Ptenochirus jagorii, Peters, 1861
|
publication ID |
https://doi.org/ 10.5281/zenodo.6448815 |
|
DOI |
https://doi.org/10.5281/zenodo.6448839 |
|
persistent identifier |
https://treatment.plazi.org/id/03AD87FA-FFCE-F621-8CAD-3D9BF597FDE3 |
|
treatment provided by |
Conny |
|
scientific name |
Ptenochirus jagorii |
| status |
|
13. View Plate 1: Pteropodidae
Greater Musky Fruit Bat
Ptenochirus jagorii View in CoL
French: Cynoptére de Jagor / German: GroRer Moschusflughund / Spanish: Ptenoquiro grande
Taxonomy. Pachysoma (Plenochirus) jagorii Peters, 1861 View in CoL ,
Daraga, Albay, Luzon, Philippines.
This species is monotypic.
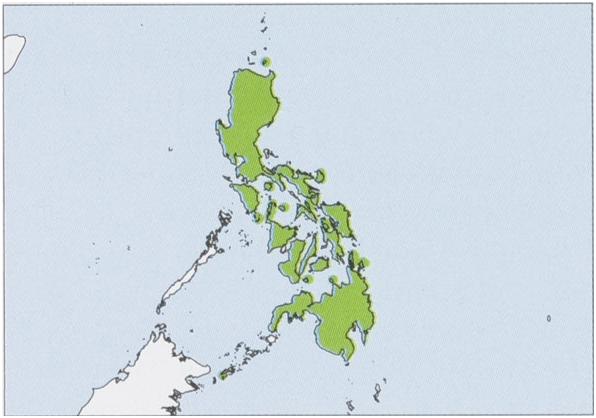
Distribution. Philippines (except Palawan faunal region), also present in the Batanes /Babuyan faunal region (Camiguin I) and the Greater Sulu faunal region (Bongao and Sanga-Sanga Is). View Figure
Descriptive notes. Head-body 114- 127 mm, tail 6-18 mm, ear 18-25 mm, hindfoot 18-22 mm, forearm 76-91 mm; weight 62-97 g. The Greater Musky Fruit Bat is medium-sized, with dark head. Adult males are slightly larger, with more conspicuous ruff than females. Head is large and wide. Muzzle is stout, with blackish brown skin; nostrils are shortly tubular and divergent; philtrum reaches upperlip, ending in two pads; two large triangular pads occur on lower lip; and both lips are lined with smaller pads. Eyes are mid-sized; iris is chestnut to reddish brown. Ears are moderately short, attenuated at tips and uniformly dark brown, and antitragus lobe is obsolescent. Head pelage is very short, generally very dark grayish brown, almost black; dorsum is grayish brown. Uropatagium and tail are well developed, and calcaris short. Ventrally, wide reddish to rusty brown ruff is present on both sexes but much more conspicuous on males, the latter with a gland beneath ruff that produces yellowish oily substance with distinctive sweet, musky cinnamon smell. Pelage on chest and belly is grayish brown, sometimes with olive tinge, extending faintlyto base of forearms. Wing membranes are dark gray, index claw is present, and wing from sides of body attach to first toe. Skull lacks basicranial deflection. Laterally, rostrum is moderately deep; forehead slopes; orbits are medium-sized, with marked rim; braincase is rounded; zygomatic rootarises well above alveolar line; and zygoma is moderately strong and only slightly arched. Dorsally, rostrum is relatively wide; paranasal recesses are inflated, passing posteriorly the level of postorbital foramina; postorbital processes are short, posterolaterally directed; temporal lines join in a low but sharp sagittal crest; braincase is oval; and nuchalcrest is pronounced. Ventrally, palate is moderately wide and flat; postdental is long and slightly convergent posteriorly; palatine spine joins sphenoidal crest; and ectotympanic is small, wide, and anteriorly edged internally by long ectotympanic. Mandible is relatively deep; coronoid is tall and sloping, with wide tip; condyle is almost level with lower alveolar line; and angle is round and salient. Upper dentition is long; I' is spatulated; I* is reduced; C' is relatively large, stout, and decurved, with groove on its convex anterior-medial surface and secondary cusp in middle of inner edge; P' is minute; and posterior cheekteeth decrease in size posteriorly, from squarish to rectangular in outline, without accessory surface cusps. Lower dentition has small and bifid-crowned Ld absent); C, is relatively small and decurved, without accessory cusps; P| is mid-sized, with triangular, labiolingually wide crown; posterior cheekteeth are large and squarish, becoming smaller posteriorly, with rectangular outline and without surface cusps; and M,is peg-like. There are nine interdental palatine ridges that are arched and undivided and three almost straight, undivided post-dental ridges. Chromosomal complement has 2n = 44 and FN = 56.
Habitat. Widely distributed in forested areas from sea level up to elevations of¢. 1950 m. The Greater Musky Fruit Bat is abundant in primary forests and common in secondary forests and disturbed/degraded forest areas. It occurs less commonly in urban areas (e.g. parks in Manila).
Food and Feeding. The Greater Musky Fruit Bat is primarily frugivorous but also eats leaves and flower products. On Panay Island, fruit from 39 genera in 28 families were eaten. Flowers from four genera in four families and leaves from seven genera in five families were used as food. Leaves used are rich in protein and other nutrients and minerals but less so than control leaves of non-used species, but used species average less in deterrent compounds like phenols. Several feeding areas are visited with high fidelity each night in a predictable fashion, indicative of trap-lining feeding behavior. Individuals fly around fruiting trees and land briefly to pluck a single fruit, which is taken to a feeding roost located 10-50 m from the fruiting tree. Ripe fruit is detected by smell and is preferred over unripen fruit. Fruit is eaten and dry pellets dropped; fresh droppings from ten or more figs indicate that this foraging pattern is repeated throughout a night. Small fig seeds that are not parasitized by fig wasps survive digestion and are defecated intact.
Breeding. The Greater Musky Fruit Bat is seasonally polyestrous, with postpartum estrus. Gestation lasts c¢.4 months. Females give birth to one offspring twice a year; less than 1% of pregnancies produce twins. Pregnant and lactating females are present in March-May and August—October. Up to five months of facultative post-implantation delay in early development (gastrulation) occurs only in primiparous females, so young females give birth only once in theirfirst year and are in synchrony with their second birth period of multiparous females. Lactation lasts ¢.3 months. Longevity is at least five years in the wild and predicted to extend up to eight years for ¢.5% of yearlings.
Activity patterns. Each night males and females leave their day roosts and visit at least two small (less than 1 ha) foraging areas. They spend most of their time near productive feeding areas and commute among them. The Greater Musky Fruit Bat frequently flies over water and roosts in tree cavities in primary forests.
Movements, Home range and Social organization. Based on radio-telemetry data from five males and five females, each followed for up to four months, the Greater Musky Fruit Bat mostly roosts alone. Small groups are reported from limestone cave entrances and under leaves of understory vegetation (e.g. Musa textilis, Musaceae ). They traveled 400-800 m /night between day roost and feeding areas. Day roost was located in a corner of an elongated home range that spanned 500-1300 m and was used from eight to at least 72 days. Changes in location occurred on average once a month, in response to depletion offruit resources and to minimize commuting; successive roosts were located within 600 m of previous roost in the forest inside. Home ranges of both sexes were 8-4-30-9 ha (mean 18-2 ha for males and 17-1 ha for females); home ranges included oneto several core foraging areas visited every night, each containing at least onefruiting tree and representing 18-55% of the home range. Pregnant females tend to occupy larger home ranges. Densities were 1-1-3-1 ind/ha (Negros Island).
Status and Conservation. Classified as Least Concern on The IUCN Red List. Although the population of the Greater Musky Fruit Bat might have declined due to ongoing land use change (deforestation), its population is presumably large, stable, and widely distributed in most Philippine faunal zones and individual islands, with fairly high levels of gene flow across islands. The Greater Musky Fruit Bat is the most frequently sampled species in many localities, and it is tolerant to disturbances such as forest conversion to cropland and rural urbanization.
Bibliography. Andersen (1912b), Giannini & Simmons (2007a), Heaney, Balete et al. (1998), Heaney, Heideman et al. (1989), Heaney, Tabaranza et al. (2006), Heideman (1987), Heideman & Heaney (1989), Heideman & Powell (1998), Jones, Bielby et al. (2009), Luft (1998, 2002), Luft et al. (2003), Mudar & Allen (1986), Ong, Rosell-Ambal, Tabaranza, Heaney, Pedregosa, Paguntalan et al. (2008), Reiter (2002a, 2002b), Reiter & Curio (2001), Reiter & Tomaschewski (2003), Relox et al. (2014), Rickart, Heaney & Rosenfeld (1989), Roberts (2006a).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Ptenochirus jagorii
| Don E. Wilson & Russell A. Mittermeier 2019 |
Pachysoma (Plenochirus) jagorii
| Peters 1861 |