Polymorphida, Petrochenko, 1956
|
publication ID |
https://doi.org/ 10.12782/sd.19.2.157 |
|
persistent identifier |
https://treatment.plazi.org/id/03A7920A-3273-FF8C-E1DB-FCC8FEF0A38C |
|
treatment provided by |
Felipe |
|
scientific name |
Polymorphida |
| status |
|
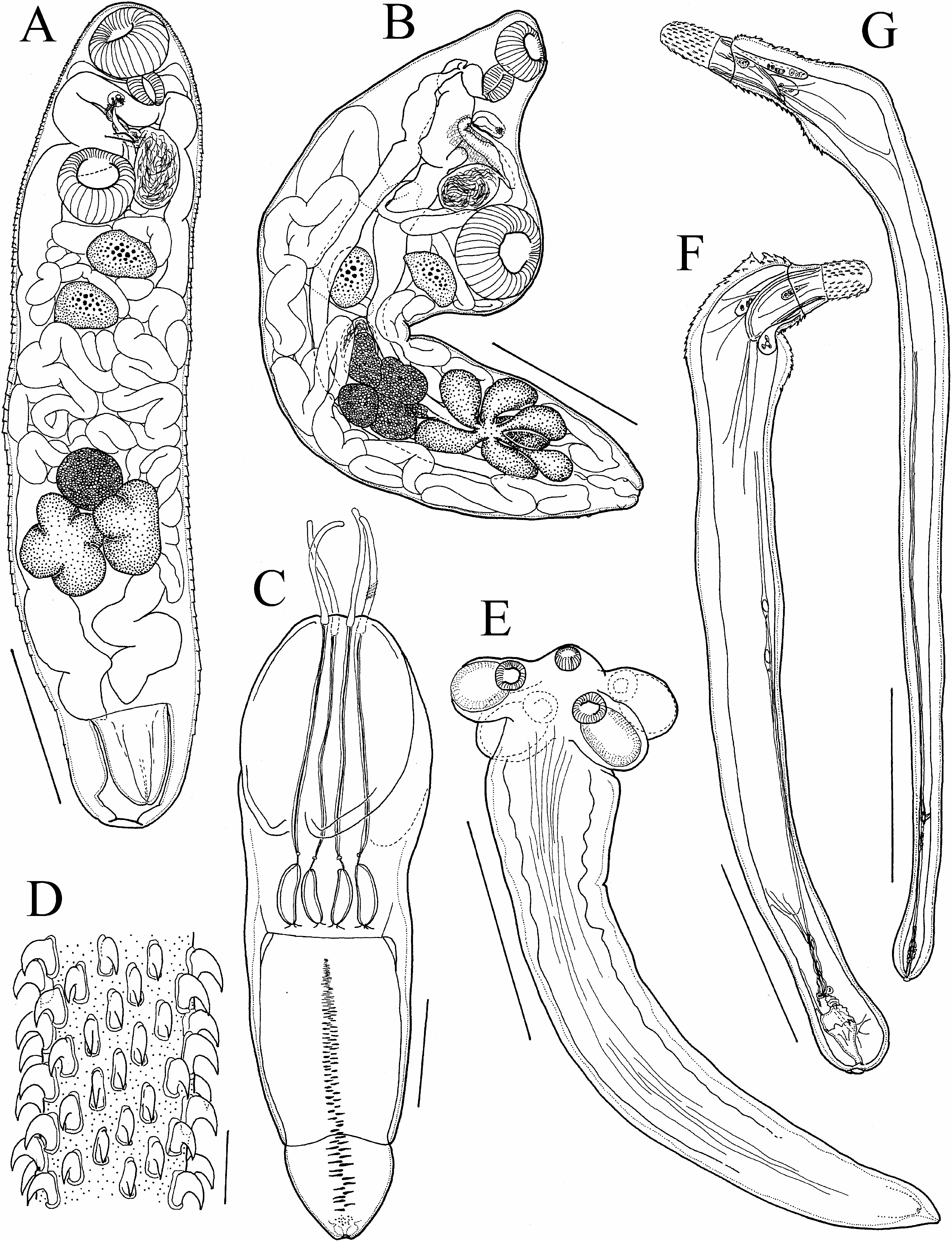
( Polymorphida View in CoL : Polymorphidae ) ( Fig. 1F–G View Fig )
Description of male. Single specimen (NSMT–As 4041) examined. Trunk elongate, slightly swollen in anterior and posterior regions with cylindrical middle region, spinose anteriorly, 8,975 long, with maximum width 1,170 at 9.7% from anterior end of trunk. Proboscis rectangular, with round anterior end, 525× 375 in length and width, armed with 20 longitudinal rows of 5 hooks each. Maximum length of hook blade 100, with root 67.5 long. Neck trapezoidal, 67.5 long. Maximum length of trunk spines 42.5. Proboscis receptacle double-walled, 470× 400 in length and width, with ganglion at midlength. Lemniscus 800 long (only one measurable). Testes oblong, undeveloped, arranged in tandem at a distance of 395 from each other, situated in middle of trunk; anterior one 165× 80 in length and width; posterior one 175× 70 in length and width. Cement glands undeveloped, indistinct, unmeasureable. Saefftigen’s pouch weakly developed, gourd-shaped, 50 in maximum diameter. Penis conical, 57.5 in maximum width. Copulatory bursa opening sub-terminally.
Description of female. Single specimen (NSMT–As 4042) examined. Trunk 10,800 long, with maximum width of 950 at 11.6% from anterior end, elongate, slightly swollen anteriorly and posteriorly, with cylindrical middle region, spinose anteriorly. Proboscis rectangular rounded anterior end, 590× 390 in length and width, armed with 19 longitudinal rows of 6 hooks each. Maximum length of hook blade 100, with root 70. Neck trapezoidal, 70 long. Maximum length of trunk spines 42.5. Proboscis receptacle 900× 350 in length and width. Lemniscus 830 (only one side measured). Uterine bell 405 long. Uterus cylindrical, 980× 42.5 in length and width. Vagina 495 long; vaginal sphincter 97.5 wide. Gonopore terminal. No eggs observed.
Remarks. Among the members of the genus Bolbosoma Porta, 1908 , three species, viz., Bolbosoma turbinella (Diesing, 1851) , Bolbosoma caenoforme (Heitz, 1919) , and Bolbosoma nipponicum Yamaguti, 1939 , have similar hook arrangements to the present specimens ( Petrochenko 1958; Yamaguti 1963). The measurements of our specimens correspond with those of B. caenoforme (see Petrochenko 1958; Arai 1989), but B. caenoforme has been suspected to be a juvenile form (post-cystacanth) of its congeners from balaenopterids and other marine mammals, especially of B. nipponicum (see Yamaguti 1963; Shimazu 1975a). Bolbosoma nipponicum is known to infect three species of balaenopterid whales, viz., the northern minke whale Balaenoptera acutorostrata Lacépède, 1804 , the sei whale Balaenoptera borealis Lesson, 1828 , and the fin whale Balaenoptera physalus (Linnaeus, 1758) ; ringed seals Phoca hispida (Schreber, 1775) ; and northern fur seals Callorhinus ursinus (Linnaeus, 1758) ( Yamaguti 1963; Kuramochi 2003); it has a large body of up to 45 mm in males and 60 mm in females. Although B. turbinella differs from B. caenoforme in that adults of the former from balaenopterid cetaceans have a characteristic trunk with a pinched anterior region and a longer proboscis (about 800 µm) ( Petrochenko 1958), it is not inconceivable that the two are conspecific. Further morphological and genetic analyses are needed to solve this taxonomic issue.
Specimens identical to B. caenoforme have been found in euphausiid crustaceans, which probably act as first intermediate hosts ( Shimazu 1975a). Pacific salmon Oncorhynchus spp. and Alaska pollock Theragra chalcogramma (Pallas, 1814) appear to be the second intermediate or paratenic hosts ( Yamaguti 1963). As was indicated above, adults of this acanthocephalan almost certainly are found from marine mammals, which act as the definitive hosts, but their presence in piscivorous birds, such as the common murre Uria aalge inornata Salomonsen, 1932 , is also possible ( Yamaguti 1963).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.