Hylobates moloch ( Audebert, 1797 )
|
publication ID |
https://doi.org/10.1093/mspecies/seac006 |
|
publication LSID |
lsid:zoobank.org:pub:9070870D-C6F6-4B1A-ACF3-80EAFADEDF02 |
|
persistent identifier |
https://treatment.plazi.org/id/03A7BE60-FFFF-833C-72A5-5A092E70FDAA |
|
treatment provided by |
Felipe |
|
scientific name |
Hylobates moloch ( Audebert, 1797 ) |
| status |
|
Hylobates moloch ( Audebert, 1797) View in CoL
Javan Gibbon
Simia Nanodes Lichtenstein, 1791:31 . Type locality “ India praefertim in Java.” Nomen oblitum.
Simia Moloch Audebert, 1797 :pl. II. Type locality “les Moluques,” amended to “Tjianten, Mt. Salak, ca. 1100 m ” by Sody (1949:121). Nomen protectum.
Simia cinerea Cuvier, 1798: 96 Type locality “Batavia.” Preoccupied by Simia cinerea Kerr, 1792 (= Mandrillus leucophaeus (F. Cuvier, 1807)) View in CoL .
Simia Leucisca Schreber, 1799 View in CoL :pl. III B (dated after Sherborn (1892)). Type locality unknown.
Pit [ hecus]. cinereus View in CoL : Latreille, 1804:277. Name combination.
? Simia hirsuta Forster , mentioned in Sonnerat, 1806:81. Type locality unknown. Nomen nudum.
Pithecus leuciscus : Geoffroy Saint-Hilaire, 1812:89. Name combination.
S [ atyrus]. Leuciscus: Oken, 1816:1226. Name combination.
Hylobates leuciscus : Kuhl, 1820:6. Name combination. Not Hylobates leuciscus Matschie, 1893:62 (= Hylobates abbotti View in CoL ).
Cheiron Leuciscus View in CoL : Burnett, 1828:307. Name combination.
H [ ybolates]. Leusiscus Geoffroy Saint-Hilaire, 1834:34 . Incorrect subsequent spelling of leuciscus Schreber, 1799 .
H [ ylobates]. leucurus Gray, 1861:136. Incorrect subsequent spelling of leuciscus Schreber, 1799 .
Hylobates javanicus Matschie, 1893:62 . Type locality “ Java.”
Hylobates lar leuciscus : Pocock, 1927:727. Name combination.
Hylobates cinereus cinereus : Kloss, 1929:119. Name combination.
Hylobates moloch View in CoL : Frechkop, 1934:23. First use of current name combination (already alluded to but not written out in Cabrera, 1930).
Hylobates moloch moloch View in CoL : Chasen, 1940:64. Name combination.
Hylobates lar moloch View in CoL : Sody, 1949:121. Name combination.
Hylobates lar pongoalsoni Sody, 1949:123 View in CoL . Type locality ”Kali Kidang, Mount Slamat, C[entral]. Java, 800 m. ”
Hylobates moloch pongoalsoni View in CoL : Supriatna, Andayani, Forstner, and Melnick in Supriatna and Manullang, 1999. Name combination.
CONTEXT AND CONTENT. Context as for genus. There has been a long debate on whether Hylobates moloch encompasses two subspecies rather than being monotypic. The two proposed subspecies H. m. moloch and H. m. pongoalsoni (originally described as H. lar pongoalsoni ) are supposed to be living in western and central Java, respectively ( Sody 1949). When named by Sody (1949), the geographic ranges of these alleged taxa were not clearly defined. Respective H. m. moloch specimens were derived from Gunung Salak and Purwakarta in the West Javan province, whereas H. m. pongoalsoni vouchers originated from Gunung Slamet and Karang Gondang in Central Java ( Sody 1949). Hylobates m. pongoalsoni is supposed to have a lighter colored back and to lack the blackish cap typical for H. m. moloch . However, later studies could not confirm a geographic pattern in fur coloration ( Groves 1972; Geissmann et al. 2002). Similarly, genetic data once treated as evidence in support for a subspecific separation ( Andayani et al. 2001; Kheng et al. 2018) failed to provide support for this assumption in a recent reevaluation and are now viewed as indicative for an isolation by distance pattern between populations ( Nijman et al. 2019; see “Population genetics”). Apart from that, geographic patterns in female song structure were discussed as potential support for a subspecific splitting of H. moloch , again pointing to a separation between individuals in western and central Java ( Dallmann and Geissmann 2009). However, song recordings are only available from few sites that do not include the central and eastern regions of the West Javan province and it remains to be clarified to which degree the recovered differences are phylogenetically significant ( Dallmann and Geissmann 2009). In consideration of all this, it is commonly argued that the available evidence regarding the intraspecific taxonomy of H. moloch is inconclusive at best and not sufficient to warrant a subspecific differentiation ( Geissmann et al. 2002; Dallmann and Geissmann 2009; Nijman et al. 2019). Most contemporary authoritative references list H. moloch as monotypic (e.g., Chivers et al. 2013; Burgin et al. 2020; Nijman 2020).
NOMENCLATURAL NOTES. The genus name Hylobates derives from Ancient Greek (ὑλοβάτης) and translates to forest walker ( Illiger 1811). The epithet moloch refers to the mythical juggernaut and stands in the Linnean tradition of naming primates after spiritual entities ( Beolens et al. 2009). The complete name may be translated to “demonic forest walker.” Javan gibbon, silvery gibbon, and moloch gibbon are established trivial names ( Groves 1972; Beolens et al. 2009). In historic accounts, the names Wouwou and Wau-wau (which since the 1820s have been applied to H. agilis [agile gibbon] as well), and to a lesser extent also Moloch and white gibbon (as opposed to H. lar , the “black gibbon”— Burnett 1828) might appear in reference to H. moloch .
The oldest available scientific name of the Javan gibbon is actually Simia Nanodes Lichtenstein, 1791 ( Caspar 2020). However, since this name vanished from the literature in the first half of the 19th century, it has been declared a “forgotten name” (nomen oblitum) following the guidelines of the International Code for Zoological Nomenclature. The junior synonym Simia Moloch Audebert, 1797 was instead accepted as valid (nomen protectum) as it has been commonly referenced as such since its introduction to the literature ( Caspar 2020).
The close resemblance between Hylobates moloch and H. abbotti from western Borneo has repeatedly confused taxonomists and led to a suit of names that served to either unite or distinguish the gibbons of Java and Borneo at the species level. Up to the middle of the 20 th century, the names H. cinereus , H. leuciscus , and H. moloch were used ambiguously to describe gibbons of both respective populations, making species assignments in numerous classic works, including the influential treatises on small apes by Adolph H. Schultz, hard to interpret (e.g., Schultz, 1933; reviewed by Groves 1971). For this reason, respective references are not discussed herein. It has even been suggested that the earliest scientific illustrations of silvery-furred hylobatids usually attributed to the Javan gibbon ( Audebert’s (1797) Simia Moloch and Schreber’s (1799) Simia Leucisca ; see Caspar 2020 for discussion of the specimens) actually refer to Hylobates from western Borneo, rendering both H. moloch and H. leuciscus unavailable names for the Javan species ( Matschie 1893). In response, the name H. javanicus was suggested ( Matschie 1893). However, the fur color traits of the illustrated gibbons are not sufficient to argue that the specimens originated from Borneo ( Groves 1972). Although doubts about its validity could theoretically still be upheld since genetic assessments of the types of Audebert are missing, H. moloch has been firmly established as the Javan gibbon’s binomen for almost a century ( Caspar 2020). After H. moloch had been frequently listed as a subspecies of either Bornean Hylobates species or H. lar over the course of the 20th century ( Pocock 1927; Sody 1949; Groves 1971), its species-level distinctiveness became gradually more accepted and is now undisputed ( Marshall and Sugardjito 1986; Geissmann 1995; Chan et al. 2010; Thinh et al. 2010; Chivers et al. 2013; Roos 2016).
DIAGNOSIS
Hylobates moloch is foremost characterized by long, silvery-gray pelage in both sexes ( Fig. 1 View Fig ). The head is typically ornamented by a dark cap and a weakly expressed white face ring which extends into a forward-projecting beard in the chin region. The great-call sequence of the female song is peculiar and diagnostic for the species (e.g., Geissmann 1995). On morphological grounds, H. moloch might well be confused with H. abbotti (Abbott’s gray gibbon) from western Borneo (Geissmann 1995). Pelage color in H. abbotti is described as medium gray or mouse gray to pale brown and duller than in H. moloch and it often, although not consistently, lacks a dark cap ( Marshall and Sugardjito 1986; Groves 2001). The fur of H. abbotti is shorter in general but longer over the ears and it does not exhibit the white pointy beard of its Javan congener ( Groves 1972; Geissmann 1995; Mootnick 2006). Additionally, the two notably differ in dental morphology, which is more derived in Bornean Hylobates ( Frisch 1973) . Assignment of infants and young juveniles to either species might be especially challenging. The identification of H. moloch in the wild is unequivocal as it is the only hylobatid inhabiting Java.
GENERAL CHARACTERS
Hylobates moloch exhibits only a single, sexually monochromatic color phase ( Groves 1972). Sexual dimorphism in appearance, body mass, and dentition is slight but noticeable (see below). Adults display a long and dense, silvery-gray pelage ( Marshall and Sugardjito 1986). The fur may show a wooly texture and is mostly plain-colored. However, it is black in the genital area and surrounding the anus ( Mootnick 2006). No genital tuft is developed ( Kloss 1929). Females may show a dark gray chest patch that can taper to the abdomen (Geissmann 1995; Mootnick 2006). A dermal gland field is present on the chest but not in the axillary or inguinal areas ( Geissmann and Hulftegger 1994). Palms of hands and soles of feet as well as ischial callosities are naked ( Groves 1972), as is the face, except for approximately 60 short vibrissae surrounding the mouth region (quantified in other Hylobates species by Muchlinski 2010). All visible portions of the skin are uniformly black. The iris of the eye is a dark amber, whereas the sclera appears dark brown ( Caspar et al. 2021).
Hair length varies dependent on body region. It is longest on the upper arm, over the ears and occiput, and between the shoulders, reaching lengths of up to 7 cm ( Groves 1972). In older adults, this can result in a prominent semicircular gray wreath of elongated peripheral scalp hair. It anteriorly frames the face and rests posteriorly on the neck. Crown hair on the scalp is typically dark gray to black and grows fanwise from the front, thus creating a more or less well demarcated cap ( Marshall and Sugardjito 1986). The expression of the cap is variable among individuals and appears not to correlate with geographic provinces ( Groves 1972), as has once been suggested ( Sody 1949). It is only faintly present in some individuals, making them resemble H. abbotti (Geissmann 1995) . Reportedly, caps tend to be darker in females (Geissmann 1995). The face is framed by a weakly expressed light gray to white face ring. Quite frequently, only a sharply tapering brow streak that might be lighter than the rest of the facial ring and the portion surrounding the mouth is present. The latter forms a forward-pointing beard on the chin in both sexes (“goatee”— Marshall and Sugardjito 1986). Hylobates moloch experiences a slight fur color change during ontogeny. Infants are born with light skin and initially develop a pelage that is paler than that of adults. Their fur is buffy gray to cream-colored and their cap is less conspicuous than in older juveniles and adults ( Groves 1972; Marshall and Sugardjito 1986; see “Ontogeny and Reproduction”).
Only few exact data on adult body mass in H. moloch are available. Except for a single female and male each ( Geissmann 1993) all refer to captive specimens. In total, data on two adult females with a mean body mass of 5,925 g ( 5,600 –6,250 g; ± SD 365 g) and four adult males with a mean body mass of 6,693 g ( 5,800 –7,270 g; ± SD 575.6 g) are available ( Geissmann 1993; Michilsens et al. 2009; Zihlman et al. 2011). Despite the small sample size given, the subtle male-biased sexual size dimorphism that can be derived from these data (113%) corresponds well to that found in other Hylobates species (e.g., 108% in the lar gibbon, H. lar — Schultz 1944). Published body mass values tentatively place H. moloch as one of the largest-bodied species of its genus ( Geissmann 1993; Zihlman et al. 2011; but see Michilsens et al. 2009).
Body proportions in H. moloch are typical of its genus ( Groves 1972). Hylobates species display slightly more massive hind limbs than other gibbons in relation to their body size and lower intermembral indices than crested gibbons ( Nomascus ) and siamangs ( Symphalangus — Zihlman et al. 2011). The following segmental indices have been reported for H. moloch ( SD presented in parentheses): intermembral index, 128.3 (2.6) and brachial index, 112.3 (2.6), derived from museum specimens ( n = 5— Groves 1972; apparently not discriminated from measurements of H. abbotti ); intermembral index, 119.3 (3.4) and humerofemoral index, 107.3 (4.0), derived from carcasses of captive specimens ( n = 3; Zihlman 2011). The mean vertebral formula of H. moloch is 7 C, 13.1 T, 4.7 L, 4.9 S, 2.7 Ca ( Groves 1972; apparently not discriminated from measurements of H. abbotti ).
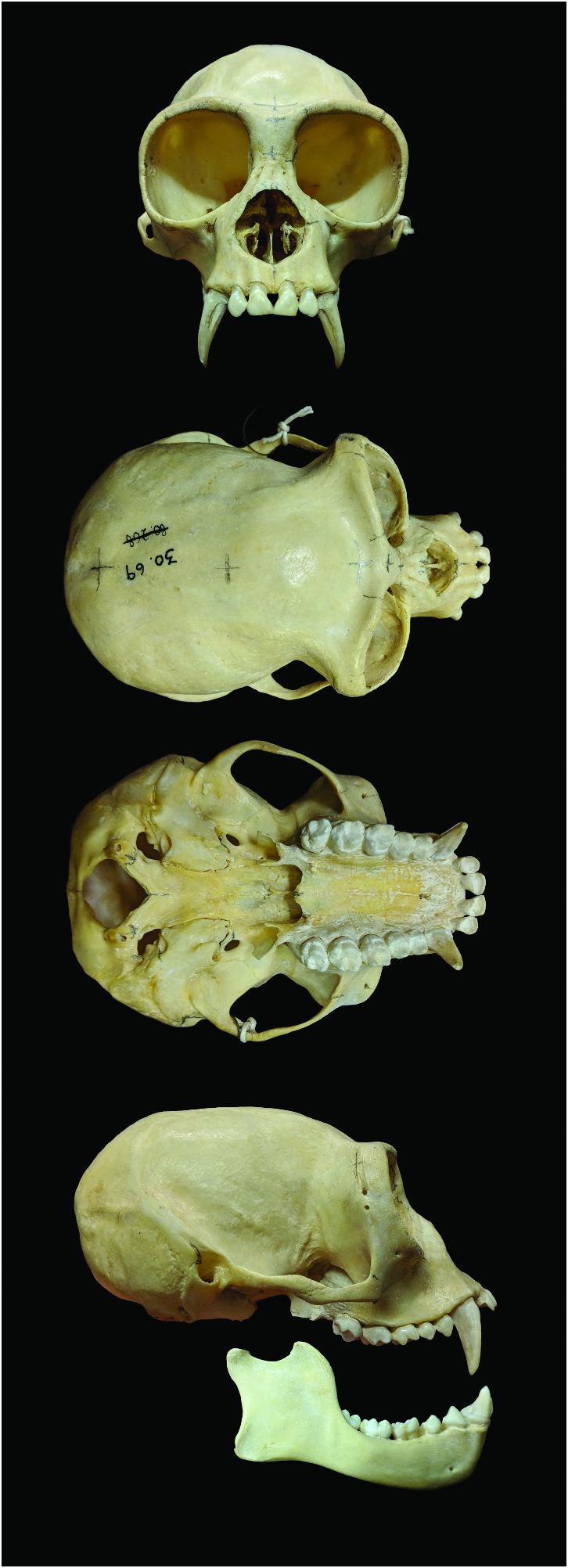
Skulls of Hylobates species (except for those of the morphologically derived Kloss’s gibbon, H. klossii ) are so similar to each other that even experts on hylobatids struggle to diagnose them ( Pocock 1927; Marshall and Sugardjito 1986). They exhibit very prominent brow ridges and a pronounced angle where nasals and frontals meet, as well as comparatively weak jaws with small teeth ( Fig. 2 View Fig ; Pocock 1927). There is broad interspecific overlap in morphometric variance of skulls within Hylobates ( Creel and Preuschoft 1976) and cranial traits are not phylogenetically informative (see “Molecular genetics”). Craniometrically, H. moloch ( n = 18) is particularly hard to distinguish from the gibbon species of Borneo (Müller’s gibbon, H. muelleri sensu lato, n = 101) and the pileated gibbon of southern Indochina ( H. pileatus , n = 7; Creel and Preuschoft 1976). Sexual dimorphism in the skull morphology of H. moloch is slight. Females ( n = 8), when compared to males ( n = 10), tend to show a shorter neurocranium with more pronounced curvature of the cranial vault along the medial parietals, a more protruding glabellar region, narrower orbits, shallower jaws, and more nimble zygomatic arches ( Creel and Preuschoft 1976). Cranial capacity was 113.3 and 98.3 in one captive male and female, respectively ( Zihlman et al. 2011). The slender but compact hyoid apparatus of H. moloch differs notably from that of congeneric species ( H. lar and H. pileatus ) studied so far ( Zihlman and Underwood 2019).
The dental characters of H. moloch are remarkably primitive and thus more distinctive and diagnostic than the bones of the skull. Conforming to the catarrhine ground pattern, each jaw quadrant houses two incisors, a canine, two premolars, and three molars ( Fig. 2 View Fig ). Only very rarely, third molars (wisdom teeth) in either the upper or lower jaw are congenitally absent ( Frisch 1973). Furthermore, the third molars seldom show signs of morphological reduction, meaning diminution in size and numbers of cusps ( Frisch 1973), a trait that separates H. moloch from all its congeners except H. pileatus . Only one upper and lower third molar, respectively, was found to show notable signs of morphological reduction in a sample of 33 such teeth ( Frisch 1973). Unique within the genus Hylobates , the molar grooves consistently conform to the ancestral hominoid condition, the Dryopithecus pattern ( Frisch 1973). Invariably, a well-developed lingual cingulum is present on the upper molars, whereas it is reduced to varying degrees in congeneric species. Supernumerary cusps commonly occur on the lower but not on the upper molars ( Frisch 1973). Both sexes exhibit dagger-like elongated upper canines that are constantly sharpened by grinding along the specialized lower first premolar ( Groves 1972).
The following basic skull and dental measurements (mm, SD in parentheses) were derived from a mixed-sex sample of nine H. moloch specimens ( Groves 1972): cranial length; 79.2 (1.45); total skull length, 100.2 (1.29); facial height, 30.9 (2.43); M 2 breadth, 5.93 (0.17); M 3 breadth, 6.47 (0.16). A data set of three-dimensional cranial landmarks of H. moloch is available for eight female and 10 male specimens ( Creel and Preuschoft 1976).
DISTRIBUTION
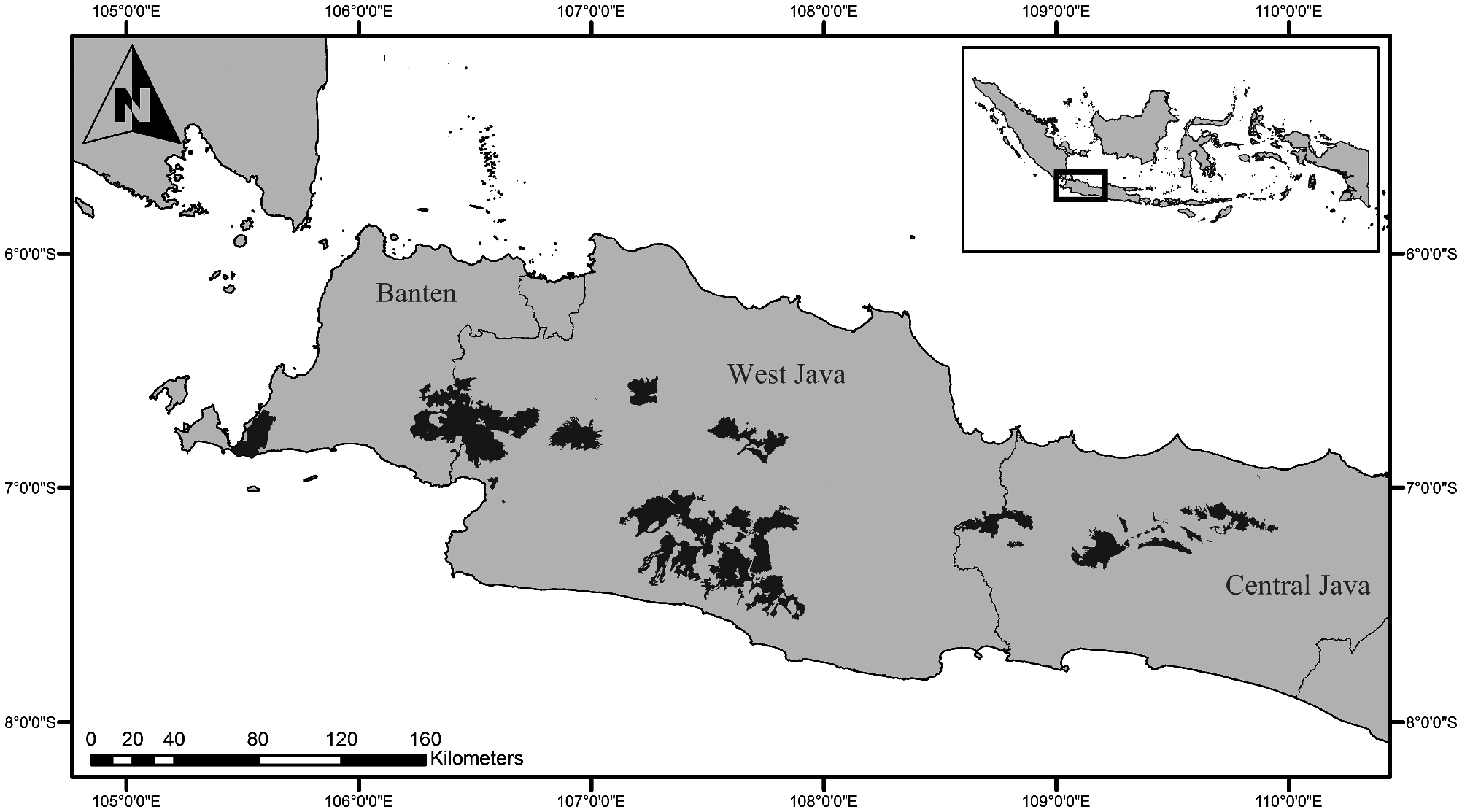
Hylobates moloch exclusively inhabits the Indonesian island of Java. It occurs in evergreen rainforests of the Banten, West, and Central provinces ( Fig. 3 View Fig — Kappeler 1984a; Nijman 1995). Although temperatures in these habitats are stable throughout the year, rainfall shows seasonal variation (S. Kim et al. 2012). For instance, at Gunung Halimun-Salak National Park in West Java, a short dry season between June and September (< 200 mm precipitation/month) and a wet season from October to December (≥ 402 mm /month) is typical (monthly precipitation average: 316 ± 175 mm, range = 63–775—S. Kim et al. 2012). Java is one of the most populated areas in the world and has lost more than 90% of its original forests, with remaining forested areas being severely fragmented ( Whitten et al. 1996; S. Kim et al. 2011; Malone et al. 2014).
Accordingly, H. moloch occurs in a number of nonconnected rainforest patches, including prominent protected areas, but also in nonprotected secondary forests, especially in Central Java ( Fig. 3 View Fig ; see “Conservation”). In western Java ( Banten and West Java provinces) it inhabits the Ujung Kulon National Park, the Gunung Halimun-Salak National Park, the Gunung Gede-Pangrango National Park, Telaga Warna, Gunung Papandayan, Gunung Buangrang, Gunung Tilu, Gunung Simpang, Gunung Wayang, and Sangga Buana. In Central Java the range includes the Dieng mountains and Gunung Slamet ( Kappeler 1981; Asquith et al. 1995; Nijman 2004; Setiawan et al. 2012; Iskandar et al. 2018).
Apart from human activity, climatic factors and elevation limit the range of H. moloch in Java. It is restricted to highcanopy lowland and submontane rainforests and does not occur in mangroves ( Kappeler 1984a). The species is only rarely found at elevations above 1,600 m ( Kappeler 1984a; Nijman 2020) but might occasionally venture into areas of up to 2,400 m (e.g., on Gunung Pangrango— Nijman 2004). At this elevation level, vegetation changes to low-canopy high montane rainforest, which cannot sustain H. moloch ( Kappeler 1984a) . Regions of the Central and East Javan provinces east to 110° longitude exhibit a significantly drier and more seasonal climate than the western portions of the island and present no suitable habitats for the species ( Kappeler 1984a). Accordingly, the restriction of H. moloch to western and Central Java primarily derives from climatic factors rather than from anthropogenic disturbance.
FOSSIL RECORD
The fossil record of small apes in general is extraordinarily sparse with the vast majority of fossils being isolated dental remains ( Harrison 2016). The oldest known hylobatids are the 12.5–13.8 million year old (middle Miocene) Kapi ramnagarensis from Jammu and Kashmir, India ( Gilbert et al. 2020) and Yuanmoupithecus xiaoyuan from the late Miocene of Yunnan, estimated to be about 7–9 million years old ( Harrison 2016). All other hylobatid fossils are noticeably younger and date to the Pleistocene or Holocene. Most fossil sites are located in continental South Asia and yield finds that are largely attributable to the extant gibbon genera ( Harrison 2016).
The oldest gibbon fossils on the island of Java date back to the early Pleistocene. With an estimated age of 0.8 million years, a partial femur from the famous Javan Trinil site, East Java (precisely the Trinil H.K. bone bed), represents the oldest hylobatid fossil known from the Sunda Islands but its generic affiliation is uncertain ( Ingicco et al. 2014). Fossils of definitive Javan Hylobates are extremely rare, as only two isolated teeth are known ( Hooijer 1960). They were found at the Sangiran and Punung A sites in Central and Eastern Java, respectively, in deposits that also bear teeth of the once abundant siamangs ( Symphalangus syndactylus ) which eventually went extinct on Java ( Hooijer 1960). An age of approximately 120,000 years is assumed for fossils of the Punung fauna, whereas 800,000 years is estimated for the Kedung Brubus fauna at Sangiran, which thus includes the oldest unambiguous occurrence of Hylobates on the island ( Ingicco et al. 2014). Although the few dental remains of Javan Hylobates are at times assigned to the extant H. moloch , their species identity remains unclear ( Hooijer 1960; Harrison 2016).
FORM AND FUNCTION
The postcranial morphology of Hylobates moloch is highly specialized for forelimb-powered suspensory locomotion in the forest canopy. As characteristic for hylobatids, it employs a combination of brachiation (arm-swinging—72%), leaping (25.6%), climbing (2%), and bipedalism (0.4%) to move through the tree tops (percentages based on observations on wild individuals by YY). Two kinds of brachiation are differentiated, the continuous and the ricochetal type. The latter is specific to the Hylobatidae and characterized by an aerial phase when swinging from one handhold to the next ( Reichard et al. 2016). This aerial phase is missing during continuous brachiation. The anatomical adaptations to support their unique style of locomotion, as described below, are uniformly shared among all small apes, but have been repeatedly described in detail for H. moloch specimens (e.g., Kohlbrügge 1890; Chapman 1900; Donisch 1973; Michilsens et al. 2009; Zihlman and Underwood 2019).
Hylobatids have strongly elongated arms and hands, which assist in brachiation ( Reichard et al. 2016). Compliant to the specific demands of brachiation, the shoulder mobility of these animals exceeds that of all other primates, including humans ( Chan 2008). The sockets of the scapulae show a permanent cranial orientation in small apes, omitting extensive rotation of the shoulder blades to raise the arms above the head ( Donisch 1973). Besides that, the hylobatid trapezius and rhomboid muscles are specially conformed to stabilize the shoulder blades against the steady gravitational pull experienced during brachiation ( Donisch 1973). A greater mobility of the upper body relative to the abdomen is realized by a caudally shortened M. latissimus dorsi, which does not insert at the pelvis, as it is the case in humans and great apes ( Donisch 1973). The humerus exhibits a 120° torsion along its axis as well as a broad trochlea, and the olecranon fossa is markedly deep, occasionally even perforated, to reinforce the elbow joint ( Groves 1972). Forelimb muscles controlling arm extension are reduced. The anconeus muscle is absent, making the M. triceps brachii, which attaches to a flat olecranon, the only elbow extensor ( Michilsens et al. 2009). On the other hand, the arm-flexing M. biceps brachii shows a unique attachment pattern in hylobatids and is specifically adapted to enhance rapid ricochetal brachiation ( Jungers and Stern 1980). Peculiarities of the hylobatid wrist were discussed by Lewis (1969).
The individual phalangeal bones of the fingers are elongated and curved to facilitate grip during swinging and climbing ( Susman et al. 1982). The well-developed thumb is highly mobile and separated from the palm of the hand by a deep cleft reaching to the base of the first metacarpal ( Zihlman and Underwood 2019). Given their elongated hands and fingers, small apes execute precision grips preferably by pressing the thumb against the radial side of the index finger ( Christel 1993). The M. accessorius interosseus, an apomorphic muscle of hylobatids, increases stability of the index finger during this type of grasping ( Susman et al. 1982). All this enables surprisingly meticulous manipulations, even of small objects such as seeds ( Christel 1993). The feet are well adapted for grasping as well and mirror the morphology of the hands in displaying curved toes and a deep cleft between the abducted opposable hallux and the remaining toes ( Vereecke and Aerts 2008). They can also be employed for object manipulation ( Torigoe 1985).
When moving on the ground or on large branches, H. moloch preferably walks bipedally. Similar to humans, small apes display a long achilles tendon that allows storage of elastic energy ( Vereecke and Aerts 2008). Nevertheless, different from humans, their feet exhibit planar soles and a flexible midfoot region which facilitate pedal grasping ( Vereecke and Aerts 2008). When walking upright, their posture is characterized by flexed knee and hip joints ( Vereecke and Aerts 2008).
There are no conspicuous specializations in regard to food processing and digestion in H. moloch . The gastrointestinal system conforms to the hominoid ground pattern and exhibits a clearly demarcated cecal appendix ( Groves 1972).
Few studies are available on the sensory biology of hylobatids and none is exclusively devoted to H. moloch . The derived morphology of the hylobatid vestibular organ facilitates their fast and agile locomotion and has been studied in detail ( Urciuoli et al. 2020). All small apes have acute trichromatic color vision. The wave length tuning of their retinal opsins resembles that of other catarrhine primates, including humans ( Hiwatashi et al. 2011). The sensitivity of other sensory channels in H. moloch and its relatives, such as hearing and olfaction, remains understudied but can be expected to largely correspond to that of great apes and humans. Despite the presence of persisting nasopalatine ducts, hylobatids lack a functional vomeronasal organ ( Maier 1997).
ONTOGENY AND REPRODUCTION
Ontogeny. —The neonatal mass of Hylobates moloch is 376 g (± SD 39.8; n = 3), falling well into the range of other Hylobates species ( Geissmann and Orgeldinger 1995). Wild infant H. moloch are weaned at an age of about 22 months (Yi 2020).
Hylobates moloch typically undergoes a noticeable fur color change during ontogeny. Infants are born with light skin and initially develop a paler fur than adults. They appear buffy gray to cream-colored and their cap is usually less conspicuous than that of older juveniles or adults ( Groves 1972; Marshall and Sugardjito 1986). Females may develop a dark pectoral patch (see “General Characters”), earliest around the age of 5 years, which initially forms at the center of the chest ( Mootnick 2006). From a behavioral perspective, around the age of 1 year, H. moloch infants acquire locomotor independence from their mothers, spending about 70% of their time farther than 1 meter away from them ( Burns 2015; Yi 2020). At Citalahab, Gunung Halimun-Salak National Park, offspring survival rate was found to be 100% until 4 years of age (0–1 years: n = 12, 1–2 years: n = 9, 2–4 years: n = 7), and 66% between 4 and 6 years of age ( n = 6), presumably due to predation (Lappan et al. in press). Immatures show a peak of solo and social play during adolescence at about 5–7 years of age ( Burns et al. 2010). When developing into subadults, they start to peripheralize themselves from the rest of the family, especially from same-sex parents ( Burns et al. 2010; also observed by YY in the wild). At this stage, they are also least groomed by other individuals among family members ( Burns 2015). Eventually, subadults disperse from their natal group and either establish territories or replace same-sex residents within established pairs. At Citalahab, Gunung Halimun-Salak National Park, a H. moloch subadult dispersed at an age of 8.8 years and three subadults of unknown age dispersed when they were assumed to be older than 9 years (Lappan et al. in press). In captivity, H. moloch females give birth to their first infant at an average age of 8.8 years (± SD 0.7; n = 11— Hodgkiss et al. 2010).
The maximum recorded longevity for H. moloch is approximately 47 years, as was reported from a wild-caught female kept at Assiniboine Park Zoo in Winnipeg, Canada ( Thompson 2018). Little is known about the life spans of wild H. moloch . Some individuals at Citalahab in the Gunung Halimun-Salak National Park are around 30 years old and are still breeding (Lappan et al. in press).
Reproduction. — Hylobates moloch forms stable breeding pairs with so far no reported cases of extrapair matings (see “Reproductive behavior”). Reproductive anatomy and physiology conform to that of other Hylobates species and have been studied in particular to inform captive-breeding efforts ( Astuti et al. 2004; Thompson 2018; Notosoediro et al. 2019). No true scrotum is developed in males, instead the testes are situated in parapenial pouches above the base of the penis ( Groves 1972). Males have a small baculum ( 4.5 mm; n = 1) while females lack a baubellum ( Groves 1972). As all haplorrhine primates, H. moloch is a spontaneous ovulator, cycling in an approximately monthly rhythm and experiencing menstruation. The average ovarian cycle calculated as days between events of menstrual bleeding is 25.6 days (± SD 1.6; n = 7) or 27.3 (± SD 3.1) days, if determined based on the recurring presence of the vulval sexual swelling ( n = 11— Hodgkiss et al. 2010). Captive H. moloch experience menarche at 6.2 years and start showing monthly sexual swellings at 6.5 years of age (± SD 1.0 and 0.7, respectively; n = 11— Hodgkiss et al. 2010). Even though sexual swellings in hylobatids are far less pronounced than in primates with naked sexual skin, such as macaques ( Macaca ) or chimpanzees ( Pan — Palombit 1995), they are conspicuous enough to be visually detected (compare H. lar — Dahl and Nadler 1992). During swelling of the vulva, the urethral eminence, the lobes of the labia minora, and portions of vaginal epithelium become exposed as tumescent pink structures, covering an area of approximately 10 cm 2 (data from H. lar — Dahl and Nadler 1992). Sexual swelling can be observed in H. moloch females throughout the entire gestation period ( n = 7 pregnancies— Hodgkiss et al. 2010), which is about 210 days ( Thompson 2018). The mean interbirth interval of captive H. moloch is 2.3 years when infants survive, and 1 year when infants die during or shortly after birth (± SD 0.4 and 0.3, respectively; n = 11— Hodgkiss et al. 2010). Wild H. moloch show an average interbirth interval of 3.65 years and no seasonal clustering of births (± SD 0.7; n = 11; Lappan et al. in press; Yi et al. 2020a). Considering the age at dispersal, interbirth intervals and an assumed longevity of 35 years, a wild female H. moloch may have around seven offspring in her lifetime. This number may be lower in reality, considering that reproductive failures have been frequently reported in other wild Hylobates species ( Palombit 1995).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Hylobates moloch ( Audebert, 1797 )
| Caspar, Kai R & Yi, Yoonjung 2022 |
Simia Moloch
| Sody H. J. V. 1949: 121 |
Hylobates lar moloch
| Sody H. J. V. 1949: 121 |
Hylobates lar pongoalsoni
| Sody H. J. V. 1949: 123 |
Hylobates moloch moloch
| Chasen F. N. 1940: 64 |
Hylobates moloch
| Frechkop S. 1934: 23 |
Hylobates cinereus cinereus
| Kloss C. B. 1929: 119 |
Hylobates lar leuciscus
| Pocock R. I. 1927: 727 |
Hylobates javanicus
| Matschie P. 1893: 62 |
Cheiron
| Burnett G. T. 1828: 307 |
Hylobates leuciscus
| Matschie P. 1893: 62 |
| Kuhl H. 1820: 6 |
Pithecus leuciscus
| Geoffroy Saint-Hilaire E. 1812: 89 |
Simia hirsuta
| Sonnerat P. 1806: 81 |
Simia cinerea
| Cuvier G. 1798: 96 |
Simia Nanodes Lichtenstein, 1791:31
| Lichtenstein A. A. H. 1791: 31 |