Punctifulvius Schmitz, 1978
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5382.1.17 |
|
publication LSID |
lsid:zoobank.org:pub:5137CFC9-7604-44DD-8D7E-80E44EB6676C |
|
DOI |
https://doi.org/10.5281/zenodo.10286875 |
|
persistent identifier |
https://treatment.plazi.org/id/03AD990D-A346-FFDC-56B2-F8F3FA73FCBA |
|
treatment provided by |
Plazi |
|
scientific name |
Punctifulvius Schmitz, 1978 |
| status |
|
Genus: Punctifulvius Schmitz, 1978 View in CoL View at ENA
Diagnosis: See the recently revised diagnosis by Namyatova & Cassis (2019).
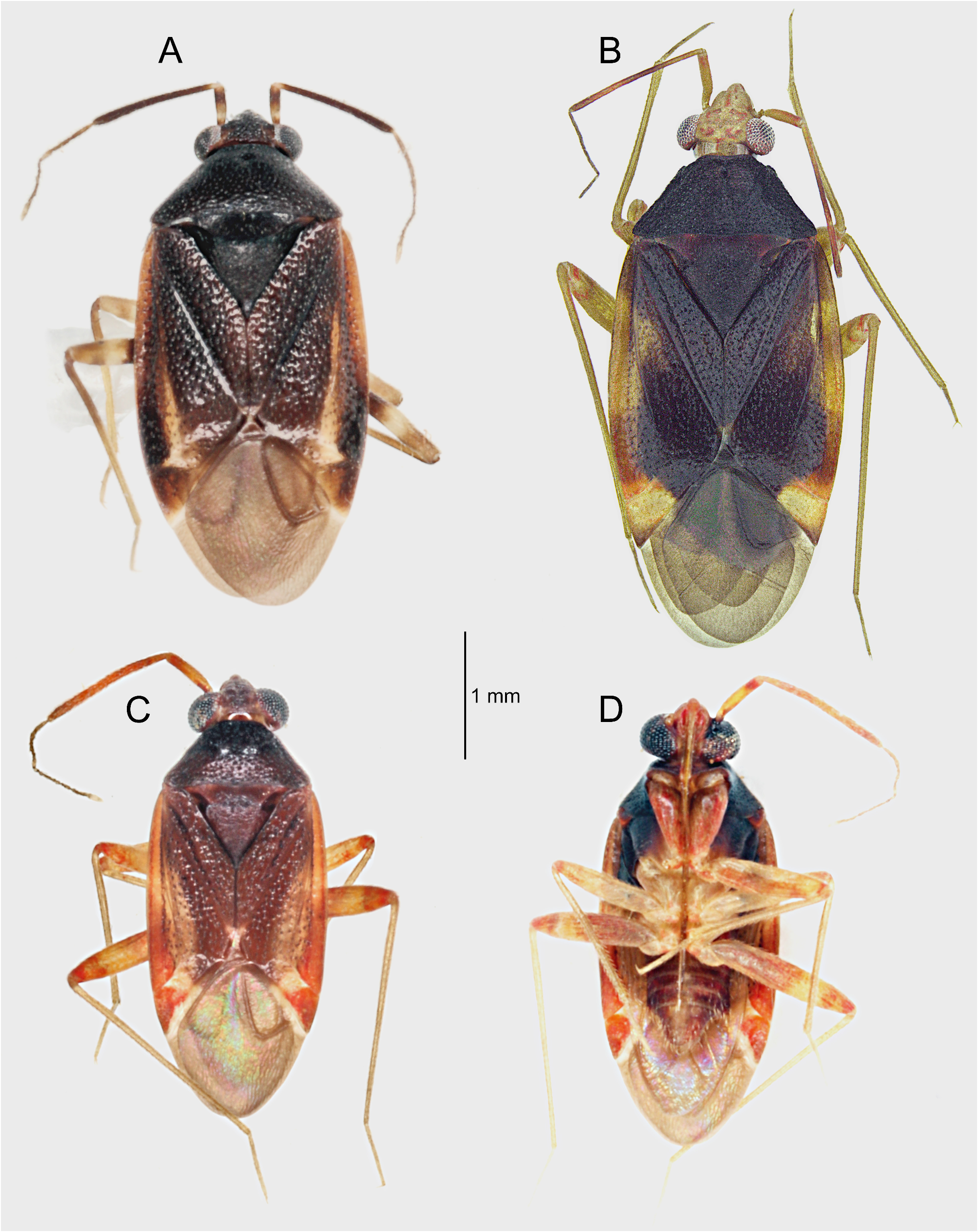
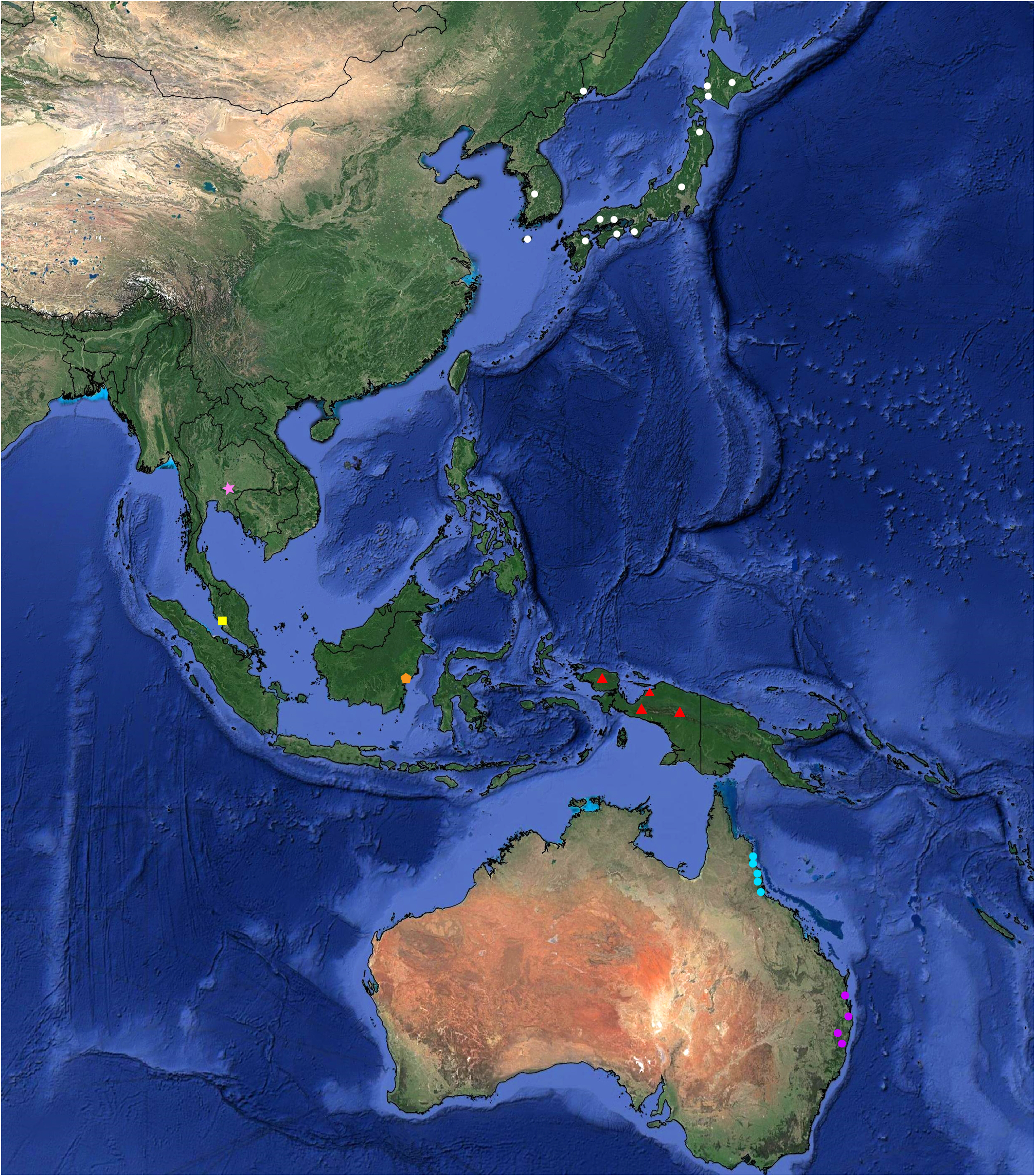
Discussion. Until now, Punctifulvius has been known from its disjunct distribution, with P. kerzhneri found in the temperate and cold temperate zones in the eastern Palearctic Region and P. aquilonius and P. austellus known from the wet tropics and temperate rainforest of Australia ( Yasunaga 2000; Kim et al. 2019; Namyatova & Cassis 2019). Our study significantly increases the distributional range for this taxon, which is now found from the Palearctic, across Southeast Asia, to Australia ( Fig. 12 View FIGURE 12 ).
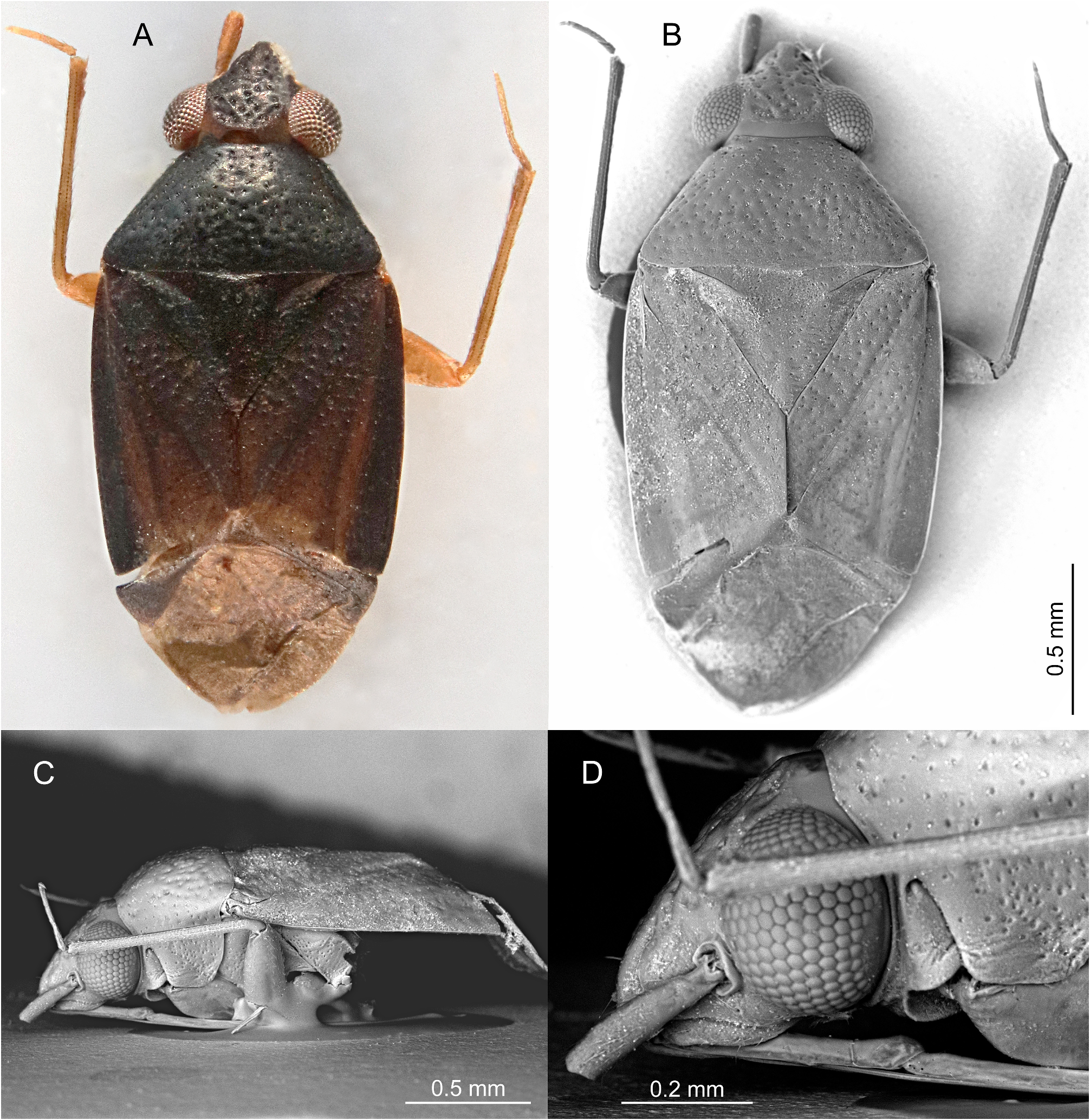
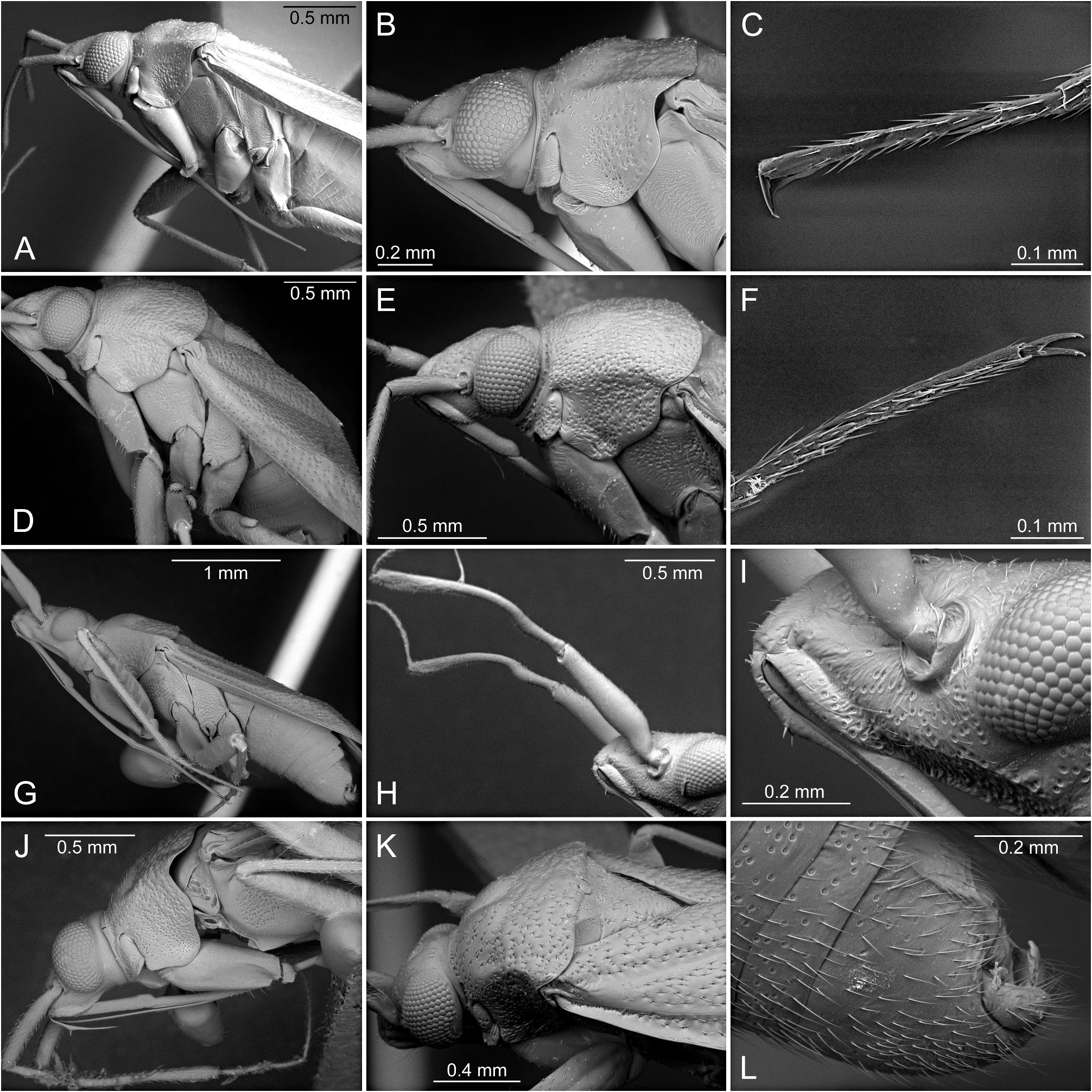
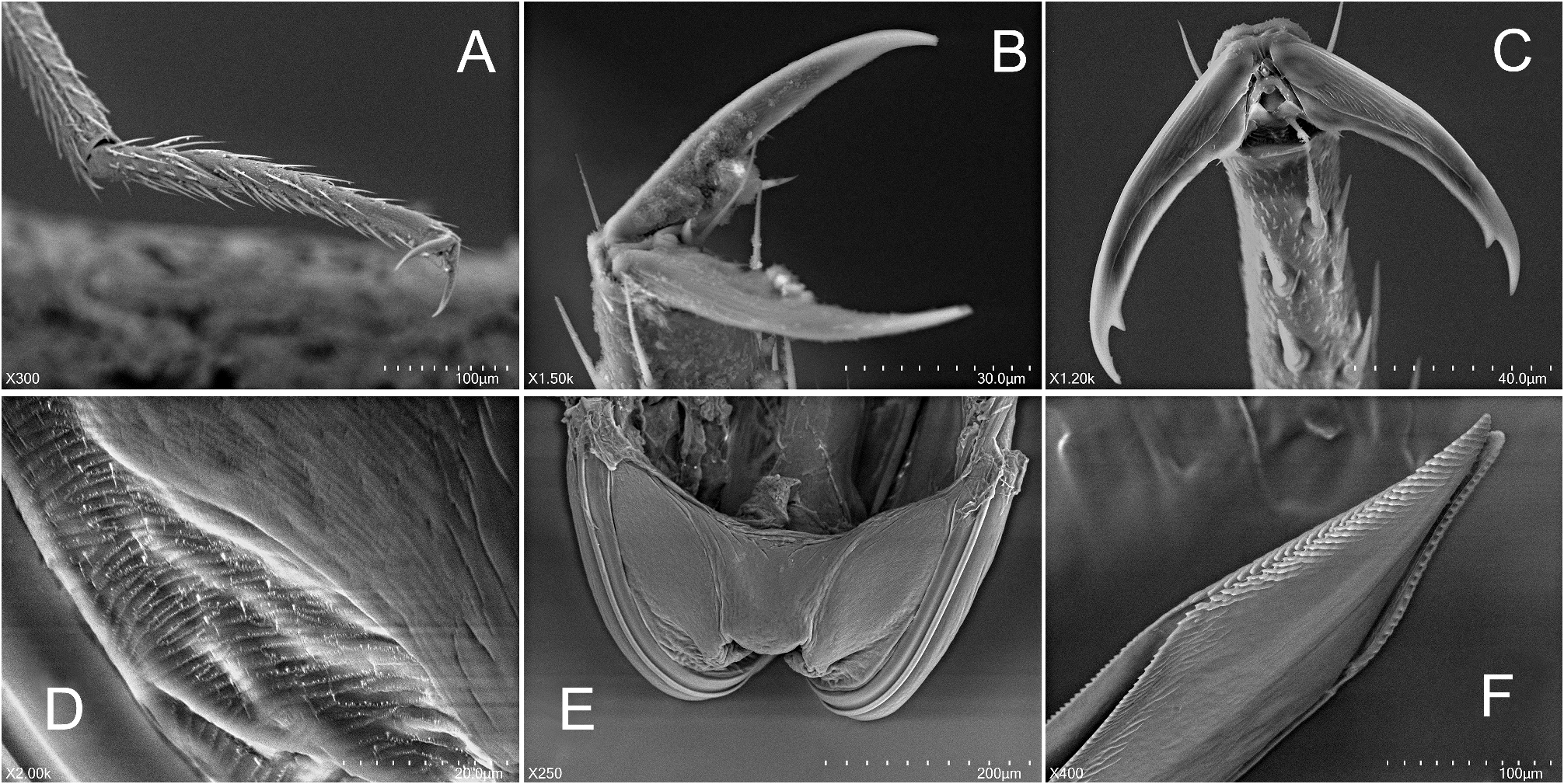
Based on similar external appearance, the closest sister genus of Punctifulvius was assumed to be Yamatofulvius Yasunaga, 2000 currently known by four Japanese species ( Yasunaga 2000). However, Yamatofulvius congeners have several unique synapomorphies, such as a frontal head with a few spines on the labrum (= epipharynx) ( Fig. 7I View FIGURE 7 ) and more or less sinuate male antennomere II ( Fig. 7H View FIGURE 7 ) ( Yasunaga 2000); these structures were reported to function during courtship. The adult males were observed to ride final instar females ( Fig. 7G–I View FIGURE 7 ). The riding males hold the partners tightly, using the labral spines ( Fig. 7I View FIGURE 7 ) and second antennomeres ( Fig. 7H View FIGURE 7 ), until the females complete final eclosion to the adults ( Yasunaga & Miyamoto 2006). Wolski (2010) observed similar spines on the labrum and sinuate antennomere II in some genera of the Rhinocylapus group as defined by Gorczyca (2000), containing Proamblia Bergroth, 1910 , Rhinocylapidius Poppius, 1915 , and Rhinocylapus Poppius, 1909 ( Wolski 2010: figs 3B, D, E, 4A–F). This author also included Mycetocylapus Poppius, 1914 in the complex. Subsequently, spines on the labrum and sinuate antennomere II were also found in the newly described genus Rhinocylapoides Wolski & Gorczyca, 2011 ( Wolski & Gorczyca 2011: figs 1, 2, 4) and it was added to the Rhinocylapus complex by the authors. Wolski (2010) presented a set of morphological features unequivocally defining the Rhinocylapus complex, the most important of which is the shape of the parameres, unique among cylapines, with left paramere distinctly rounded, apical process flattened, with short, rounded process at extreme apex ( Wolski 2010: figs 6C, D, 8C, D, E, 10B, C, 11B, C, 12B, C, 13B, C, 14B, C, 15C, D) and right paramere distinctly smaller than left paramere, flattened, margins of paramere body more or less parallel ( Wolski 2010: Figs 6E View FIGURE 6 , 8H View FIGURE 8 , 10D View FIGURE 10 , 11D View FIGURE 11 , 12D View FIGURE 12 , 13D, 14D, 15E). Such shape of the parameres is also found in Punctifulvius and Yamatofulvius ( Figs 6C–F View FIGURE 6 , 8A–D View FIGURE 8 ; Yasunaga 2000: figs 50, 51, 66, 67, 70, 74; Yasunaga & Miyamoto 2006: figs 6K, N) and Wolski (2010) suggested their affinity to the genera of Rhinocylapus complex. Recently, Namyatova & Cassis (2019) and Tyts et al. (2022) placed Punctifulvius and Yamatofulvius in the Rhinocylapus complex based on the results of their molecular and morphology-based phylogenies, with Punctifulvius being positioned as a sister group to other genera of the complex. They presented its revised diagnosis and listed the following characters for distinguishing Punctifulvius from other members of the complex: punctured thoracic pleura (Figs; 3D, 5C, 7A, B; Namyatova & Cassis 2019: figs 10E, F, P), pit between pronotal calli ( Fig. 3B View FIGURE 3 ; Namyatova & Cassis 2019: figs 10B, C), antennomere I subequal in length to or narrower than antennomere II ( Figs 1A, B View FIGURE 1 ; Namyatova & Cassis 2019: fig. 4), subdivided labial segment I ( Figs 3E View FIGURE 3 , 5D View FIGURE 5 , 7B View FIGURE 7 ; Namyatova & Cassis 2019: 10 O), left paramere sensory lobe with a basal outgrowth ( Fig. 6C View FIGURE 6 : Yasunaga 2000: fig. 51; Namyatova & Cassis 2019: 11 D, J). Additionally, species of Punctifulvius have endosoma with a single, curved, sharply pointed sclerite ( Figs 6B View FIGURE 6 , 8E View FIGURE 8 ; Yasunaga 2000: fig. 52; Namyatova & Cassis 2019: figs 11A, B, F).
Namyatova & Cassis (2022) paid attention that most of these features are also present in Teratofulvioides punctatus Carvalho & Lorenzato, 1978 , a single representative of the genus Teratofulvioides Carvalho & Lorenzato, 1978 known from Papua New Guinea. Their observations were based on the original description of Carvalho & Lorenzato (1978), without any specimen of T. punctatus at hand. Our study of the single specimen of this species collected in the Indonesian Irian confirms the close similarity to the Punctifulvius species, with the only differing character, i.e., the lack of the long process on the left paramere sensory lobe ( Carvalho & Lorenzato 1978: 85, 86). On the other hand, P. sakaerat n. sp. lacks the long process on the left paramere sensory lobe as well ( Figs 8A–B View FIGURE 8 ). Unfortunately, we could not study the male genitalia as the abdomen was partly damaged in the examined specimen of T. punctatus . Nevertheless, given the known distributional range of Punctifulvius and the fact that most of the diagnostic characters for Punctifulvius are found in T. punctatus , it is very likely that both genera are synonymous.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Cylapinae |
|
Tribe |
Fulviini |