk sac displayed approximately the same shape as the developing sexual females reported by Huys et al. (1993b) and Ohtsuka and Boxshall (1998). Posteriorly, the presumptive abdomen is partly separated from the remaining body ( Fig. 17 View FIGURE 17 C, arrow). Two other individuals had a similar trunk sac with undifferentiated tissue inside ( Fig. 16 View FIGURE 16 A and 16B), and resembled the potential sexual female ( Fig. 17 View FIGURE 17 C). No further observations were made on this specimen.
Etymology. The species is named after Prof. Reinhardt Møbjerg Kristensen (Zoological Museum, Copenhagen), in honour of his work on marine invertebrates in Greenland. He was the first to catch the species described herein.
Discussion. The Tantulocarida are at present divided into five families, the Basipodellidae (with 8 species), Deoterthridae (with 11 species), Microdajidae (with 5 species), Onceroxenidae (with 2 species), and Doryphallophoridae (with 3 species). With this new species ( Arcticotantulus kristenseni sp. nov.) included and with A. pertzovi assigned to the Deoterthridae , the family Basipodellidae contains 7 species, and the Deoterthridae , 13 species ( Table 1). The present description of A. kristenseni sp. nov. from the coastal waters of West Greenland broadens the geographical distribution of the Tantulocarida but is not the first discovery of this group in Greenland. Polynyapodella ambrosei Huys, Møjbjerg , and Kristensen, 1997 (q.v.) has previously been described from Northeast Greenland. Of all described tantulocaridans A. kristenseni sp. nov. is most similar to A. pertzovi and is therefore described as a second species in the same genus.
Similarities between A. kristenseni and A. pertzovi . Both species of Arcticotantulus are found on a harpacticoid copepod species of the genus Bradya [not Pseudobradya Sars, 1911 as mentioned for A. pertzovi by Kornev et al. (2004) (Huys, pers. com. 2006)]. The two species are found in different Arctic regions far from each other (White Sea and off the coast of West Greenland) and are considered separate. A comparison between A. kristenseni sp. nov. and the published information about A. pertzovi reveals several similarities ( Table 4) that justify their placement in the same genus. Both A. kristenseni and A. pertzovi possess a limited number of pore pairs, and both species have the D I - and L I -pore pairs. In both A. kristenseni and A. pertzovi the cephalic lamellae are arranged longitudinally. Each of the exopods on the first to fifth thoracopods of both species is divided into two segments. Each of the endopods on the third to fifth thoracopods has two setae attached. The sixth thoracopods are similar in both species. A ‘w-shaped’ ornamentation and some minor denticles are present on the abdomen of both species.
Differences between Arcticotantulus kristenseni and other Arctic and North Atlantic Tantulocarida. Among other Arctic species of the Tantulocarida, A. kristenseni is clearly different from Polynapodella ambrosei with respect to the two-segmented urosome (superficially multisegmented in P. ambrosei ) and in having fewer pore pairs on the cephalon (as concerns the A-, D-, and L-pore pairs). On the cephalon of A. kristenseni the A I - - and A II -, the D I -, D II -, and D III -, and the L I -pore pairs are present, but on the cephalon of A. Pertzovi , only the A III -, the D I - and the L I -pore pairs. Furthermore, A. pertzovi also has a D IV -pore pair (see Table 4). Another difference concerns the lamellae on the cephalon of the tantulus larvae. In addition to the longitudinal lamellae on the cephalon in both A. kristenseni and A. pertzovi , A. kristenseni has some lamellae arranged in polygons. The setation of the thoracopods in the two species is also different. In A. kristenseni , the protopods of the first and second thoracopods are unsegmented (two-segmented in A. pertzovi ), the endopods Character Arcticotantulus kristenseni Arcticotantulus pertzovi Total body length 147–192 µm 140 µm Size of oral disc 10–12 µm in diameter 7 µm in diameter Length of cephalic stylet Approx. 60 µm 30 µm Cephalic pores Anterior pores A I, A II A III
Dorsalpores D I, D II, D III D I, D IV
Lateralpores L I L I
Swelling between cephalon and first tergite in Not parted significantly Not parted significantly developing male
Cephalic rostrum Absent Absent Cephalic lamellae ornamentation Longitudinal and polygonal Longitudinal Thoracopod 1 No. of segments of protopod 1 2
No. of endopod setae 1 0
No. of exopod setae 4 2
No. of segments of exopod 2 2
No. of segments of endopod 2 1
Thoracopod 2 No. of segments of protopod 1 2
No. of endopod setae 1 0
No. of exopod setae 4 2
No. of segments of exopod 2 2
No. of segments of endopod 2 1
Thoracopod 3 No. of segments of protopod 1 2
No. of endopod setae 2 2
No. of exopod setae 4 3
No. of segments of exopod 2 2
No. of segments of endopod 2 1
Thoracopod 4 No. of segments of protopod 1 2
No. of endopod setae 2 2
No. of exopod setae 4 3
No. of segments of exopod 2 2
No. of segments of endopod 2 1
Thoracopod 5 No. of segments of protopod 1 2
No. of endopod setae 2 2
No. of exopod setae 4 3
No. of segments of exopod 2 2
No. of segments of endopod 2 1
Thoracopod 6 No. of segments of protopod 1 1
No. of endopod setae No endopod No endopod
to be continued.
carry one seta (absent in A. pertzovi ), the exopods carry four setae (two in A. pertzovi ), and the endopod is divided indistinctly into two segments (unsegmented in A. pertzovi ). Furthermore, in A. kristenseni , the protopods of the third to fifth thoracopods are undivided (two-segmented in A. pertzovi ), the exopods carry four setae (three in A. pertzovi ) and the endopods are divided into two segments (unsegmented in A. pertzovi ) ( Table 4).
Relationship of the genus Arcticotantulus and comments on the description of Arcticotantulus pertzovi . Arcticotantulus was assigned to the Basipodellidae by Kornev et al. (2004), but based on the information on A. kristenseni provided in this paper and the previously published information on A. pertzovi , and by using the family characteristics provided by Huys (1990a), we argue that the genus is better assigned to the Deoterthridae ( Table 2 View TABLE 2 ). The classification of the Tantulocarida has mostly been based on external characteristics of the tantulus larvae, such as pore patterns of the cephalon, segmentation of the urosome, and setation of the thoracopods. In the following these characteristics are listed and discussed.
Cephalon of Arcticotantulus . The single pair of subdorsal cephalic pores found on Arcticotantulus is similar to the single pair (or more) of subdorsal cephalic pores found in Deoterthridae ( Huys 1990a) . The tantulus of A. kristenseni has a very characteristic pore pattern on the cephalon, a pattern which to our knowledge, is not found in any other tantulocaridans. There is a total of six pairs: four pairs close to the posterior margin of the cephalon and two additional pairs anteriorly ( Figs 3 View FIGURE 3 A, 3B, 10C, 12). One of the anterior pore pairs and two of the posterior pore pairs has an emergent sensillum, consistently found in the same position in all examined specimens. The total number of pore pairs in Arcticotantulus deviates from the ten pairs usually found in the Deoterthridae , with only six pairs in A. kristenseni and only five pairs in A. pertzovi ( Kornev et al. 2004) , but since this number is known to vary within the Deoterthridae , we do not see the low count of pore pairs hindering a placement within this family (see Table 2 View TABLE 2 ). The absence of a cephalic rostrum favours the placement of Arcticotantulus within the Deoterthridae (see Table 2 View TABLE 2 ) ( Huys 1990a).
Thoracopod setation in Arcticotantulus . Usually there is only one seta on the endopod of the first thoracopod in Deoterthridae , or setation is absent there ( Table 2 View TABLE 2 ) ( Huys, 1990a). This is consistent with the single seta found on the endopod of this limb in A. kristenseni and with the lack of setae seen in A. pertzovi . In contrast, Basipodellidae are characterised as carrying two setae on the endopod of the first thoracopod. Most aspects of the setation of the trunk limbs in Arcticotantulus fit some of the existing families as they are defined by Huys (1990a), but the setation of one limb in Arcticotantulus is a notable exception. In addition to an elongate process with a spatula-shaped tip, the endopod of thoracopod 2 has only one seta, which is contrary to the situation in the Deoterthridae , Onceroxenidae , Basipodellidae , and Doryphallophoridae , in all of which two setae are present ( Huys 1990a). In Microdajus langi Greve, 1965 (Microdajidae) , two setae appear to be present as well, but without free rami ( Boxshall & Lincoln 1987) it is difficult to infer where these setae inserted originally. This makes it difficult to compare Arcticotantulus with Microdajus langi Greve, 1965 . The endopod of thoracopods 3–5 in Arcticotantulus has two setae, as is the case for all families: Microdajidae being a possible exception. The exopods of the thoracopods in Arcticotantulus have a well-developed setation that falls within the range of variation of the Deoterthridae ( Huys, 1990a) , but this differs only slightly from that of the Doryphallophoridae , the Onceroxenidae , and the Basipodellidae . Thus, the setation of thoracopods 3–5 is not usable for identifying the correct family ( Table 2 View TABLE 2 ).
Abdomen and urosome of Arcticotantulus . Because of confusion in defining the terms ‘abdomen’ and ‘urosome’ in the Tantulocarida, there have continuously been difficulties assigning species to different families. When Huys (1990a) characterized the different families he regarded the abdomen as comprising the seventh, limbless, thoracic somite together with the part of the tantulus larvae posterior to it. In contrast, Boxshall and Vader (1993) used the term ‘urosome’ for this region (similar to Huys’ (1990a) use of ‘abdomen’), and restricted the term ‘abdomen’ only to more posterior parts of the tantulus larvae. As Boxshall and Vader’s terminology (1993) has been the most commonly used in descriptions after 1993, we have decided to apply it in the descriptions herein. Difficulties arise when tantulocaridans are assigned to families only based on the characteristics mentioned by Huys (1990a). Using the terminology applied by Boxshall and Vader (1993), Basipodellidae are characterized as having a multi-segmented urosome and Deoterthridae as having a two-segmented urosome. Arcticotantulus has an unsegmented abdomen and a seventh limbless thoracic somite. This implies that the urosome is two-segmented, and that Arcticotantulus therefore should be assigned to Deoterthridae . The abdomen (second segment of the urosome) of Arcticotantulus kristenseni resembles the abdomen of Deoterthron dentatum Bradford and Hewitt, 1980 and Deoterthron lincolni (Boxshall, 1988) , two other species of Deoterthridae (see Huys 1990a), in having (1) a pair of semi-circular lamellae anteriorly, (2) being most narrow posteriorly, and (3) by being partly covered by cuticular hairs in the posterior half. The abdomen of Doryphallophora harrisoni ( Boxshall and Lincoln, 1987) , which is a species of the family Doryphallophoridae , resembles that of A. kristenseni to a lesser extent in being relatively unornamented with cuticular hairs only ( Boxshall & Lincoln, 1987). In most other species of the Tantulocarida the abdomen of the tantulus larvae is more heavily ornamented, and often superficially subdivided into several ‘segments’. This is not the case in Arcticotantulus , which has an unsegmented abdomen (and a two-segmented urosome) with little ornamentation apart from a ‘w-shaped’ ridge in the cuticle (Fig. 10, Kornev et al. 2004: figs. 1A, 3B). One other potentially taxonomically important structure is the honeycomb-like ornamentation on the thoracic tergites, which hitherto have been only known from Aphotocentor styx Huys, 1991 (q.v.) and Campyloxiphos dineti Huys, 1990 a (q.v.). Both of these are assigned to the Deoterthridae . This ornamentation is only conspicuous on Arcticotantulus kristenseni and not on A. pertzovi .
The developing male of Arcticotantulus . Huys (1990a) suggested that the three different types of thoracic swelling during male development are taxonomically significant at the family level. In Arcticotantulus kristenseni there is only one expansion zone of the tantulus trunk sac during male development - behind the sixth tergite. This is similar to that shown for Microdajus (Microdajidae) , Onceroxenus (Onceroxenidae) , and Deoterthron (Deoterthridae) , while the trunk sac expansion in Doryphallophora and the Basipodellidae takes place between other tergites ( Huys 1990a). Similarly, A. pertzovi ( Kornev et al. 2004: fig. 1B) only has an expansion zone behind the sixth tergite (see Huys 1990a: fig. 7). The expansion zone in Acrticotantulus can thus be compared with the expansion zone observed in Microdajidae , Onceroxenidae , and Deoterthridae . According to Kornev et al. (2004), the developing male of A. pertzovi has its first tergite widely parted from the cephalon ( Kornev et al. 2004: fig. 1B) as in Basipodellidae ; however, as far as we can tell, this partition is not nearly as large as would be expected for Basipodellidae (see Huys 1990a). On the contrary, the partition of the first tergite from the cephalon seen in A. pertzovi resembles the partition seen in Deothertridae (see Huys 1990a).
The host species of Arcticotantulus . Bradya sp. (Huys pers. com) appears to be hosting all the specimens of A. kristenseni and the same harpacticoid genus appears to be infected by A. pertzovi . The host species of A. pertzovi was originally reported as Pseudobradya sp. ( Kornev et al. 2004) but this may be incorrect as the host resembles Bradya sp. (Huys pers. com).
Taxonomic conclusions. In summary, it is not a straightforward matter to assign Arcticotantulus kristenseni to any existing family of the Tantulocarida. Since most characters are shared with species of Deoterthridae (see above), we assign this new species to this family, and also assign it to the genus Articotantulus in light of various similarities with A. pertzovi . We are aware that the pore pattern on the cephalon and the presence of only one seta on the endopod of thoracopod 2 do not fit the characteristics of the Deoterthridae as defined by Huys (1990a).
Internal structures of Arcticotantulus kristenseni sp. nov. The tubular structures inside the head of the tantulus larvae apparently do not connect with any superficial openings. Conversely, although, the developing male has superficial openings on the head shield, it does not have any clearly visible internal connecting structures ( Fig. 7 View FIGURE 7 A). The existence of such connections can probably only be verified by transmission electron microscopy (TEM). The so-called tubular structures within the head could be a misinterpretation of glandular structures, but to confirm this TEM would be required as well. Glandular structures associated with the stylet have previously been recognized by Boxshall and Huys (1989), Huys (1991), and Huys et al. (1994). The purpose of these glandular or tubular structures remains uncertain. It is presumed that muscles associated with the cephalic stylet degenerate after it has served its purpose by piercing the host when the tantulocaridan attaches itself ( Boxshall 1991). This could explain the asymmetrical placement of the stylet in the head. The purpose of the umbilical cord-like structure reported in this work and previous papers connecting the male’s head with the head of the attached larvae is uncertain, but Boxshall and Lincoln (1987) suggested that it provides the male inside with nourishment extracted from the host. This work documents the presence of a tube-like structure crossing the cuticle of the host ( Fig. 16 View FIGURE 16 D) of an undeveloped female. Huys (1991) suggested that a branching network of cells spreads out from the tantulocaridan and inside the host.
| ZMUC |
Zoological Museum, University of Copenhagen |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Family |
|
|
Genus |