Megalanceoloides remipes ( Barnard, 1932 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4178.1.7 |
|
publication LSID |
lsid:zoobank.org:pub:D45BF4A8-E989-450D-804A-3FFAA2AAFAFF |
|
DOI |
https://doi.org/10.5281/zenodo.6088184 |
|
persistent identifier |
https://treatment.plazi.org/id/03A93570-FFE4-6970-1FAB-4EA8FCB53961 |
|
treatment provided by |
Plazi |
|
scientific name |
Megalanceoloides remipes ( Barnard, 1932 ) |
| status |
|
Megalanceoloides remipes ( Barnard, 1932) View in CoL
( Figures 1–4 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 )
Lanceola remipes Barnard 1932 ; Megalanceola remipes Vinogradov 1964 ; Vinogradov et al. 1996.
Material examined. Adult female, collected 8 March 2015 in in Farallon Basin (25o 27’ 109 o 51’N), southern Gulf of California, Eastern Pacific by ROV submersible, depth: 2,0 94 m.
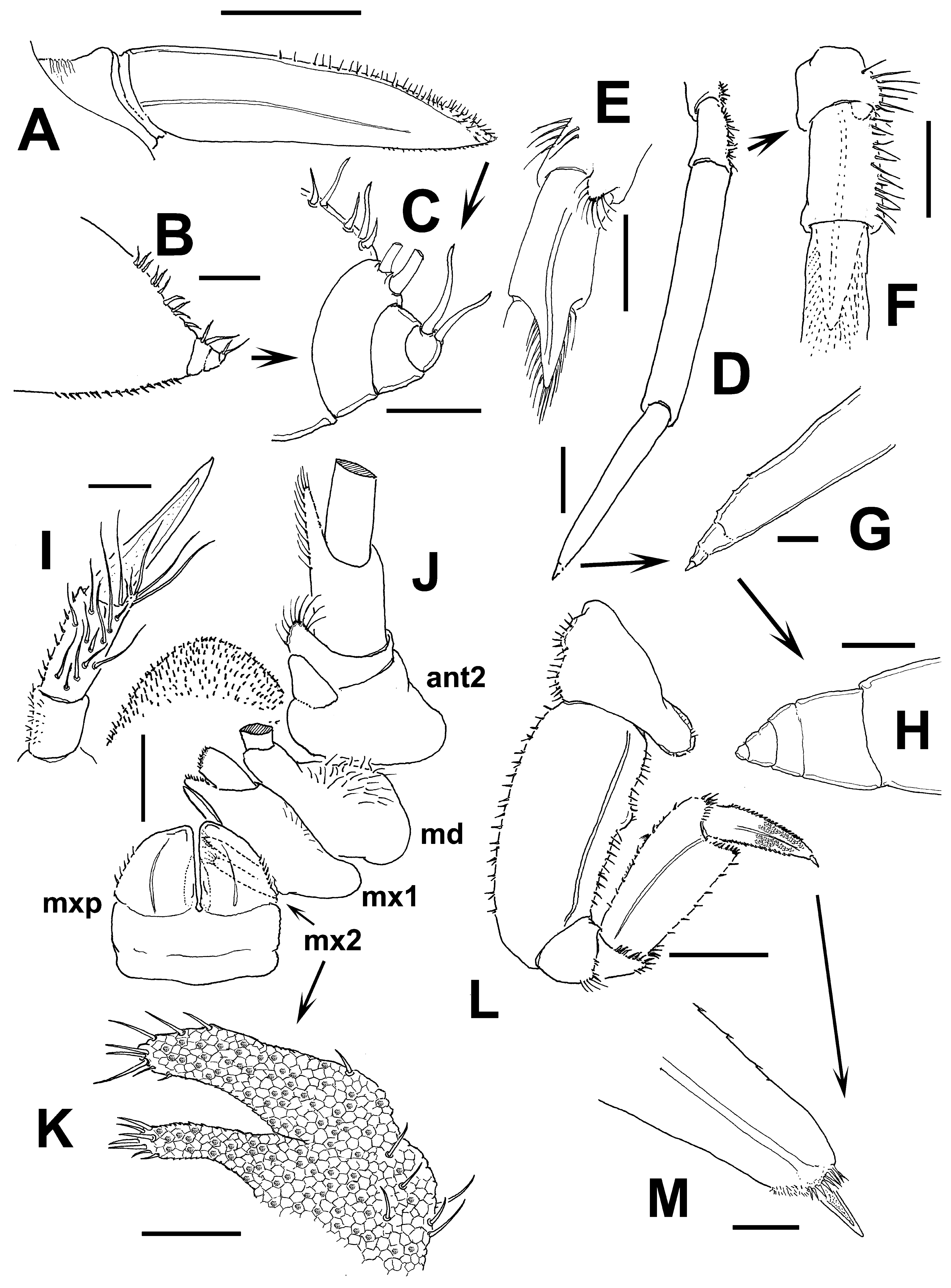
Remarks. This species was redescribed by Zeidler (2009) based on the holotype female from south–west Atlantic, but some of the appendages were missing in this specimen and were not described in the original work by Barnard (1932). Our morphological comments emphasize these appendages/characters in order to complement the description of the species.
The specimen examined is an ovigerous female. Total length: 25 mm Some of the characters of previous descriptions ( Barnard 1932, Vinogradov 1964, Zeidler 2009) can be clearly seen in the illustrations, like the relatively slender pereon, not laterally broadened; however, the specimen from the Gulf of California was carrying eggs and its body is dorsoventrally broadened ( Fig. 3 View FIGURE 3 ). Most appendages are identical to previous descriptions. Particularly, the complete second antenna was not seen before by any of the other authors. This appendage is much longer than the first antenna, ensiform, with a characteristic pointed lobe on S2 and a larger lobe on S3 ( Fig. 2 View FIGURE 2 , 3 View FIGURE 3 E, F), S4 elongated, S5 0.62X, S4 with 4 terminal segments ( Fig. 3 View FIGURE 3 D, G, H). Some doubts about the structure and segmentation of the maxillae 2 ( Mx 2) were left in Zeidler (2009) and only a general view was provided by Vinogradov (1964). The bilobed Mx 2 is here illustrated showing the spinulation pattern and the reticulate surface ( Fig. 3 View FIGURE 3 K), the outer lobe has 9 spinules, 4 of which are terminal; the inner lobe has 7 spinules set in a terminal cluster. The basal segment has 6 long terminal spinules. In the specimen from the Gulf of California the basis of P VI and VII are not as abruptly narrowed proximally ( Fig. 4 View FIGURE 4 J, L) like in previous descriptions ( Zeidler, 2009, fig. 28). Uropods1–3 with external margins denticulated as well as both sides of endopods and exopods.
Differences from previous specimens. The size of our specimen (25 mm) is intermediate between the female collected from the Indian Ocean (19 mm) ( Vinogradov, 1964) and the type specimen (40 mm) from the South Atlantic Ocean ( Barnard, 1932).
In our specimen the A1 has a reticulate surface, with three distal segments; Barnard (1932) reported no minute apical joints in the holotype, a character that was recently corrected by Zeidler (2009) by mentioning that these small segments are subequal in length. The Californian ( Fig. 3 View FIGURE 3 B,C) and the Indian Ocean ( Vinogradov 1964, fig. 4) specimens clearly have three subequally long apical segments. The terminal segment has five setae in the Indian Ocean specimen ( Vinogradov 1964, fig 4), but only two long setae in the California specimen ( Fig. 3 View FIGURE 3 B, C). Also, the penultimate A1 segment is unarmed in our specimen from California ( Fig. 3 View FIGURE 3 C) and has at least two setae in the Indian Ocean specimen ( Vinogradov 1964, fig. 4).
Mandible palp. The terminal segment of the palp represents about the 50% of the appendage ( Zeidler 2009, fig. 28C), but some variation was found in the other specimens; it is slightly longer (55.1% of palp length) in the Indian Ocean specimen ( Vinogradov 1964, fig. 4) and in the Pacific Ocean female (52.3%) ( Fig. 3 View FIGURE 3 I). Also, the second segment is hirsute in both the Indian ( Vinogradov 1964, fig. 4) and Pacific specimens ( Fig. 3 View FIGURE 3 I), but appears to have a weaker ornamentation in the holotype ( Zeidler 2009, fig. 28C).
Based on the examination of the holotype specimen and with reference to the Indian Ocean specimen described by Vinogradov (1964), Zeidler (2009) stated that the second maxillae has four long apical setae and the inner lobe is armed with 3 setae ( Zeidler 2009); in our specimen from California the outer lobe has also 4 subequally long apical setae plus other 4 subapical ones. Also, the inner lobe has 7 subequally long apical setae ( Fig. 3 View FIGURE 3 K), thus differing from the other specimens.
The basis of pereopod 2 has two distal setae but these are relatively short, unequally long in the Indian Ocean specimen ( Vinogradov 1964, fig. 4) whereas these elements are equally long and longer in the Californian specimen ( Fig. 4 View FIGURE 4 A). Pereopod 3 was not illustrated by Vinogradov (1964), but in the holotype the small apical dactylus arises from within a distal brush of short hair-like elements ( Zeidler 2009, fig. 28); in the Pacific Ocean specimen the insertion area of the dactylus is naked ( Fig. 4 View FIGURE 4 F).
In the Indian Ocean specimen the dactylus of pereopod 5 is very small, the terminal margin of the S6 is rounded ( Vinogradov 1964, fig. 5); in the Californian specimen the dactylus is more prominent and the distal margin of the S6 is relatively acute, not rounded ( Fig. 4 View FIGURE 4 H,I).
In the Indian Ocean specimen the dactylus of pereopod 6 is simple, claw-like ( Vinogradov 1964, fig. 5), whereas it has also a small curved adjacent element in the specimen from California ( Fig. 4 View FIGURE 4 K). The dactylus of pereopod 7 has also some additional differences: in the holotype the small apical claw-like dactylus arises alone from a heavily hirsute surface ( Zeidler 2009, fig. 28), but in the Pacific Ocean specimen the insertion area of the dactylus is naked and the dactylus has a few accompanying setae ( Fig. 4 View FIGURE 4 M).
Symbiosis. The amphipod was found grasping a siphonophore of the physonect genus Apolemia (Eschscholtz, 1829) with the dactyls of P VI and VII. The siphonophore was not identified but could be one of the species recently described for the zone ( Siebert et al., 2013). It was not collected because it was lost during the capture process as can be seen in the supplementary online video (http://w2.ecosur-qroo.mx/cna/rebeca/ D722%20D8%20Amphipod.mov). Digital photographs of M. remipes were taken when alive ( Figs 1 View FIGURE 1 , 2 View FIGURE 2 ). The in vivo color of the hyperiid was very similar to some parts of the siphonophore (i.e. gastrozoids).
Distribution. This is the first record of this species from the Pacific Ocean. The only additional records are from the South Atlantic (41°43’S 42°20’W) and the Indian Ocean (03°11’N 67°02’E); in both cases it was found at depth samplings from 2000 m to surface ( Zeidler 2009).
| ROV |
Museo Civico di Rovereto |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Megalanceoloides remipes ( Barnard, 1932 )
| Gasca, Rebeca & Haddock, Steven H. D. 2016 |
Megalanceola remipes
| Vinogradov 1964 |
Lanceola remipes
| Barnard 1932 |