Anthaxia midas subsp. midas, Kiesenwetter, 1857
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4370.3.1 |
|
publication LSID |
lsid:zoobank.org:pub:1990812B-965B-4015-AE78-A40058B0E4CD |
|
DOI |
https://doi.org/10.5281/zenodo.5681396 |
|
persistent identifier |
https://treatment.plazi.org/id/03AF8517-FF45-9308-2DCE-FD9E99F4174B |
|
treatment provided by |
Plazi |
|
scientific name |
Anthaxia midas subsp. midas |
| status |
|
The Anthaxia (Anthaxia) midas Kiesenwetter, 1857 View in CoL species-group
Historical taxonomic references. In the careful study of the bibliography of this group, we ascertained that the first unquestionable reference of A. midas sensu lato, was published by Gory & Laporte (1839: 15), who reported this species from south France as A. croesus (Villers) .
Some years later, Kiesenwetter (1857: 83) wrote that, in his opinion, A. croesus (Villers) sensu Gory & Laporte was a different species from the species originally described by Villers, and eventually changed its name into A. midas ( Kiesenwetter, 1857: 90) .
Incidentally, since Kiesenwetter expressed his opinion, the problem of the real identity of A. croesus (Villers) had remained somehow unresolved. In fact, A. croesus auctorum has been widely quoted in literature, always with various incorrect original spellings, and not only in relation to A. midas , but also to various species from different subgenera. Therefore, to avoid confusion, before we deepen the taxonomic history of the A. midas group, it will be appropriate to dedicate a few lines to explain the taxonomical position of A. croesus sensu lato.
It is well known that, at the time of publication of the work by Kiesenwetter, there was no universally recognized taxonomic regulation. Therefore, when Kiesenwetter (1857: 82) synonymized A. (Haplanthaxia) scutellaris (Gené, 1839) , A. (H.) viminalis Gory & Laporte, 1839 , and A. (H.) fulgidipennis Lucas, 1846 under A. croesus (Villers) , his opinion raised a debate on the real identity of A. croesus . The discussion lasted for a long time, becoming in some cases rather controversial. In absence of any illustration of this species in the original work of Villers, various authors speculated on its real taxonomic position, associating it with different species.
Indeed, the few characters outlined by Villers, are objectively applicable to various species from different groups, all with similar patterns of colouration. In the attempt to assert their respective point of view, Kiesenwetter (1857: 83) and Marseul (1865: 230) even discussed at length on the comments made by Villers about the beauty and size of his species, or the alleged hidden meanings of its distribution. Eventually, most of the early authors followed the opinion of Kiesenwetter. Among them, we find Jacquelin Du Val (1861: 141), Redtenbacher (1874: 511), Stierlin (1886: 10), Kerremans (1892: 119, 124; 1903: 174, 176), Fauconnet (1892: 250, 251), Acloque (1897: 277) and others. Jacquelin Du Val (1861: Pl. 25, Fig. 22 View FIGURES21‒30 ) also published an illustration of A. croesus (Villers) , perhaps the only faithful one ever published. The specimen pictured in his work undoubtedly represents a male of the species formerly known as A. scutellaris , now A. croesus (see Kubáň et al., 2016: 22), and illustrates the typical bent shape of its legs, and its exact pattern of dorsal colouration.
We know that, before his death, Villers had passed his collection to a fellow citizen of Lyon, but since then, there have been no more information about its final destination, and it is presumed to have been destroyed. Nevertheless, it is conceivable that at the time during which Jacquelin Du Val was preparing his work, the collection still existed, and he might have had a chance to see the type, and illustrate it in his work.
Amongst more recent authors who discussed the matter, we find Obenberger (1917: 46, 133), Schaefer (1938: 216) and Richter (1949: 149). Each of them gave a different interpretation, associating A. croesus (Villers) to different forms of A. salicis (Fabricius, 1776) , but probably they did not take into consideration the fact that, together with A. croesus , in the same work Villers had separately treated also A. salicis ( Villers, 1789: 337) . Schaefer in particular, followed the opinion of Abeille (1904: 217), who had associated A. croesus (Villers) with the species currently known as A. semicuprea Küster, 1852 .
In the absence of a recognized type, the various interpretations were destined to remain merely individual speculations, and the problem has long remained unresolved. In modern Catalogues ( Bílý, 1997: 61; Bellamy, 2008: 1465), A. croesus (Villers) was still considered a synonym of A. salicis , while A. croesus auctorum (not Villers), was treated as an unavailable name for A. salicis . In our opinion instead, based on the above, and in particular on the illustration by Jacquelin Du Val, the description of A. croesus (Villers) is more pertinently applicable to A. scutellaris . Therefore, with consensus of some specialists, in the latest edition of the Palaearctic Catalogue, A. croesus (Villers) has been resurrected from synonymy, and A. (H.) scutellaris (Gené, 1839) was reconfirmed as a synonym of A. croesus (Villers) ( Kubáň et al., 2016: 22).
With regard to A. croesus (Villers) sensu Gory & Laporte , it seems obvious that the authors had never seen the type of that species before using its name in reference their specimen. According to what Schaefer assumed (1938: 209), they based their identification only on the description and distribution given by Villers (1789: 339), and were probably misled by the alleged same provenance of both specimens. In fact, Gory & Laporte had received their specimen from Solier, who lived in Marseille, and this led them to believe that the specimen had been collected in southern France.
Kiesenwetter (1857: 83) contested their identification mainly by focusing on the significance of the comments made by Villers about the size of his species. Hence, by renaming it A. midas , he implicitly considered the species by Gory & Laporte a homonym.
Marseul instead, whom at first seemed to agree with Kiesenwetter ( Marseul, 1863: 136), after a short time changed his mind, and adopted a point of view diametrically opposed ( Marseul, 1865: 229). In fact, based on his personal interpretation of the comments by Villers and Gory & Laporte, he challenged the arguments of Kiesenwetter, and reversed the situation, placing A. midas Kiesenwetter in synonymy with A. croesus (Villers) sensu Gory & Laporte.
Like Marseul, Obenberger also showed a wavering opinion about the validity of A. midas , and at first he seemed to consider it as a valid species ( Obenberger, 1912: 66, 67). Some years later though, in his "Holarktische Anthaxien" ( Obenberger, 1917: Table A. Fig. 5 View FIGURES 1‒9 ), he illustrated what clearly appears to be a specimen of Balkan origin, identifying it as A. croesus Gory & Laporte. In the same paper, Obenberger also begun to further complicate the situation by applying the same name to different species, and considering A. croesus (Villers) as a variation of A. salicis . In a subsequent work, Obenberger (1925: 56) changed his mind once again, describing A. midas muelleri and implicitly reassessing the status of the species. Incidentally, the type of A. midas muelleri Obenberger, 1925 , could be the same specimen that he had formerly illustrated as A. croesus Gory & Laporte in his previous work ( Obenberger, 1917).
In the meantime Obenberger had also described another taxon of this group, currently known as A. spathuligera Obenberger, 1924 , the taxonomic position of which the author was doubtful. In fact, he first described the taxon as a subspecies of A. salicis ( Obenberger, 1924: 27) , and then he considered it a subspecies of A. midas ( Obenberger, 1938: 192‒193). A longtime after these change, Sakalian (2007) wrongly elevated the taxon to the rank of species.
A further species, actually the first of the A. midas species-group described by Obenberger, is A. (A.) holoptera Obenberger, 1914 . The author described this species together with several other new species conserved in the NHMW, and in the description, he specified that the type be conserved in the Daniel collection. In the absence of illustrations, many early authors based their concept of A. holoptera , and its taxonomic position, only on its description. Thus Schaefer (1936: 330, 347) tried to associate it with A. (Melanthaxia) morio (Fabricius, 1793) . Later, it was more objectively compared to A. (A.) reitteri Obenberger, 1913 ( Bílý, 1980: 405) , and eventually placed in the A. plicata Kiesenwetter, 1859 species-group ( Bílý 1991).
Among the various authors that in the twentieth century published more or less important papers on A. midas, Schaefer is the one who has most often treated of this species in his works. His first study regarding this species was the description of a melanic aberration that he named A. midas ab. fagniezi ( Schaefer, 1933: 100). A few years later, in his “ Anthaxia de France” ( Schaefer, 1938: 206), he described his emblematic A. midas oberthuri , and already in old age, a further aberration named A. midas ab. massanensis ( Schaefer, 1968: 76). From the middle of the last century onwards, several authors have published papers on species herein associated with the A. midas species-group, including the description of A. patsyae from Southwestern Iran ( Baiocchi, 2008). However, with a few exceptions, they were mostly referring to biogeographic data.
Taxonomic comments. In his work, Kiesenwetter (1857: 83) first gave an explication of why, in his opinion, Gory & Laporte had wrongly identified their specimen as A. croesus (Villers) , and a few pages later, considering it a different species, he renamed it A. midas ( Kiesenwetter, 1857: 90) .
Indeed, from a careful assessment of what Gory & Laporte (1839) wrote in their work, it is clear that they did not intend to describe a new species, but simply report from Southern France the species already described by Villers. Therefore, although being the first account in literature for A. midas , their citation cannot be considered as a valid description of a new species, but merely a wrong identification of a species.
On the other hand, Kiesenwetter (1857: 90) did not give a precise description of the species, but only compared some morphological characters with other species, and concerning the distribution, he stated that the species was to be found in Southern France, Italy and Dalmatia. Although poorly detailed, what Kiesenwetter wrote matches the least requirements of the ICZN (1999) to define it as a valid description. Therefore, as A. croesus sensu Gory & Laporte, 1839 (not Villers, 1789) is not an actual described taxon, the act by Kiesenwetter (1857) must not be considered as a replacement of an unavailable name, but the description of a new taxon.
According to the distribution given by Kiesenwetter (1857), it is conceivable that he had considered not only the specimen of Gory & Laporte, but also further specimens from different localities. However, he did not designate any type from a precise locality, because in those times it was not necessary to do so. It is worth noting how all subsequent authors, including one of the authors of the present study ( Baiocchi, 2008) overlooked the lack of a type designation by Kiesenwetter.
The current taxonomical state of A. midas sensu lato, was outlined by Schaefer (1938: 207‒208) after long studies of this species. Schaefer in fact, in the absence of a type designation by Kiesenwetter, regarded the specimen of Gory & Laporte as the only type of A. midas . This specimen, which is conserved in the Oberthur collection in the MNHN, is not originally from France as initially stated by Gory & Laporte. In fact, Schaefer, who was a keen expert of Buprestidae with a particular fondness for this species, had noticed the incongruence of what Gory and Laporte had written about the provenance of their specimen ( Schaefer, 1938: 208‒209). In his careful evaluation of the dorsal chromatic pattern, Schaefer noticed the differences between the specimen that Gory & Laporte had received from Solier, who lived in Marseille, and the specimens that he had personally collected in Southern France. To emphasize these differences, and the fact that it could be a case of “ patria falsa ”, Schaefer pointed out that the specimen of Gory & Laporte was identical to A. midas muelleri Obenberger, 1925 , a taxon described from Istria, that he eventually considered a junior synonym of A. midas ( Schaefer, 1938: 208) . Hence, having identified in the populations from Istria and Dalmatia the nominal form, Schaefer described his subsp. oberthuri from the Western Mediterranean. In the interest of nomenclatorial stability, in this study we follow this long-accepted taxonomical decision of Schaefer.
Concerning the name-bearing type of A. midas , we realized that given the present taxonomical state of the species, A. midas is currently represented by an incorrect type specimen. Therefore, in order to ascertain the actual provenance of the current type and of the specimen of Gory & Laporte, we proceeded to the extraction and preparation of their genitalia. In fact, during the study of this group, we realized that a reliable identification of the species could be achieved only through the examination of the male genitalia. We thus verified that, as correctly supposed by Schaefer, the specimen of Gory & Laporte belongs to the form from the Balkans, while the type in the ZSMG is a specimen of the subsp. oberthuri , most probably from France.
We know that after the death of Kiesenwetter, part of his collection was acquired by C. Muller, and subsequently it was eventually deposited in the ZSMG. It is hard to say when and whom may have decided to consider the specimen in question as the holotype of the species. We can be quite sure though, that this was not done by Kiesenwetter or by Muller, because comparing the original labelling with samples of their respective handwriting ( Horn et al., 1990: 201, 272, 496, 497, 508, 509), we verified that neither of them did so. This said, it follows that it is now necessary to designate a new name-bearing type, namely a lectotype, which can correctly represent the species.
As mentioned above, in the original description Kiesenwetter gave the distribution of A. midas sensu lato, while Schaefer subsequentely recognized two forms and defined the real distribution of the species ( Schaefer, 1938: 208). Consequently, according to the taxonomic state of the species as defined by Schaefer, the correct type of A. midas must necessarily be a specimen from the Balkans, and must be chosen among the original material actually studied by Kiesenwetter, or at least mentioned in his description. In the historical material from private collections and museums that we have studied, only the specimen of Gory & Laporte matches these requirements. In fact, besides being actually a specimen from the Balkans, it was also mentioned by Kiesenwetter (1857: 83, 90) in his description of A. midas , and even if the author did not have the opportunity to physically study the specimen, he must have unequivocally compared the faithful illustration published by Gory & Laporte (1839) with his material. Therefore, we designate this specimen ( Figs. 10, 11, 12, 18 View FIGURES 10‒20 ) as the lectotype of A. midas Kiesenwetter, 1857 . Concerning the specimen formerly regarded as the type of A. midas ( Figs. 13‒17 View FIGURES 10‒20 ), although we ascertained that it belongs to a different species, it is however a specimen certainly studied by Kiesenwetter, and included in the distribution he gave. It is thus a true syntype, and it is here designated as a paralectotype.
In the study of the morphology of the species belonging to this group, we noticed substantial differences in the aedeagus of the various taxa. We usually consider the shape of male genitalia as the principal character to distinguish the different species in many species-groups. In this case, we noticed that the shape of the apical portion of the penis is particularly diagnostic. In the four Balkan and Middle-Eastern species of this group, the apical borders are finely spiny ( Figs. 110, 111, 112, 114 View FIGURES110‒115 ), while in the Western Mediterranean species, this border is completely smooth ( Fig. 113 View FIGURES110‒115 ). Therefore, based on this, and on other lesser morphological and chromatic characters, we elevate A. midas oberthuri Schaefer, 1938 to the rank of species.
Some previous works and catalogues reported an incorrect year of description for this species. Although the volume 106 of the Annales de la Société entomologique de France is referred to the year 1937, the correct year of description of A. oberthuri is 1938, since the fasciculum containing the description was actually issued on February 10th of 1938.
As in many of his descriptions, Schaefer (1938) did not fix a holotype for this taxon, nor a type locality, which however was subsequently fixed by Cobos (1986: 164). We were already in possession of some of the syntypes indicated in the description, and during our last visit to the MNHN we have found the remainder of the 35 ones mentioned by Schaefer (1938) in his paper. In accordance with the type locality fixed by Cobos (1986), we designate one of the males collected in the locality of Sainte Baume on 24 May 1936, and conserved in the MNHN, as lectotype of A. (A.) oberthuri ( Figs. 31‒33 View FIGURES 31‒39 ). The remaining 34 specimens from Sainte Baume, and one of the first two specimens collected in La Massane by Mayet, namely the one examined by Schaefer (1938: 210), are designated as paralectotypes.
With regard to the synonyms of A. midas , the one that has probably undergone the greatest number of taxonomic changes is A. spathuligera . This species was originally described as a variation of A. salicis ( Obenberger, 1924) and, as in the case of A. holoptera , it remained very poorly known. Based only on the description, its original taxonomic combination was accepted by other authors for many years, until Obenberger (1938: 192) changed its status into a subspecies of A. midas . The species was synonymized for the first time with A. croesus (Villers) by Richter (1949: 149), and then with A. midas by Cobos (1986: 309). It was again considered a subspecies of A. midas by Bílý (1997: 28, 117), and once again synonymized with A. midas by the same author ( Bílý, 2006: 372). Sakalian (2007: 14) removed this taxon from synonymy, and elevated it to the rank of species, but it was treated again as a synonym of A. midas in the World Catalogue ( Bellamy, 2008: 1426), and once again as a full species, in the latest edition of the Palaearctic Catalogue ( Kubáň et al., 2016).
The great ambiguity that has reigned over this taxon is because the specimen is actually an aberrant dark individual. Indeed, its dark colouration is one of the reasons that induced Sakalian to raise this taxon to the rank of species, together with additional characters like the narrower head vertex, the supposed lack of the green scutellar patch, the shape of the apex of median lobe, and the singular distribution. Indeed, although more consistent in the Balkan and Turkish species, the slightly narrower head vertex is found within the intraspecific variability that we observed in all species of the group. The dark colouration, and the less evident scutellar green patch, which is anyhow present, are obviously due to the fact that it is a melanic specimen. Melanic specimens are known also from Southern France ( Fig. 37 View FIGURES 31‒39 ) and Italy ( Fig. 53 View FIGURES 52‒57 ), and may potentially occur anywhere, probably depending on the temperature conditions during the pupation of single specimens. This also explains the unique distribution emphasized by Sakalian, actually a single record from a single locality. Regarding the different morphology of the penis, Sakalian was probably misled by a wrong evaluation of the genitalia. In fact, a careful examination of the penis, after its separation from the tegmen, led us to verify that the apex is not differently shaped as stated by Sakalian, but simply damaged ( Figs. 25 View FIGURES21‒30 , 115 View FIGURES110‒115 ).
We assume that the type of A. spathuligera was the only specimen studied by Obenberger (1924) in its description, because as far as we know no other similar specimen has ever been conserved in the Obenberger collection. However, since in his description Obenberger (1924) did not state the number of specimens he studied, we cannot be sure of this, hence, we designate the type conserved in the NMPC (inv. nr. 22252) as the lectotype of A. salicis var. spathuligera Obenberger, 1924 ( Figs. 21‒25 View FIGURES21‒30 , 115 View FIGURES110‒115 ), as originally named by the author.
Regarding the type locality of this species, the lectotype bears a data label stating “Trebinje”, a locality in the southernmost part of Herzegovina, as stated by Obenberger in the description. Based mainly on the morphology of the aedeagus, we reconfirm that A. spathuligera Obenberger, 1924 is conspecific and a junior synonym of A. midas Kiesenwetter, 1857 .
The study of several specimens from different Balkan populations, pointed out a high consistency in the morphology of male genitalia, as well as substantial differences to other species of the group. In addition, the normal chromatic pattern of this species, although slightly variable, also presents a difference of specific importance in the shape of the green basal macula of the elytra, as already observed by Obenberger (1925: 57) and Schaefer (1938: 207).
Obenberger (1925: 56) did not realize that the type of his A. salicis var. spathuligera was just an aberrant specimen, and redescribed the same species as A. midas muelleri ( Obenberger, 1925) . In fact, the same study that led us to reconfirm the synonymy of A. spathuligera , also proved the exact correspondence of morphological characters of A. midas muelleri with the nominotypical form of A. midas . Therefore, we reconfirm that A. midas muelleri Obenberger, 1925 is conspecific and a junior synonym of A. midas Kiesenwetter, 1857 .
The type of A. midas muelleri ( Figs. 28‒30 View FIGURES21‒30 ) is a female from Istria conserved in the NMPC (inv. nr. 22251), which bears a label “Istria Reitter”. Since in his description Obenberger (1925) did not make clear if this was the only specimen he had studied, we designate this specimen as the lectotype of A. midas muelleri Obenberger, 1925 .
Concerning A. holoptera , although Obenberger (1914) did not make it clear in the paper, we verified that the type ( Fig. 26 View FIGURES21‒30 ) bears a label partly handwritten by Obenberger ( Fig. 27 View FIGURES21‒30 ), which identifies it as a unique type, therefore a holotype by monotypy. From its original labelling, we also deduced that Daniel had originally decided to call it A. hoploptera , while Obenberger instead, intended to name it A. danieli . Eventually, Obenberger (1914) opted to call the species with the name chosen by Daniel, but likely because of a typological error or something else, the species was eventually published with the name A. holoptera . In fact, in some subsequent works, Obenberger refers to it as A. hoploptera ( Obenberger, 1917: 59, 84; 1926: 649). Although cited by Obenberger (1917: 18), the name Anthaxia danieli was never published, and is therefore a nomen nudum, as also considered by Obenberger in the Coleopterorum Catalogus ( Obenberger, 1930: 490).
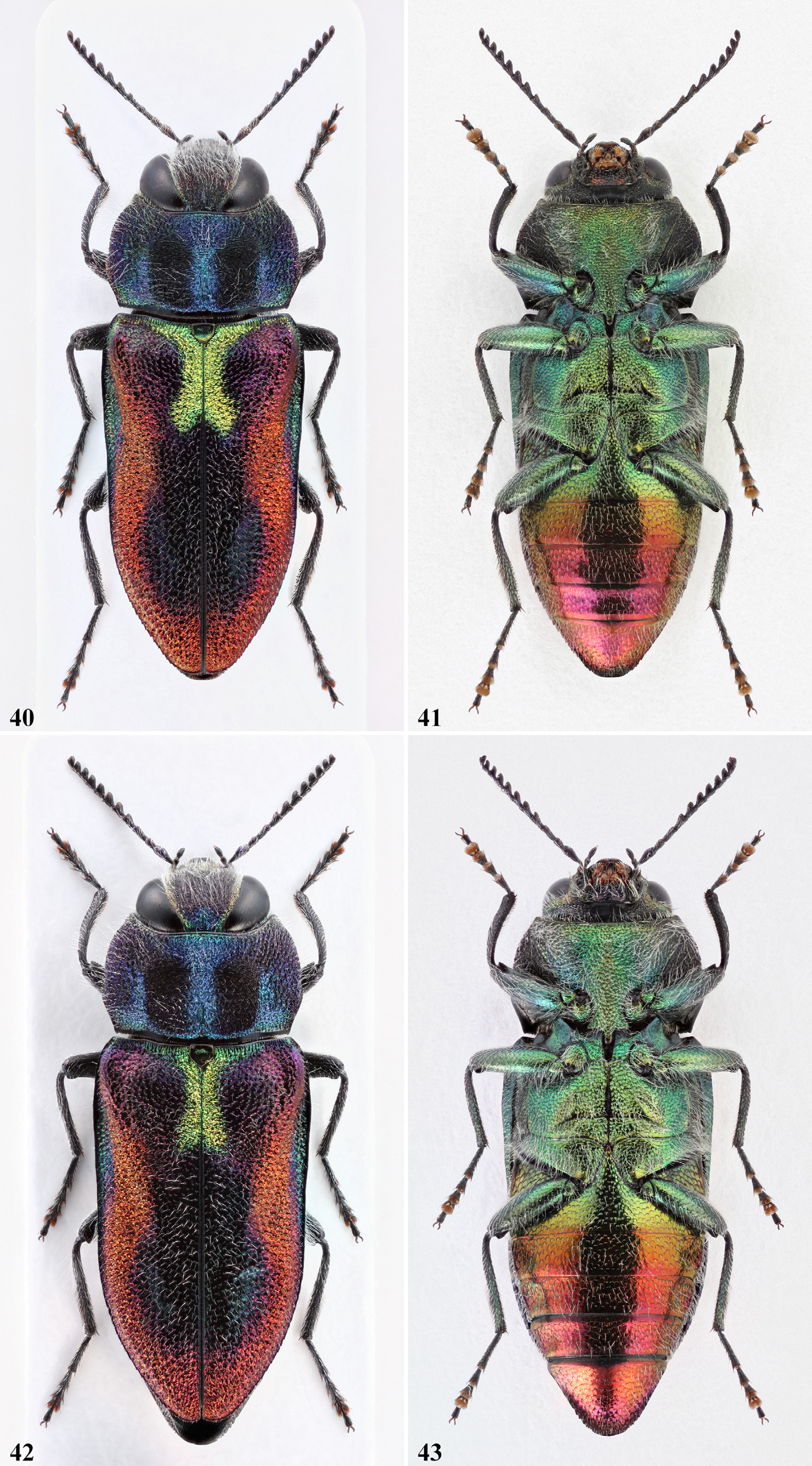
Definition of the species-group. Medium to large size species; length: 5.0‒8.4 mm, width: 2.2‒3.8 mm, length to width ratio: 2.2‒2.4 times longer than wide; body strongly flattened dorsally ( Fig. 138 View FIGURES 136‒138 ), sub-parallel to slightly wedge-shaped, widest immediately behind humeri; pubescence white, moderately raised to fully erect, mostly of woolly appearance, long to very long on head, pronotum, elytral base, sternal parts and legs, shorter and sparser on elytral discal area and on abdomen ( Fig. 49 View FIGURES 48‒51 , 138 View FIGURES 136‒138 ).
Dorsal aspect brightly coloured in most species (largely dark brown in A. holoptera ); frons colouration very variable, green with golden tinge, or completely golden with irregular dark tinge, or completely purple-black; vertex mostly brightly coloured of bluish-green; antennae black with dark purple shine, basal two antennomeres usually more brightly coloured; pronotum cobalt blue ( Fig. 40 View FIGURES 40‒43 ), or green ( Fig. 65 View FIGURES 62‒65 ), or golden reddish, often with purple shine ( Fig. 76 View FIGURES 74‒77 ), occasionally almost completely dark ( Fig. 77 View FIGURES 74‒77 ); two large discal spots present on pronotum (missing in A. patsyae ), oblong, well separated in middle, mostly connected to the anterolateral dark coloured area ( Fig. 1 View FIGURES 1‒9 ); elytral colouration either uniformly dark brown ( A. holoptera ), or brightly coloured with a more or less strong red background colour, resembling the pattern of the A. candens species-group; an obvious green basal macula is present in all species (mostly residual in A. holoptera ), variously extended on elytral base, around the scutellum and along the basal part of the suture; a dark blueish or greenish discal macula shaded with violet extends from the sides of the green basal macula along the elytral suture, never reaching the elytral apex. Ventral colouration: sternum golden green, abdomen green with golden reddish lustre ( Fig. 14 View FIGURES 10‒20 ) to completely red with purple shine ( Fig. 67 View FIGURES 66‒69 ). Legs blackish dorsally, with dark green shine on underside.
Head ( Fig. 1 View FIGURES 1‒9 ) mostly large, never wider than anterior pronotal margin; eyes large, slightly pear-shaped, widest in lower part, usually slightly projecting beyond outline of head; vertex flat to feebly depressed, 0.27‒0.31 times as wide as width of head; frons flat to slightly depressed, rarely slightly convex, 0.50‒0.60 the width of head; inner ocular margins ( Fig. 78‒87 View FIGURES 78‒83 View FIGURES 84‒89 ) irregularly S-shaped, slightly divergent in lower 1/3, sub-parallel in middle, strongly directly convergent on the vertex; clypeus moderately long, weakly prominent, lateral margins short and subparallel, anterior margin flattened, slightly arched to bilobate; fronto-clypeal area visibly depressed, deeply sunken near the antennal fossae. Sculpture of vertex with strongly irregular and lengthened sculpture, converging towards a median line; sculpture of frons areolate to foveate-reticulate, inconsistent, rather deep and irregular, consisting of a tight network of polygonal and sub-round cells of various size, with narrow borders; cell bottom smooth, brilliant, with irregular central grain; frontal pubescence very long, dense and thin.
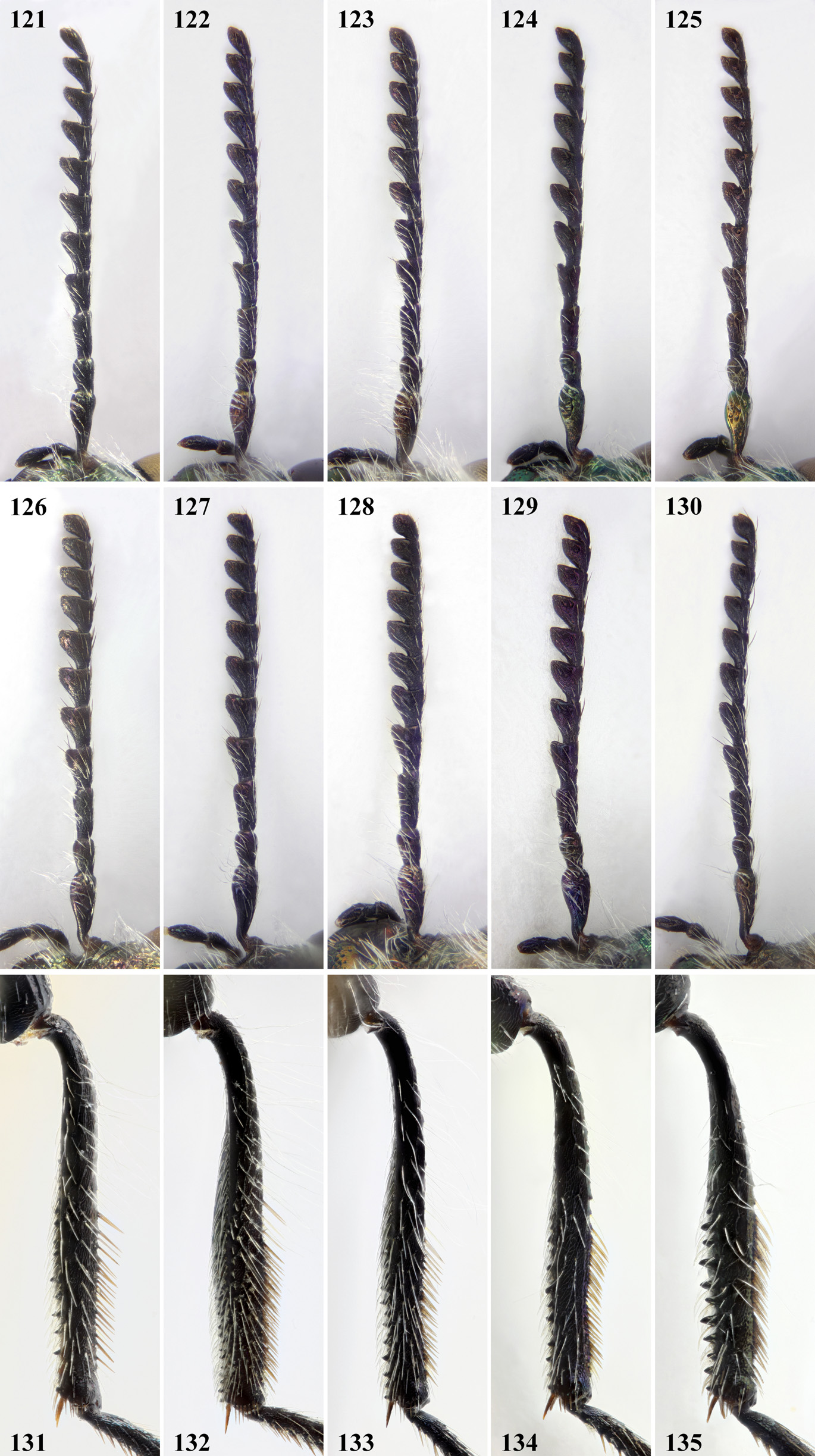
Antennae ( Figs. 121‒130 View FIGURES 121‒135 ) long, 1.5‒1.6 times longer than pronotal length in midline in males, slightly shorter in females; antennomeres 3‒10 sub-triagular to sub-trapezoidal, slender at base, slightly longer than wide.
Pronotum ( Fig. 1 View FIGURES 1‒9 ) transverse, 1.7‒2.0 times wider than long, widest at mid length or in posterior 1/2; lateral margins weakly incurved on anterior 1/3, sub-parallel and somewhat indented at mid length, carinate and strongly narrowed on posterior 1/3; anterior margin strongly incurved backwards, weakly bisinuate; posterior margin slightly arched backwards in middle, with a thick black smooth border; posterior angles obtuse; lateroposterior depressions usually absent, or at most wide and very shallow; pronotal sculpture composite, areolate and irregularly polygonal on anterolateral part, sub-round or irregularly streched on lateroposterior corners; cell borders narrow, cell bottom mostly smooth, occasionally uneven, with irregularly shaped central grain; sculpture of anterocentral part transverse and strongly irregular, with an elongate and strongly bisinuate shape, rest of the discal area characterized by a shallow central furrow, from which diverges a wave like system of very fine and tight striae, distally incurved towards the posterior margin; pubescence very long, withish, denser along lateral margins, very sparse on the disc.
Scutellum ( Fig. 1 View FIGURES 1‒9 ) sub-pentagonal, finely microsculptured.
Elytra 1.65‒1.85 times longer than wide, uneven because of various depressions, strongly narrowed at base, usually sub-parallel to 2/3 of their length, weakly tapered on distal 1/3; elytral base as wide as posterior pronotal margin, lengthwise striate; laterobasal angles obtuse; basal transverse depressions rather shallow, not reaching the scutellum; humeral swellings well raised, obliquely stretching backwards; a well raised callus is present at the base of each elytron; lateral elytral groove shallow, widest in post-humeral part, progressively narrowing until expiring at 2/3 of the elytral length; lateral elytral margin smooth on basal 1/2, progressively serrate until the apex; epipleura not serrate, strongly widened opposite the humeral swellings, then parallel until disappearing at the apex; elytral apices slightly sloping, sub-round to sub-angulate, with sutural junction obtusely angled; elytral sculpture roughly scabriculous, smoother and slightly punctured in the area of the dark elytral macula; pubescence very long and fully erect basally, thin, long and semi-erect on the rest of the surface.
Ventral surface ( Fig. 49 View FIGURES 48‒51 ). Anterior prosternal margin weakly arched backwards; prosternal surface visibly depressed at mid length; sculpture scabriculous along the anterior margin, densely foveate in middle and on prosternal process; rest of the sternal surface more or less densely foveate; posterior end of central metasternal suture narrowly open, not raised; abdominal surface with sparse variolate sculpture and fine basal microreticulation, especially on posterior border of segments; anal ventrite sub-truncate in males, sub-round in females, with dense imbricate sculpture; margins flat, usually briefly serrate or crenulate on preapical part.
Legs long, slender; inner margin of all tibiae slightly to very deeply serrate in males; tarsal segments rather long; all legs with a fine, sparse imbricate sculpture and very fine basal microsculpture; inner posterior angle of the metacoxal plate gibbous; all trochanters simple in females, metatrochanters slightly protruding in males. In males of A. holoptera , the metafemora are stronger than in the other species, being considerably hollow posteriorly, with thinned edges, more roundly expanded at the tibial joint ( Fig. 89 View FIGURES 84‒89 ) than in the other species ( Fig. 88 View FIGURES 84‒89 ).
Aedeagus ( Figs. 100‒109 View FIGURES100‒109 ). Phallobase always hyaline, oblong, occasionally ovoidal, with posterior border always more or less raised, dorsal apophyses poorly developed, nearly truncate or slightly emarginate. Paramers hyaline at base and apex, more or less chitinous laterally; basal 1/2 sub-cylindrical, anterior 1/2 strongly to very strongly narrowed, with wide dorsal cleavage and apex flattened and moderately expanded ( Fig. 100 View FIGURES100‒109 ) or sharply narrowed ( Fig. 103, 104 View FIGURES100‒109 ); membranaceous setigerous area transparent, considerably large when fully hydrated, enclosed in the outer pre-apical part, bordered by distinctly serrate edges. Median lobe stout, slightly crenulated ( Fig. 114 View FIGURES110‒115 ) or serrate laterally ( Fig. 113 View FIGURES110‒115 ), flattened on apical part, rather gibbous and deeply depressed lengthwise at mid length; apex acute to sharply pointed, slightly bent upwards, with borders smooth to strongly spiny; sculpture of dorsal surface usually slightly variolate; basal apodemes long, representing about 4/10 of total length.
The ovipositor ( Figs. 116‒120 View FIGURES 116‒120 ) is similar in all species of this group, much more robust if compared with that of the species belonging to the A. salicis species-group.
Sexual dimorphism. Females differ from males in their stouter and more parallel body, frons and vertex slightly wider, shorter antennae with less elongate last antennomere, simple metatrochanters, smooth tibiae, and sub-round anal ventrite. Sexual dichromatism absent.
Remarks. Although some external characters enable a relatively easy identification of the species of this group, an obvious level of intraspecific variability is present, and in some populations it turned out to be quite misleading, as in the case of specimens from Gargano and Sicily.
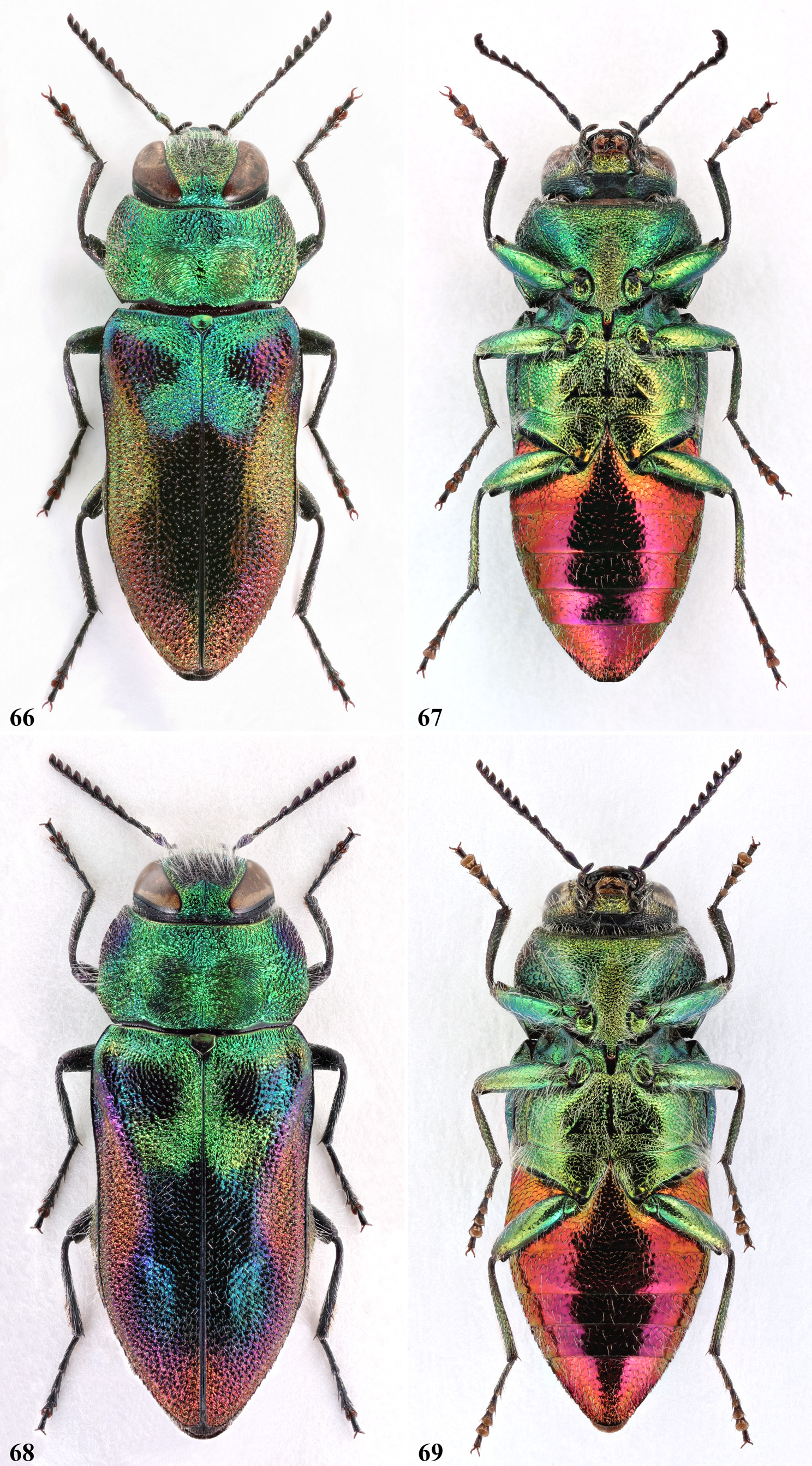
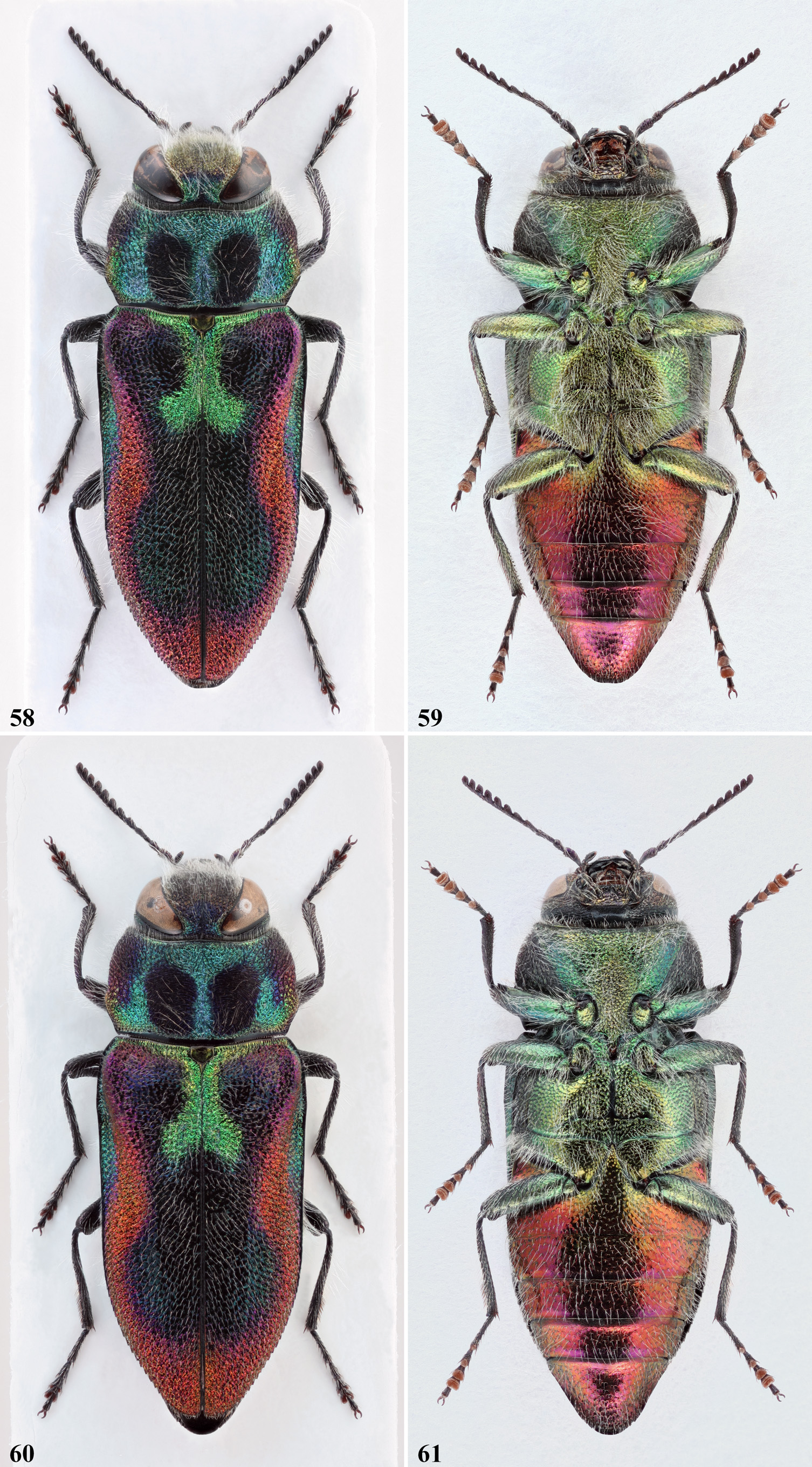
Concerning the habitus, A. midas , A. oberthuri , and A. cebecii sp. nov. are richly coloured species, and very much resemble each other. The other two species represent the brightest and the darkest forms of the group. A. patsyae usually shows a much brighter appearance, and while the elytral pattern is the same than in the latter three species, its colouration instead tends to change deeply, according to the incidence of the light. In this species, the discal spots are missing ( Fig. 66 View FIGURES 66‒69 ). In A. midas , A. oberthuri and A. holoptera the pronotal spots are always more or less confluent with the dark coloured anterolateral area ( Figs. 40 View FIGURES 40‒43 , 48 View FIGURES 48‒51 , 74 View FIGURES 74‒77 ), while in A. cebecii sp. nov. they are mostly separate ( Fig. 58 View FIGURES 58‒61 ). In A. holoptera in addition, the dark colouration of the elytra is occasionally extended to most of the pronotum ( Fig. 77 View FIGURES 74‒77 ). It is worth noting that from eastern Iran eastwards, in the distribution of closely related species of the A. plicata species-group, there are only found dark species (e.g. A. (A.) auriventris Ballion, 1871 , A. (A.) reitteri Obenberger, 1913 and A. (A.) plavilscikovi Obenberger, 1935 ).
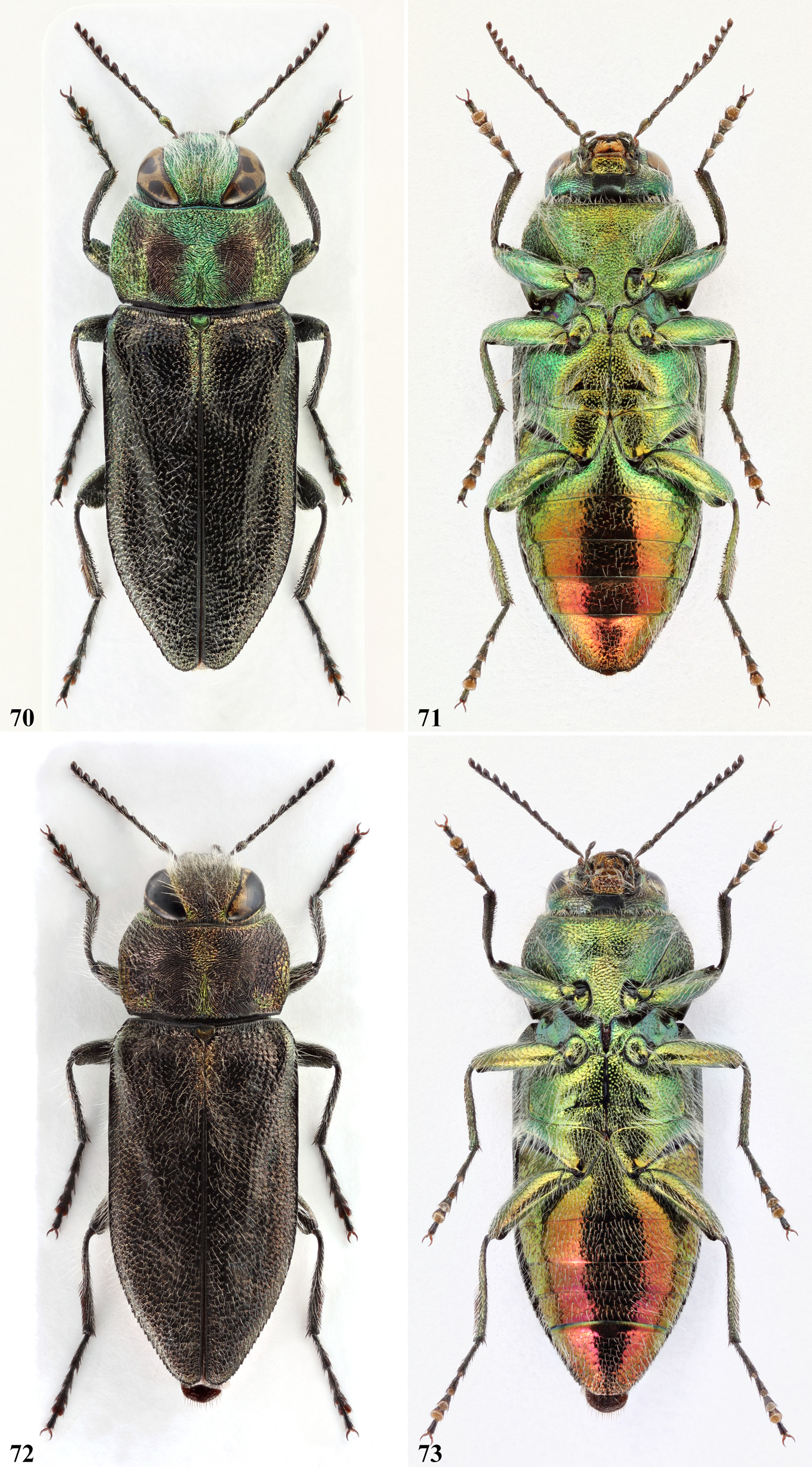
Previous authors gave a great importance to the basal green elytral macula, which indeed is somehow diagnostic. This patch is only vestigial and mostly coppery coloured in most specimens of A. holoptera , especially in its easternmost populations, being appreciable only along a short basal part of the suture ( Fig. 70 View FIGURES70‒73 ). In the other species, differently from what stated by previous authors, we have observed that the extension of the green colouration along the elytral base is somewhat variable even within a same species. The sutural part instead is conspicuous and more consistent, differently expanded at the apex, depending on the species. We have also observed that the more we move eastward, following the line of distribution, the more the sutural part of the green spot takes on a strongly divergent shape at the apex. Infact, in A. oberthuri the sutural part is mostly sub-parallel, neatly truncate or slightly tapered at the apex ( Fig. 48 View FIGURES 48‒51 ), occasionally very feebly divergent ( Fig. 55‒56 View FIGURES 52‒57 ). Instead, in A. midas ( Fig. 40 View FIGURES 40‒43 ), in A. cebecii sp. nov. ( Fig. 58 View FIGURES 58‒61 ) and in A. patsyae ( Fig. 66 View FIGURES 66‒69 ), it becomes gradually wider, until becoming a large x-shaped macula. The wrong assessment of the shape of the sutural part in A. oberthuri is most probably at the origin of the wrong identification of the Gargano’s populations, which until now have been considered trans-adriatic and belonging to the Balkan species ( Curletti, 1994: 94, 95).
In the three species with the typical colouration, the red background colour of the elytra can be more or less deep, and can occasionally turn to gold ( Fig. 45 View FIGURES 44‒47 ), or even golden-green ( Figs. 44 View FIGURES 44‒47 , 52 View FIGURES 52‒57 ). The large dark elytral macula is rather variable in extension and colour, being usually bluish-black, occasionally with greenish lustre or completely dark violet as in most Moroccan specimens. We have also observed that, in specimens of A. oberthuri from Sicily, the elytral macula may be completely missing, leaving the elytra nearly entirely ochraceous ( Fig. 56 View FIGURES 52‒57 ). However, this form is found together with normally coloured specimens ( Fig. 55 View FIGURES 52‒57 ), and therefore represent individual chromatic aberrations without any taxonomic importance. In A. holoptera , the elytra are invariably completely dark brown, while in A. patsyae , along with some typically coloured specimens, like the holotype ( Fig. 68 View FIGURES 66‒69 ), most individuals show a golden elytral background colour, and the discal macula is strongly shaded in reddish purple ( Fig. 66 View FIGURES 66‒69 ).
Concerning body proportions, we observed that A. oberthuri shows a somewhat more sub-parallel shape of the body, compared to the other species which appear to be slightly more wedge-shaped, especially in males.
With respect to the genitalia, the species that shows the most widely enlarged aedeagus is A. oberthuri , in which the parameres are progressively expanded until the apex, and not very sharply pointed. ( Fig. 100 View FIGURES100‒109 ). In A. cebecii sp. nov. ( Fig. 101 View FIGURES100‒109 ) and A. patsyae ( Fig. 102 View FIGURES100‒109 ) the preapical portion of the parameres is more sinuously enlarged, and then strongly narrowed to the very sharp apex. In A. midas ( Fig. 103 View FIGURES100‒109 ) and A. holoptera ( Fig. 104 View FIGURES100‒109 ), the anterior part of parameres is not expanded, nor particularly flattened, but progressively sharply pointed. In some European specimens of A. oberthuri , the apical part of the parameres is more largely expanded, but this form is found together with normal individuals, and does not indicate specific separation. In A. oberthuri , the penis is usually stouter and wider than in the other species ( Fig. 105 View FIGURES100‒109 ), with apex acute but not sharply pointed, and slightly bent upward. The apical part of the lower lamella in this species has completely smooth edges ( Figs. 113 View FIGURES110‒115 ). In the other four species the penis is somewhat more slender, and the apex is more acute, with apical part of the lower lamella visibly spiny laterally ( Figs. 110, 111, 112, 114 View FIGURES110‒115 ).
Bionomy and distribution. The larvae of all species of this group develop in various species of Acer (Sapindaceae) . We observed that the size of the attacked branches is usually from moderate to large size, probably because during the larval cycle, which lasts at least two years, the larva needs plenty of space to dig its typical large and sinuous galleries under the bark.
As already stated by Izzillo (2010: 4) for the Italian populations, in all species the egg deposition occurs in April / May, and the larva feeds under the thick bark for at least one year, until the next spring, when it digs a rather deep pupal cell. The adult is usually completely formed already during the summer, but stays in its pupal cell until the next spring, thus passing a second winter in the wood, and emerging after two years from the egg deposition.
As happens in all species, some larvae develop over a longer period of time, up to three years. This may be a mechanism of defense against extreme natural events that may put at risk the survival of the entire population, as well for a genetic reshuffling as to avoid the split into separate and different biennial species. For this reason, larvae of different age are usually found to share the same branch. The duration of life cycle is probably the same also for species living in dry and hot biotopes, like in Iran.
As in the vast majority of species with a sub-cortical larval development, during the extended time in which the larvae dig their galleries under the bark, they are greatly at risk of parasitism by a number of endoparasitoid Hymenoptera , or to be attacked by mites and other deadly parasitoids, e.g. Hymenoptera Bethylidae , or to predation by larvae of Cleridae . Infact, adults of Bethylidae and larvae of Cleridae , reach the larvae by digging their tunnels through the larval galleries filled with the larval frass. Also woodpeckers have proved to play their role in the reduction of the number of larvae that achieve a complete development.
Adults usually emerge as early as late April, and can be found until mid-June. However, in the last decades, likely because of drastic climate changes, they have also been occasionally found in the warmest days of winter, as early as February. Adults are rather elusive, and can be seen almost exclusively on warm sunny days, mostly in the late morning. After this time, they usually stay hidden under the bark of dead branches. Observations made on A. oberthuri by various authors, indicate that this species frequents fresh clearings or paths with plenty of flowers on which they feed on the petals, especially yellow Asteraceae and Ranunculaceae , although they also appreciate Dog-Rose flowers (Rosa canina).
Regarding the presumed rarity of the species of this group, although they are somewhat elusive and difficult to locate, a deeper knowledge of their biology will ensure that in the future they can be discovered in new places, even in already well-known areas, as witnessed by the recent discovery of the Turkish populations. In fact, in most previous works, the few formerly known species of this group have been treated as relict species in danger of extinction, because of their presumed scarcity in nature. In fact, in most of the places where we have collected the species, with the exception of A. patsyae , we have found healthy and rather numerous populations, with the number of active individuals estimated at least in hundreds, and certainly not in danger of imminent extinction. As in all highly parasitized species, there are fluctuations in density of populations, with peaks of numbers either in the host species, or in the parasitoid species.
The distribution of this species-group is in the southwestern 25% of the Palearctic zone, ranging from Western Maghreb, across all Northern Mediterranean countries, reaching to Iran and Turkmenistan. Although this vast area longitudinally overlaps the West Palearctic distribution of the hostplant, namely the genus Acer , we have found only a comparatively modest number of precise collecting localities, both from the literature and from material studied in this work, even after two centuries of research by a great multitude of European entomologists. We may thus assume that the distributional limits are already rather well defined, since the genus Acer is also present in Central Asia and along the lower slopes of the various Himalayan mountain ranges, and is widely spread in the temperate zone of the Far East, but despite the increasing degree of knowledge of the oriental fauna, no other species of this group has been found so far in the Far East.
The A. midas species-group can thus be considered to be of the Turano-Mediterranean distributional chorotype ( Vigna Taglianti et al., 1999). Although occasionally found at altitudes around 1800 meters, all species seem to prefer more temperate environments at lower altitude, along the perimeter of Mediterranean and Caspian seas, with good populations of aged trees.
Despite their superficial similarity, the various species of this group are referable to distinct groups of populations with well-differentiated male genitalia. On the other hand, the same homogenous pattern of colouration for most of the species is also a clear indication of a common origin. Because of their present residual and fragmented distribution, the morphology and the chromatic pattern of these species are somewhat variable. In our opinion though, this variability must be considered as intraspecific variability, and is not indicative of subspecific differentiation.
According to Hervé (1953: 562‒563), the particular biocenosis necessary for the existence of A. oberthuri , testifies to its ancient origin. In our opinion, the current geographic separation of the different taxa is more recent, and may date back to the Pleistocene, at the time of the end of last Glacial Period, about 12000 years ago. During the last glaciation, the southern limits of the Scandinavian Glacier reached as far south as Northern Europe. During this period, great extensions of land south of the Würm line took on the aspect and the climate of a tundra, where only plants able to grow at low temperatures survived. Areas of the original forest vegetation, including the genus Acer , would have survived only much further south, where we can presume that small groups of the ancestral form of the current A. midas species-group survived, especially in the Maghreb, and in southern parts of Spain, Italy and Balkans. Southern Turkey and Iran however, seem to have been less influenced by the decrease in temperatures. Having been temporarily pushed so far south, the original continuous distribution of the ancient ancestor was broken up, and with the melting of glaciers, and the consequent rise in sea level, the Balkan populations have remained isolated, especially from the Western Mediterranean ones, giving raise to the differentiation into the different species treated in this work.
Key to species of the Anthaxia (Anthaxia) midas View in CoL species-group
1 Elytra dark brown ( Fig. 70 View FIGURES70‒73 ), metafemurs enlarged ( Fig. 89 View FIGURES 84‒89 )........................ A. (A.) holoptera Obenberger, 1914 View in CoL
- Elytra brightly coloured ( Figs. 40 View FIGURES 40‒43 , 48 View FIGURES 48‒51 , 58 View FIGURES 58‒61 , 66 View FIGURES 66‒69 ), metafemurs normally developed ( Fig. 88 View FIGURES 84‒89 )............................. 2
2 Pronotum without dark discal spots ( Fig. 66 View FIGURES 66‒69 )........................................ A. (A.) patsyae Baiocchi, 2008 View in CoL
- Pronotum with two large dark discal spots ( Figs. 40 View FIGURES 40‒43 , 48 View FIGURES 48‒51 , 58 View FIGURES 58‒61 ).................................................... 3
3 Sutural part of the basal green elytral macula sub-parallel, very slightly ( Fig. 55 View FIGURES 52‒57 ) or not enlarged posteriorly ( Fig. 48 View FIGURES 48‒51 ), aedeagus with parameres moderately enlarged at apex ( Fig. 100 View FIGURES100‒109 ), median lobe with smooth apex ( Fig. 113 View FIGURES110‒115 ).......................................................................................... A. (A.) oberthuri Schaefer, 1938 stat. nov.
- Sutural part of the basal green elytral macula more or less enlarged at apex ( Figs. 40 View FIGURES 40‒43 , 58 View FIGURES 58‒61 ), aedeagus with parameres slightly ( Fig. 101 View FIGURES100‒109 ), or not enlarged at the apex ( Fig. 103 View FIGURES100‒109 ), median lobe with apex finely spiny ( Figs. 110, 114 View FIGURES110‒115 )................... 4
4 Pronotal dark discal spots always connected to the anterolateral dark colouration ( Fig. 40 View FIGURES 40‒43 ), apex of parameres not enlarged ( Fig. 103 View FIGURES100‒109 ).................................................................. A. (A.) midas Kiesenwetter, 1857 View in CoL
- Pronotal dark discal spots usually not connected to the anterolateral dark colouration ( Fig. 58 View FIGURES 58‒61 ), parameres slightly enlarged before the apex ( Fig. 101 View FIGURES100‒109 ).............................................................. A. (A.) cebecii sp. nov.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |