Leptodactylus thomei, Almeida, Antonio De Padua & Angulo, Ariadne, 2006
|
publication ID |
https://doi.org/ 10.5281/zenodo.174274 |
|
DOI |
https://doi.org/10.5281/zenodo.5612859 |
|
persistent identifier |
https://treatment.plazi.org/id/03A9B966-245C-3C66-FEA3-FBE9FBA3FD2E |
|
treatment provided by |
Plazi |
|
scientific name |
Leptodactylus thomei |
| status |
sp. nov. |
Leptodactylus thomei View in CoL sp. nov.
( Figures 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 )
Holotype. MBML 2521, adult male, collected at Povoação, state of Espírito Santo, Brazil, on 28 August 2002, by Antonio de Padua Almeida, and recorded by Ariadne Angulo.
Paratypes. Twenty-seven specimens collected at the type locality: MBML 2515–2517, 25 August 2002; MBML 2518–2520, 26 August 2002; MBML 2522–2526, 27 August 2002; MBML 2527, 28 August, 2002, collected by Antonio de Padua Almeida and some recorded by Ariadne Angulo; MBML 2302–03, 19 September 2001; MBML 2306, 21 September 2000; MBML 2307, MBML 2311, MBML 2314, 0 2 May 2001; MBML 2309–10 and MBML 2312–13, 18 September 2001, collected by Antonio de Padua Almeida and Rogerio Penha da Silva; MBML 2297, MBML 2300, 0 1 May 2002; MBML 2304–05, 0 7 June 2001; MBML 2308, 16 September 2001, collected by Antonio de Padua Almeida.
Diagnosis. The advertisement call of Leptodactylus thomei differs from the advertisement calls of all other described species of the L. marmoratus group with known vocalizations. Leptodactylus thomei further differs from Leptodactylus andreae Müller, 1923 by the lack of flattened toe discs and the presence of a mask-like pattern formed by an inverted triangle found in the interorbital region. The new species differs from L. araucaria ( Kwet & Angulo, 2002) in: (1) size (maximum SVL of L. thomei males 23.2 mm and females 23.1, in L. araucaria males 18.8 mm and females 19.9 mm), (2) the presence of visible white tubercles on tibia (absent in L. araucaria ), and (3) lack of dorsal longitudinal dark marks and light dorsolateral and mid-dorsal stripes in L. thomei (present in most L. araucaria ). Leptodactylus thomei differs from L. bokermanni Heyer, 1973 by its marginally smaller size and by having a mask-like pattern embedded in the tan triangle patch of the interorbital region (but see discussion). It can also be distinguished from Leptodactylus diptyx Boettger, 1885 by the advertisement call and egg clutch size [58 unpigmented eggs in L. diptyx ( De la Riva 1995), 29-33 cream-coloured eggs in L. thomei ( Almeida & Angulo 2002 and this study)]. It can be distinguished from Leptodactylus heyeri (Boistel, Massary & Angulo, 2006) by: (1) advertisement call, (2) the lower surface of the foot with several small and distinct tubercles, (3) males lacking a yellow throat, and (4) a distinct dorsal colouration pattern. Leptodactylus thomei can be distinguished from Leptodactylus hylaedactylus ( Cope, 1868) by its aquatic reproductive mode ( Almeida & Angulo 2002). The new species differs from Leptodactylus lutzi ( Heyer, 1975) given that L. lutzi is a larger species (males 30 mm and females up to 34 mm) and has a characteristic spotting on the posterior face of the thigh ( Heyer 1975). It can be distinguished from Leptodactylus marmoratus ( Steindachner, 1867) by (1) not possessing flattened toe discs, (2) possessing a mask-like patch in the interorbital region, and (3) having an aquatic reproductive mode. Leptodactylus thomei lacks the four longitudinal series of black dorsal spots that characterize L. martinezi Bokermann, 1956 .
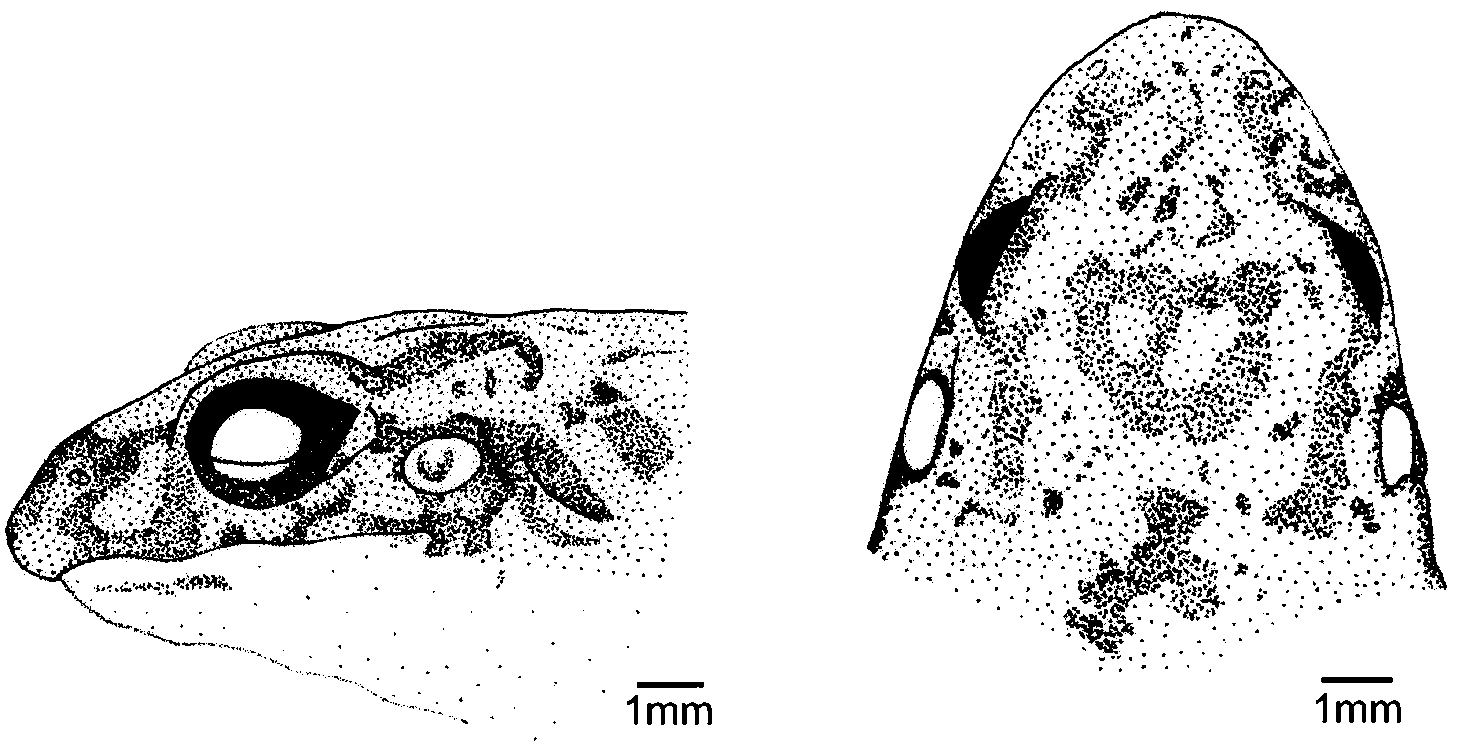
Description of holotype. Body small, robust, with short limbs (see Table 1 View TABLE 1 ). Outline of snout subelliptical to subovoid, laterally the snout is acuminate, head as long as broad ( Fig. 1 View FIGURE 1 ). Nostrils anterior, dorsolateral, equally distant from snout and eye. Tympanum distinct, tympanum diameter almost half of eye diameter. Supratympanic fold poorly developed, but crevice extending from back of tympanum to arm insertion. Supratympanic melanophore markings dark and continuous from back of eye to back of tympanum, sparser and discontinuous from back of tympanum to arm insertion. Canthus rostralis indistinct, loreal region slightly concave. A rounded, cream-coloured oval gland is present at the angle of jaw. Vocal sac single and internal, a pair of vocal slits present; tongue oval to elongate. Short series of vomerine teeth posterior to choanae. Arms short, robust. Finger lengths III>I>II>IV; finger tips rounded, not expanded; fingers without webbing or fringes ( Fig. 2 View FIGURE 2 a). Two large metacarpal tubercles; size of outer, circular, metacarpal tubercle about three times that of inner, ovoid, metacarpal tubercle; prominent, rounded subarticular tubercles on fingers, very pronounced on the thumb. Nuptial asperities absent. Hindlegs short, thigh and shank of similar length. Toe lengths IV>III>V>II>I; toe tips rounded, neither expanded nor flattened; toes without webbing or fringes ( Fig. 2 View FIGURE 2 b). Two distinct, light, metatarsal tubercles; inner metatarsal tubercle ovoid, about twice as large as outer, rounded metatarsal tubercle. Sole of foot and lower surface of tarsus with several small, but relatively distinct tubercles. Upper shank surface smooth, with perceptible but sparse white tubercles. Dorsal texture slightly rugose, with more conspicuous tubercles placed in two longitudinal, discontinous, and glandular lateral folds extending from tympanum to inguinal region. A pair of cream oval glands present on either side of the anus. Ventral surface smooth.
Colour in life. The dorsum is greyish-brown, with two dark dorsolateral melanophore lines running discontinuously from the back of the eye to thigh insertion, with a greater discontinuous patch in the shoulder area. Medially, the shoulder area also has a chevron shaped mark. There are two light and irregular blotches within the dark triangular patch of the interorbital region, giving the patch the appearance of a mask. This patch extends from the interorbital region to the back of head, roughly terminating at the tympanum level. Posteriorly and dorsally, there is a tan blotch on either side of the insertion of the legs. There are faded to bright orange patches on the heels, flanks, and dorsal scapular area. Venter is immaculate white. Iris is reticulated and copper-like in colour.
Colour in preservative. Dorsum greyish-brown, with dark melanophore marks of dorsal pattern as previously described. Venter white, immaculate; throat white, with a slight shadow following the contour of lower lip.
Measurements of holotype (in mm). SVL 21.2, HL 5.9, HW 5.9, ED 2.4, TYD 1.1, END 1.52, IOD 1.9, IND 2.3, FAL 1.4, HDL 3.6, TL 8.6, SL 8.1, TSL 3.9, FL 8.2.
Morphological variation. Two irregular lighter-coloured blotches within the dark triangular patch of the interorbital region, which give the patch the appearance of a mask, are commonly found in members of the new species (93% of examined specimens, n =28, in other specimens the interorbital triangular patch is not continuous, as the inner blotches can be joined to the lighter dorsal pattern outside the triangle). Most specimens examined Males (n = 20) Females (n = 4)
commonly have a light mid-dorsal hairline stripe whose extension can vary from above the vent to the sacral region (79% of examined specimens, n =28, absent in the other 21%); white or cream oval glands on either side of the anus (60% of examined specimens, n =28, not visible in the other specimens), toe tips either slender, pointed, or with very slight expansions (stages 1A and B of Heyer 1973), without discs.
Larval morphology. The tadpole of Leptodactylus thomei is illustrated in Figure 4 View FIGURE 4 . Body piriform in dorsal view, almost twice as long as it is wide; snout narrowly rounded in profile; nostril marginally nearer to tip of snout than to eye; distance between nares slightly less than interorbital width; nares somewhat triangular, dorsolateral and directed anterolaterally; eyes dorsal; opening of the lateroventral sinistral spiracle directed posterolaterally, with short spiracular tube, positioned around halfway between tip of snout and body-tail juncture. Vent tube short and median; maximum body height slightly greater than maximum tail height; dorsal fin origin on the body-tail juncture; dorsal and ventral fins slightly arched; tail musculature reaching the tail tip; myotomes distinct over the last two thirds of tail; tail tip rounded to pointed. Mouth anteroventral; oral disc Range Mean S
Total length (TL) 11.3–17.9 16.1 1.45
Body length (BL) 4.3–6.4 5.7 0.48
Tail length (TAL) 7.0–11.8 10.3 1.06
Maximum tail height (MTH) 2.0–3.4 2.8 0.32
Tail muscle height (TMH) 0.8–1.8 1.4 0.21
Tail muscle width (TMW) 0.7–1.4 1.2 0.19
Body height (BH) 2.5–3.8 3.1 0.29
Body width (BW) 3.0–4.4 3.9 0.33
Internarial distance ( IND) 0.5–1.0 0.8 0.12
Interorbital distance (IOD) 0.8–1.1 0.9 0.09
Snout-nostril distance (SND) 0.3–0.6 0.4 0.08
Snout-eye distance (SED) 0.8–1.2 1.1 0.11
Eye-nostril distance ( END) 0.3–0.6 0.5 0.08
Oral disc width (OD) 1.1–1.8 1.6 0.16
Eye diameter (ED)* 0.52–0. 62 0.58 0.06
ED/BL* 0.09–0.12 0.1 0.01
OD/BL 0.22–0.34 0.28 0.02 surrounded by a single row of rounded marginal papillae with a broad rostral gap; submarginal papillae absent; tooth row formula 2(2)/3; rows of similar length; beak cornified, serrated; lateral line system not visible. Measurements are presented in Table 2; an illustration of the oral disc can be seen in Figure 5 View FIGURE 5 .
Larvae colour in life. Dorsum tan to dark-brown, with scattered golden speckles; spiracle transparent; ventral wall transparent; internal organs visible; the posterior half with golden spots which present an iridescent aspect under stereomicroscopic examination; tail musculature mottled, with the fins slightly mottled; conspicuous mottling present in upper fin. Dorsal aspect of hind limbs with dark-brown bands alternated with unpigmented bands; this pattern is also found in the toe rudiments.
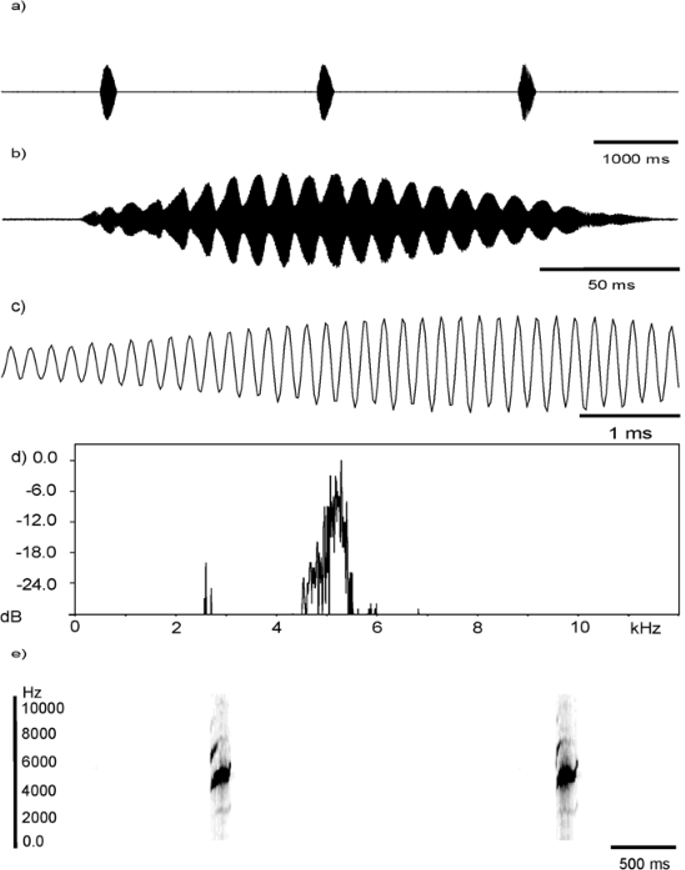
Advertisement call. This call was first described and figured in Angulo (2004) under Adenomera “Linhares”. One of the recordings used in Angulo’s (2004) analysis was replaced with another one in this study, given that the removed recording could potentially constitute an intermediate signal or a signal with a different function. The holotype MBML 2521 was recorded at 01:08 hrs on August 27, 2002, at an air temperature of 17.7ºC and 92% relative humidity. A total of six calls were recorded for this male and were used to describe the acoustic parameters measured herein (see Table 3 View TABLE 3 , Figure 6 View FIGURE 6 ). Call length average is 189.5 ms (range 165-210 ms), emitted at a low call rate (18 calls/min), with a mean call rise time of 71.73 ms, but ranging from 53-90 ms. The call is distinctly pulsed, audible to the human ear with a deep and regular amplitude modulation, consisting of 17–21 pulses/call, an average pulse rate of 111 pulses/s, average pulse duration of 9 ms, and average pulse rise time of 6 ms. Pulses rise gradually in increasing order of amplitude over the first third of the call, are roughly sustained over the middle third, and then smoothly decrease in amplitude, tapering off to background noise levels over the final third of the signal. The waveform representation of the call looks, overall, symmetrical. The dominant frequency oscillates around 5223 Hz and is approximately twice the fundamental frequency (average of 2505 Hz), comprising a harmonically-related formant. The maximum number of harmonically-related frequencies detected for this call is six or seven. The call has an average upward change in dominant frequency of 1881 Hz, but ranges from 1809–1981 Hz.
Advertisement call variation. Table 3 View TABLE 3 shows measurements of acoustic parameters of calls for five vouchered males, including the holotype, and three additional unvouchered recordings (but see Table 3 View TABLE 3 for unavailable voucher 1). Temperatures are also provided for each set of recordings. Overall, the call of L. thomei is of intermediate to long duration (call length range 120–210 ms), when compared to advertisement calls of other Leptodactylus of the L. marmoratus group; it is emitted at low call rates (10–24 calls/min), albeit widely variable, very likely influenced by the motivation of callers and interactions with neighbouring males. All advertisement calls examined exhibit a pulsed structure, although it is not always possible to distinguish individual pulses from the waveform. Calls usually sound pulsed due to the very deep and regular amplitude modulation, although the pressure level of this modulation does not go down to zero in examined calls. If pulses can be discretely observed, the number varies from 10–21 per call, and ranges *One of these callers is in fact vouchered (MBML 2520); however, since both were recorded simultaneously, and since it is difficult to determine which of the calls was issued by the voucher, we have not assigned calls to this voucher.
from 97–154 pulses/s. The amplitude patterns of calls vary according to the individual caller. The dominant frequency ranges from 4566 to 5562 Hz, and it corresponds to the next harmonic following the fundamental frequency (range 2153–2811 Hz). Between three to possibly seven harmonics can be detected for this call. The call shows upward change in the main carrier which varies from mild to very strong modulation (range 603 to 2153 Hz). This change in dominant frequency is variable both within and among individuals.
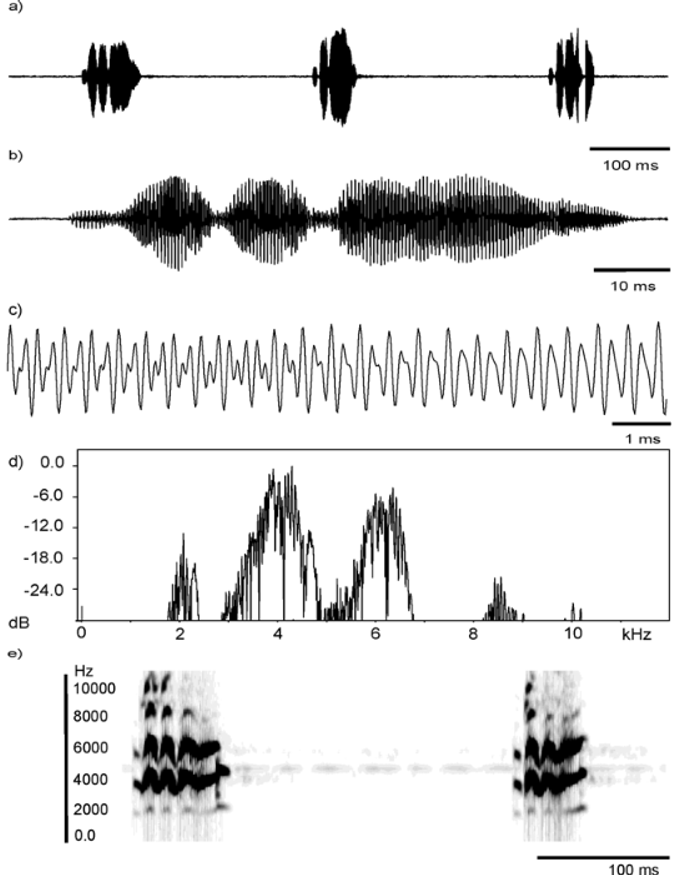
Agonistic calls. When offered playbacks of their own call type or when neighbouring males call in close proximity, often males will change their call type. The acoustic signals produced under these circumstances differ from the advertisement call and do not resemble any other known advertisement call. There appear to be at least two types of vocalizations in situations of conflict for this species. One is a short “chuckle” (depicted in Figure 7 View FIGURE 7 ), comparable to aggressive signals in other species of the L. marmoratus group ( Angulo 2004), while the other is a signal which seems to be intermediate between the “chuckle” and advertisement call. The “intermediate” signal is often times heard as a single call, whereas “chuckles” have been heard as quick, consecutive calls or issued in groups of three. Agonistic signals were recorded for three males on 28 August 2002 at 20.4 º C (n =1 call), 19.9ºC (n =7 calls) and 21.5ºC (n =7 calls). Of these recordings, one set corresponds to what we believe may be “intermediate” calls (n =7 recorded at 21.5ºC) and the other two to “chuckles”. Angulo (2004) provided descriptions for both these calls types, which are summarised herein. The “intermediate” call length varies from 28–127 ms with a mean duration of 101 ms. The call rate is ca 53 calls/minute, call rise time varies from 3 to 66 ms. There is pulse structure, with about 3–13 pulses per call. The fundamental frequency varies between 2173 and 2253 Hz, and dominant frequencies can range between 4526 and 4705 Hz; the dominant frequencies are the second harmonicallyrelated bands of the signal. Change in dominant frequency varies from moderate to intense (range 948–1723 Hz), and the number of harmonic bands detected is about 3. “Chuckles” (see Figure 7 View FIGURE 7 ), on the other hand, are shorter (average 64 ms, range 50–91 ms), and with a much higher call rate, ranging from 205–227 calls/min if they were to be issued continuously. The number of pulses per call decreases to 3–6, and pulses seem to change in amplitude pattern as compared with the advertisement call. With two exceptions, where the main carrier seems to become the third harmonically-related frequency (6041 and 6300 Hz), the dominant frequency is the second harmonically-related band (range 3848–4885 Hz), and a fundamental frequency that ranges between 1874–2412 Hz. “Chuckles” contain up to five or six emphasized harmonic bands, with the first three harmonically-related frequencies with the greatest energy content. “Chuckles” are noisier across the frequency spectrum (see Figure 7 View FIGURE 7 d). This acoustic signal also shows an upward change in dominant frequency towards the end of the call.
Natural history. Individuals of the new species are found inhabiting the extensive leaf litter carpet which covers cocoa plantations within alluvial forests, although they display a patchy distribution during the reproductive season, with males aggregating into vocalizing groups. Reproductive activity starts around mid-August and continues until March, which coincides with the rainy season in this area. Calling activity can usually start around midafternoon or dusk, sometimes as early as midday during rainy or cloudy days, and continue well into the night. It increases dramatically at the beginning of and after showers. The species is fairly abundant during the rainy season. No other Leptodactylus of the L. marmoratus group is known to occur in this habitat.
Reproduction. Reproduction of this species involves egg deposition in foam nests in earthen chambers and a subsequent aquatic phase necessary for the tadpoles to complete development ( Almeida & Angulo 2002). During field work, we collected a gravid female (MBML 2522), which upon capture expelled an egg. She was kept in a plastic bag overnight. On the following day, the female was sitting on about 0.5 cm of a layer of foam, where all remaining eggs (n =32) had been released. Eggs were cream-coloured, with an average diameter of 2.3 mm (range 1.1–2.85 mm, SD=0.36).
Etymology. The new species is named after João Carlos Alciati Thomé, in recognition for his conservation efforts on the Doce River Coastal Plain, northern Espírito Santo, which encompasses the type locality of the new species.
Known geographic distribution. The new species has been collected at one locality in the state of Espírito Santo (Povoação, municipality of Linhares) and it probably also occurs in Governador Lindemberg, Espírito Santo (based on heard calls and one collected specimen from this site—MBML 5736) and in southern Bahia (based on examination of museum specimens—see Appendix). Although uncommonly encountered in the dry season, in the rainy season this species is frequently heard and observed.
TABLE 1. Morphometric measurements for Leptodactylus thomei sp. nov. All measurements expressed in mm. Abbreviations are as follows: n = sample size, S = standard deviation.
| Range | Mean | S | Range | Mean | S |
|---|---|---|---|---|---|
| Snout-vent length 19.18–23.21 | 21.82 | 1.13 | 20.37–23.06 | 22.03 | 1.16 |
| Head length (HL) 5.19–6.38 | 5.83 | 0.29 | 5.38–6.06 | 5.78 | 0.29 |
| Head width (HW) 5.50–6.75 | 5.99 | 0.28 | 5.56–6.50 | 6.02 | 0.38 |
| Eye diameter (ED) 1.92 –2.40 | 2.21 | 0.12 | 2.16–2.40 | 2.26 | 0.10 |
| Tympanum diameter (TYD) 0.92–1.20 | 1.06 | 0.08 | 0.92–1.20 | 1.11 | 0.12 |
| Eye-nostril distance (END) 1.52–1.88 | 1.71 | 0.09 | 1.72–1.88 | 1.82 | 0.06 |
| Interorbital distance (IOD) 1.69–2.06 | 1.86 | 0.10 | 1.69–2.06 | 1.88 | 0.16 |
| Internarial distance (IND) 2.04–2.36 | 2.19 | 0.10 | 1.88–2.32 | 2.14 | 0.19 |
| Forearm length (FAL) 1.28–1.48 | 1.37 | 0.06 | 1.18–1.45 | 1.34 | 0.12 |
| Hand length (HDL) 3.13–3.88 | 3.48 | 0.20 | 3.31–3.75 | 3.63 | 0.21 |
| Thigh length (TL) 6.25–8.63 | 7.16 | 0.60 | 6.38–8.25 | 7.34 | 0.77 |
| Shank length (SL) 6.75 –8.63 | 7.71 | 0.45 | 7.63–8.38 | 8.03 | 0.34 |
| tarsus length (TSL) 3.56–4.25 | 3.80 | 0.20 | 3.69–4.31 | 4.03 | 0.27 |
| Foot length (FL) 7.63–9.25 | 8.29 | 0.47 | 7.13–9.50 | 8.59 | 1.02 |
| continued. |
TABLE 3. Measurement of acoustic parameters for recorded males of Leptodactylus thomei sp. nov. MBML 2521 is the holotype. Numbers linked by a hyphen are ranges, numbers in bold are means followed by standard deviations in brackets. All temporal parameters are expressed in ms (except for call rate, expressed as calls / min, and pulse rate, expressed as pulses / s) and frequency parameters in Hz. “ Noisy signal ” indicates that amplitude readings were too noisy to provide certain temporal parameters.
| Recordings | MBML 2515 | MBML 2516 | MBML 2517 | MBML 2518 |
|---|---|---|---|---|
| Air temp. (ºC) | 19.7 | 19.7 | 18.6 | 17.7 |
| N (calls) | 10 | 10 | 10 | 10 |
| Call length | 138.21 (8.25) 127.39–152.04 | 140.79 (9.84) 122.04–157.14 | 169.3 (8.10) 158.82–182.34 | 171.01 (20.62) 127.64–198.73 |
| Call rate (calls/min) | 23 | 17 | 19 | 14 |
| Call rise time | 51.64 (12.94) 38.23–73.95 | 62.29 (19.03) 37.94–87.1 | 31.92 (8.25) 14.54–41.43 | 77.28 (27.59) 13.54–110.29 |
| Pulse rate/s | 118.7 (4.12) 113.64–124.53 | 120.52 (5.53) 111.4–129.7 | 109.57 (8.18) 96.88–125.72 | 123.93 (13.10) 106.77–153.7 |
| No. pulses/call | 11.9 (0.88) 10–13 | 15.14 (1.35) 13–17 | 16.67 (1.22) 15–19 | 16.75 (1.36) 15–18 |
| Pulse duration | 8.13 (0.44) 7.44–8.75 | 8.24 (0.53) 7.48–9.12 | 8.45 (0.90) 6.96–9.57 | 8.00 (0.75) 7.01–8.93 |
| Pulse rise time | 5.33 (1.09) 3.54–6.96 | 4.17 (1.26) 2.43–6.19 | 5.74 (1.49) 2.31–7.98 | 3.88 (1.32) 2.36–6.78 |
| Dominant frequency | 5352.99 (59.61) 5263.3–5442.7 | 5374.94 (217.85) 4964.2–5522.5 | 4711.05 (117.04) 4565.5–4864.6 | 5205.48 (241.71) 4605.4–5382.9 |
| Fundamental frequency | 2577.83 (99.49) 2372.5–2691.5 | 2695.44 (111.11) 2432.3–2811.1 | 2385.88 (70.91) 2325.59–2497.85 | 2560.76 (62.33) 2432.3–2631.6 |
| Other frequencies | 7376.6–8074.4 | 7097.5–8198.4 | 5961–6818.4 | 6878.2–7815.2 |
| Maximum # of harmonics detected | 5 | Possibly 7 | 3 | 5 |
| Change in dominant frequency | 956.07 (75.42) 861.32–1033.59 | 1163.80 (301.81) 602.92–1636.53 | 1421.19 (199.95) 1119.72–1808.79 | 1808.7 (243.59) 1291.99–2153.32 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |