Macroglossus minimus (E. Geoffroy, 1810)
|
publication ID |
https://doi.org/ 10.5281/zenodo.6448815 |
|
DOI |
https://doi.org/10.5281/zenodo.6794679 |
|
persistent identifier |
https://treatment.plazi.org/id/03AD87FA-FFE9-F607-899E-35F2F714F3D5 |
|
treatment provided by |
Conny |
|
scientific name |
Macroglossus minimus |
| status |
|
73. View Plate 5: Pteropodidae
Lesser Long-tongued Blossom Bat
Macroglossus minimus View in CoL
French: Petit Macroglosse / German: Kleiner Langzungenflughund / Spanish: Macroglosus pequeno
Other common names: Daggertoothed Flower Bat, Daggertoothed Long-nosed Fruit Bat, Daggertoothed Longtongued Blossom Bat, Lesser Long-nosed Fruit Bat, Lesser Long-tongued Fruit Bat, Lesser Long-tongued Nectar Bat, Northern Blossom Bat
Taxonomy. Pteropus minimus E. Geoffroy Saint-Hilaire, 1810 View in CoL ,
Java, Indonesia.
Macroglossus minimus and M. sobrinus are currently recognized as distinct species based on morphology, but they cannot be differentiated genetically in Indochina and might be a single species. Further genetic and morphometric assessment with more complete geographical sampling is needed. Four subspecies recognized.
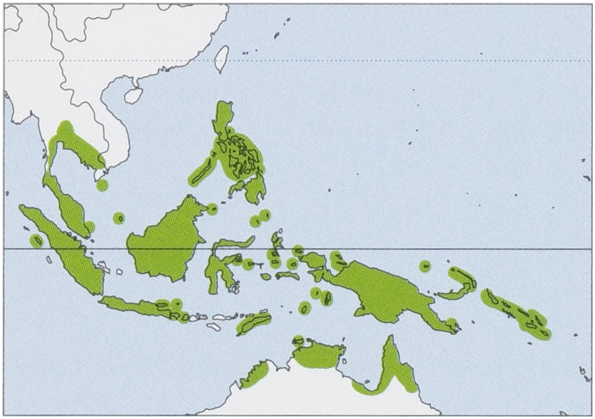
Subspecies and Distribution.
M.m.minimusE.GeoffroySaint-Hilaire,1810—Java,MaduraI,BaliI,KangeanIs,andLombokI.
M.m.booensisKompanje&Moeliker,2001—BooIsintheRajaAmpatIsoftNWNewGuinea.
M. m. nanus Matschie, 1899 — New Guinea, Yapen I, Geelvink Is, Raja Ampat Is, Fergusson I, Bougainville I, Aru Is, Bismarck Archipelago, Kai Is, Admiralty Is, Solomon Is, and N Australia (NE Western Australia, N Northern Territory, Melville I, and N Queensland). View Figure
Descriptive notes. Head-body 49-77 mm, tail c. 0-4 mm, ear 11-18- 5 mm, hindfoot 10-3-23- 3 mm, forearm 37- 4—45 mm; weight 8-25 g. Males average larger than females. The Lesser Long-tongued Blossom Batis the smallest species of pteropodid. It has elongated muzzle with very long papillae-tipped tongue, extremely reduced tail, reduced uropatagium along inner legs, and claw on second digits of wings. It is very similar to the Greater Long-tongued Blossom Bat ( M. sobrinus ) but is generally smaller with shorter muzzle, less prominent chin, slightly more side-facing nostrils with small groove under each nostril, and internarial medial groove that distinctly extends to upper lip. Head and muzzle are elongated, and nostrils are rounded and face forward. Dorsal pelage varies across populations and can be buffy brown, yellowish/golden brown, or russet-brown, with hairs having pale bases; ventral pelage is generally paler and grayer, being almost creamy in some individuals. Adult males have V-shaped apocrine gland complex running across chest that resembles a thick pink welt, giving adult males a pungent strong musky smell; Vsshaped gland is absent in females and young. Glands are apparently absent from populations on the Bismarck Archipelago but are found in most populations. Ears are relatively long, rounded, and dark brownish; eyes are large, with dark rufous brown irises. Tail is very minute and difficult to see or completely lacking. Uropatagium is highly reduced, attaching at base where tail would be and following leg up to ankles where it attaches to highly reduced calcar. Second digit of wing has a claw; third and fifth metacarpals are subequal in size, and wing is dark with fur extending over upper arm dorsally and ventrally. Skull is long, with very long and narrow rostrum; braincase is strongly deflected downward. Jaw is thin and comparatively weak. Dental formula for all species of Macroglossusis12/2,C1/1,P 3/3, M 2/3 (x2) = 34. Molars and premolars are largely reduced and flattened; large gap occurs between P' and next premolar (P%); C, is long, thin, and strongly curved outward; extra molars have been reported in some specimens; and upper incisors are very reduced, project slightly forward, and are separated from one another and canines by small gaps. Chromosomal complement has 2n = 34 and FN = 60 (Indochina) or 62 (Java).
Habitat. Primary and secondary tropical moist forests, preferring secondary, agricultural, and disturbed habitats, and reportedly woodlands, mangroves, swamp forests, various plantations, and urban areas from sea level up to elevations of ¢. 2250 m (lower densities at high elevations). Lesser Long-tongued Blossom Bats occurin virtually every habitat type in the Philippines and are commonly found in various plantations (e.g. durians and bananas) and any environment that includes plants with large flowers to feed on.
Food and Feeding. Lesser Long-tongued Blossom Bats are nectarivorous and frugivorous, feeding on a large variety of plants and acting as a major pollinator for many of them;it occasionally eats fruits. They forage by landing on a flower or hovering nearit and lapping up nectar with their tongue. Their face is often covered with pollen from plants they foraged on. Diets change seasonally and vary widely based on region and what species of trees flower there. They seem to favor Musa (Musaceae) throughout their distribution, which is a major agricultural crop throughout Australasia. They also feed on nectar of Durio (Malvaceae) , flowering coconut ( Cocos , Arecaceae ), Sonneratia (Lythraceae) mangroves, and jambu ( Syzygium , Myrtaceae ). In Australia, they feed on flowering Eucalyptus (Myrtaceae) , Melaleuca (Myrtaceae) , Sonneratia mangroves, Barringtonia (Lecythidaceae) , and bottlebrushes ( Callistemon , Myrtaceae ), all of which have large flowers. They pollinate various flowering trees including Musa and Sonneratia throughout Australasia. They occasionally feed on overripe fruit of a few trees, including some dioecious Ficus (Moraceae) and Timonius (Rubiaceae) . They will also swallow seeds of these fruits (which are proportionally large for their body size) and act as a seed disperser. Some insect material has been found in stomach samples, but this evidently does not make up a large portion of the diet.
Breeding. Breeding of Lesser Long-tongued Blossom Bats is relatively asynchronous, occurring year-round, but in areas with more seasonality, they seem to be seasonally bimodal polyestrous. In Brunei, most births occurred in November-December and April-May during periods of greatest rainfall. A similar trend was recorded in Australia, where births seem to occur during dry seasons in February-March and August— October. One female in Australia produced three successive litters in April, December, and March. Reproduction in Papua New Guinea and the Philippines seems to occur asynchronously year-round. Breeding occurs when a male approaches a roosting female and copulates for 10-15 seconds after mounting her, flying off shortly after. Lesser Long-tongued Blossom Bats seem to have delayed implantation (at least in Papua New Guinea) because sperm storage is evident in uteri. In Brunei, delayed embryonic development (probably as a result of sperm storage) was recorded after mating; mating occurred shortly after parturition of the last pregnancy. There are generally two litters/year, with one young/litter. Gestation appears to be c.120 days, although this has not been confirmed. Young cling to mothers constantly for the first 6-10 days and then stay in roosts while females forage. They become volant by ¢.40 days old but continue to hang onto their mothers in roosts until they are c¢.6 months old (based on captive individuals). Males seem to become sexually mature after c¢.7 months in Australia, which is when they developed a V-shaped gland complex on their chest. Females probably take ¢.9 months or more to reach sexual maturity. Use of V-shaped apocrine gland complex on chest of adult breeding malesis still uncertain; it might be important in sexual selection or other breeding purposes.
Activity patterns. The Lesser Long-tongued Blossom Bat is primarily nocturnal, foraging throughout the night and roosting throughout the day, although individuals have been captured during the day. It can be found roosting under large leaves, branches, and loose bark and in hollow bamboo, hollows in trees, clusters of dead leaves, or abandoned buildings. While in day roosts, it can enter shallow torpor. Body temperature decreases during this time, and duration of torpor bouts is longer when ambient temperatures are lower. Body temperature also lowers in direct correlation with ambient temperatures. The Lesser Long-tongued Blossom Bat has much lower metabolic rate than it is expected for a mammal of its weight. When foraging,its metabolic rate doubles that ofits resting metabolic rate, which is why entering torpor is important, dropping its energy expenditure to 60-80% ofits resting energy expenditure. Lesser Long-tongued Blossom Bats apparently make an audible metallic call when flying at night, at least in Australia.
Movements, Home range and Social organization. Lesser LLong-tongued Blossom Bats mostly roosts alone or in small spaced-out groups in small roosts. They do not seem to move long distances but will travel fairly long distances to food. There is evidence for a significant amount of gene flow between islands of the Philippines, indicating that individuals do make large movements between islands relatively regularly. Density on NegrosIsland in habitat that has been 15-30% deforested was 2 ind/ha. Home ranges of 18 individuals in Papua New Guinea were 1-9-15-1 ha, with some overlap among adults and young, primarily around gardens and other feeding areas. Adult males in the same study appeared to exclude conspecifics from richest feeding areas in primary forests.
Status and Conservation. Classified as Least Concern on The IUCN Red List. The Lesser Long-tongued Blossom Bat has a wide distribution and is considered common.It might be threatened by local habitat destruction, butit is common in secondary habitats and agricultural areas.
Bibliography. Achondo et al. (2014), Alviola et al. (2011), Ando et al. (1980a), Bartels et al. (1998), Bergmans & Rozendaal (1988), Bonaccorso (1998), Bonaccorso & McNab (1997), Churchill (2008), Corbet & Hill (1992), Esselstyn, Widmann & Heaney (2004), Flannery (1995a, 1995b), Francis (2008a), Francis, Borisenko et al. (2010), Francis, Rosell-Ambal, Sedlock et al. (2008), Furey et al. (2012), Geiser & Kortner (2010), Giannini & Simmons (2007a), Goodwin (1979), Gunnell et al. (1996), Heaney, Balete, Gee et al. (2005), Heaney, Balete & Rickart (2016), Heaney, Gonzales et al. (1991), Heideman & Heaney (1989), Heideman & Utzurrum (2003), Hood (1989), Hood & Smith (1989), Khan et al. (2007), Kitchener, Boeadi et al. (1990), Kofron (2008), Kompanje & Moeliker (2001), Kruskop (2013a), McKenzie & Rolfe (1986), McKenzie, Gunnell et al. (1995), McNab & Bonaccorso (2001), Roberts (2006a), Sedlock et al. (2008), Shafie et al. (2011), Syafiq et al. (2016), Webber (1992), Winkelmann & Goedeke (2000), Winkelmann et al. (2003), Yong & Dhaliwal (1976).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Macroglossus minimus
| Don E. Wilson & Russell A. Mittermeier 2019 |
Pteropus minimus
| E. Geoffroy Saint-Hilaire 1810 |