Cynopterus horsfieldii, J. E. Gray, 1843
|
publication ID |
https://doi.org/ 10.5281/zenodo.6448815 |
|
DOI |
https://doi.org/10.5281/zenodo.6448831 |
|
persistent identifier |
https://treatment.plazi.org/id/03AD87FA-FFCC-F622-89B0-3D43F684F552 |
|
treatment provided by |
Conny |
|
scientific name |
Cynopterus horsfieldii |
| status |
|
Horsfield’s Short-nosed Fruit Bat
Cynopterus horsfieldii View in CoL
French: Cynoptére de Horsfield / German: Horsfield-Kurznasenflughund / Spanish: Cynéptero de Horsfield
Other common names: Codot Horsfield, Horsfield's Fruit Bat
Taxonomy. Cynopterus horsfieldii J. E. Gray, 1843 View in CoL ,
Java, Indonesia.
Four subspecies are recognized.
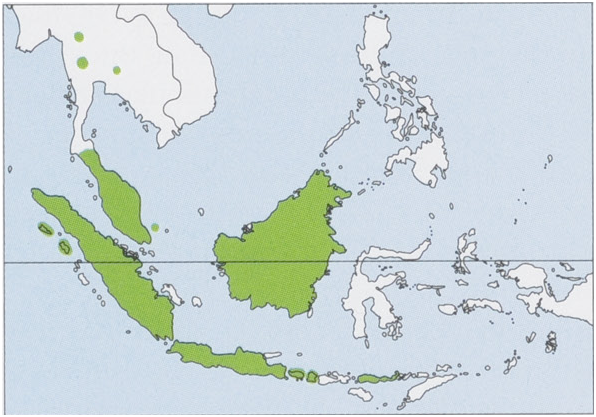
Subspecies and Distribution.
C.h.horsfieldiiJ.E.Gray,1843~Java,Bali,Lombok,andFloresIs;itmaybealsopresentonSumbawaI.
C.h.persimilisK.Andersen,1912—Borneo.
C. h. princeps G. S. Miller, 1906 — Nias I. Population on Simelue I may also represent this subspecies. View Figure
Descriptive notes. Head—body 85-120 mm,tail 12-19 mm, ear 19-22 mm, hindfoot 14-16 mm, forearm 74-3 mm (64-5— 71 mm in nominate horsfieldii , 87-89-5 mm in princeps); weight 43-63 g. Horsfield’s Short-nosed Fruit Bat is medium-sized, with rimmed ears and surface cusps on lower cheekteeth. Muzzle is short and deep; nostrils are shortly tubular and divergent, and their rims are thickened; and muzzle skin is brown. Eyes are large; iris is warm brown or cocoa-brown. Ears are long and oval, with slightly attenuated tips and white rims. Head is wide, and pelage is brown or rust-brown, longer on nape and lighter on dorsum. Tail is longer than broad uropatagium rim, and calcar is short. Pelage on throat is sparse. Conspicuous reddish brown to orange ruff extends to sides of neck and chest;it is stiffer, brighter, and richer on males. Lower chest and belly are light gray-brown; flanks have yellowish tinge. Genitals are dark brown. Wing membranes are dark grayish brown, originate on sides of body, and attach to first toe; index claw is present; and metacarpals and phalanges are dorsally white. Skull lacks basicranial deflection. Rostrum is short, forehead gently slopes, orbitis large, braincase is slightly flattened anteriorly and more rounded posteriorly, zygomatic root is above alveolar line, and zygoma is much arched and wide open. Dorsally, rostrum is wide and short; paranasal recesses are inflated and surpass posteriorly supraorbital foramina; postorbital processes are relatively thick and pointed posterolaterally; postorbital constriction is small; sagittal crest is low; braincase is oval; and nuchal crest present. Palate is flat, post-dental palate is long and convergent; palatine spine is joined to midsphenoidal ridge. Ectotympanic is small and wide anteriorly; entotympanic long and thin. Mandible has moderately thick body; coronoid is tall and rather steep, with wide tip; condyle is above lower alveolar line; and angle is round off, with demarked rim. Upper incisors are small and long; C' is long and strongly decurved, with marked cingula and strong secondary cusp on inner edge; P' is minute; and posterior cheekteeth are tall and strong, with marked cusps, from almost square to rectangular outline posteriorly. Lower incisors are small; C,is strong but short; P| is well formed, with cusp; next premolar (P,) is large and astall as C,, following teeth decreasing in height; P, and M have conspicuous additional surface cusp (especially in princeps); and M, is small, with normal cusps distinguishable. Chromosomal complement has 2n = 34 and FN = 58, with eleven metacentric or submetacentric, two subacrocentric, and three acrocentric autosomes. X-chromosome is subacrocentric, and Y-chromosome is small acrocentric.
Habitat. Lowland to montane forests, mangrove forests, and transition from forest to open/disturbed forest or cultivated land from sea level to elevations of ¢. 1460 m. Horsfield’s Short-nosed Fruit Bat uses understory and subcanopy strata and prefers to roost in forest edges and secondary forests.
Food and Feeding. Horsfield’s Short-nosed Fruit Bat is mainly frugivorous. It is transient, with aseasonal changes in abundance, and uses mainly large-crop, big-bang fruiting plants. Single fruit are taken from a tree and consumed in nearby day or feeding roost where seeds and dry pellets are discarded. Feeding roosts are located in foliage c. 3 m aboveground. Plants consumed include mainly Ficus (FE fistulosa and FE variegata ) and Artocarpus (both Moraceae ); Elaeocarpus (Elacocarpaceae) ; Payena (Sapotaceae) ; Plernandra ( Melastomataceae ); and introduced Piper aduncum ( Piperaceae ). Fruits from gardens and orchards are often eaten. Flower products are important during dry season when fruits are scarce, with up to eleven pollen types eaten (e.g. Parkia ; Fabaceae ) and unidentified leaves.
Breeding. Mating system of the Horsfield’s Short-nosed Fruit Bat is polygynous. Females are polyestrous, with postpartum estrus. Reproduction is largely aseasonal, with pregnant females found throughout the year. Litter size is one, with up to two litters per year.
Activity patterns. Activity pattern is bimodal. Horsfield’s Short-nosed Fruit Bats are most active 2-4 hours after sunset and again c.3 hours before sunrise, with resting period around midnight.
Movements, Home range and Social organization. Horsfield’s Short-nosed Fruit Bats mainly move between day roosts and feeding areas,traveling 400-1600 m. Mean home range is 8 ha for adult males and 6 ha for adult females. Home ranges widely overlap among males and females and with individuals of other species of Cynopterus (e.g. Lesser Short-nosed Fruit Bat, C. brachyotis ). Social organization is harem-based, with a single male associated with 2-5 reproductive females and their young. Harem group roosts in a tent built by the male or in unmodified roosts. In Peninsular Malaysia, banana ( Musa , Musaceae ) leaves are preferred roosts; ¢.50% of them is modified as tents by chewing mid-rib midway between base and tip. Other roosts include withering roots of epiphytic ferns ( Asplenium , Aspleniaceae ) and palm fronds ( Arenga , Cocos , Corypha , and Nypa ; Arecaceae ). Roosts are spatially clumped in forests. Males and females have low roost fidelity, and harem system is labile. Males change roosts every 1-7 days, and females change roosts every 3-14 days. Alternative roost sites include cave entrances and limestone solution cavities, where Horsfield’s Short-nosed Fruit Bats are more gregarious.
Status and Conservation. Classified as Least Concern on The IUCN Red List. Horsfield’s Short-nosed Fruit Bat is abundant and uses modified forests as roosting and feeding habitat. Population trend is unknown.
Bibliography. Andersen (1912b), Bates, Francis, Gumal & Bumrungsri (2008), Campbell (2008), Campbell & Kunz (2006), Campbell, Reid et al. (2006), Campbell, Schneider et al. (2006), Fletcher et al. (2012), Francis (1994), Funakoshi & Zubaid (1997), Hodgkison (2001), Jones, Bielby et al. (2009), Kingston et al. (2006), Kitchener, Gunnell & Maharadatunkamsi (1990), Tingga et al. (2012), Yong et al. (1973).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Cynopterus horsfieldii
| Don E. Wilson & Russell A. Mittermeier 2019 |
Cynopterus horsfieldii
| J. E. Gray 1843 |