Platevindex coriaceus coriaceus ( Semper, 1880 )
|
publication ID |
https://doi.org/ 10.5852/ejt.2021.737.1259 |
|
publication LSID |
lsid:zoobank.org:pub:FE4ED74A-3FE6-4CA6-A116-CB3AF46826F7 |
|
DOI |
https://doi.org/10.5281/zenodo.4602467 |
|
persistent identifier |
https://treatment.plazi.org/id/03A6D248-FFBF-8B65-DEC1-F9FAFD78D1AA |
|
treatment provided by |
Plazi |
|
scientific name |
Platevindex coriaceus coriaceus ( Semper, 1880 ) |
| status |
|
Platevindex coriaceus coriaceus ( Semper, 1880) View in CoL
Figs 11–21 View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig
Onchidium coriaceum Semper, 1880 View in CoL : pl. 19, figs 1, 16, pl. 23, fig. 12.
Onchidella condoriana Rochebrune, 1882: 67 View in CoL . Syn. nov.
Oncis semperi Plate, 1893: 192–193 View in CoL . Syn. nov.
Onchidium coriaceum View in CoL – Semper 1882: 271–273, pl. 21, fig. 7.
Oncis coriacea View in CoL – Plate 1893: 190–191. — Stantschinsky 1907: 395.
Oncis semperi View in CoL – Stantschinsky 1907: 395.
Platevindex coriaceus View in CoL – Baker 1938: 88.
Platevindex mortoni – Sun et al. 2014: 63 [ non Platevindex mortoni Britton, 1984 ].
Material examined
Lectotype of Onchidium coriaceum
PHILIPPINES • lectotype (here designated; 20/ 16 mm); ZMB/Moll 39028a .
Paralectotypes of Onchidium coriaceum
MALAYSIA • 1 paralectotype; Penang; ZMB/Moll 39029 .
PHILIPPINES • 6 paralectotypes; ZMB/Moll 39028b .
SINGAPORE • 2 paralectotypes; Semper leg.; ZMB/Moll 39030 .
Holotype of Onchidella condoriana
VIETNAM • holotype (23/ 16 mm), by monotypy; Poulo-Condor [Côn Son, the largest island of the Côn Ðảo archipelago, off the coast of southern Vietnam]; 1876; Dr Harmand leg.; MNHN-IM-2000-22952 .
Syntypes of Oncis semperi
PHILIPPINES • 2 syntypes (25/20 and 21/ 20 mm); Mindanao; ZMB/Moll 45661 .
Notes on type material
Onchidium coriaceum . According to the original description, the lectotype and paralectotypes from the Philippines were collected from Bohol, Manila (Luzon) and Zamboanga (Mindanao). However, they are all mixed together in the same jar and nothing allows us to assign them to Bohol, Manila or Zamboanga. Thus, the type locality is simply “ Philippines ”. The lectotype is selected because it is well preserved and was dissected for the present study (all internal organs remain in the specimen); the anatomy of the lectotype matches perfectly the characters observed in our recently-collected material (retractor muscle inserting at the posterior end of the visceral cavity, penis almost as long as the visceral cavity, intestinal loops of type II, deferent duct attached to the surface of the oviduct and tightly coiled with many U-turns). All other syntypes become paralectotypes. All six paralectotypes (30/22 to 23/ 16 mm) from the Philippines (ZMB/Moll 39028b) were dissected previously, likely by Semper himself or Plate (1893). Three of these paralectotypes (30/22, 28/28 and 23/ 16 mm) are missing most of the reproductive and digestive systems, but parts of the oviduct, intestine and rectal gland remain; one paralectotype (23/ 16 mm) corresponds to only the left half of a slug with some organs remaining; two paralectotypes (27/26 and 20/ 16 mm) were previously dissected but all organs remain. The large (22/ 10 mm) paralectotype from Singapore (ZMB/Moll 39030) was previously dissected and its penial apparatus is missing; the notum of a small (20/ 17 mm) paralectotype from Singapore (ZMB/Moll 39030) was cut open for the present study to check several characters. The paralectotype (27/ 25 mm) from Penang (ZMB/Moll 39029) was previously dissected and the penial complex is missing; the deferent duct in its posterior reproductive system is not attached to the oviduct, indicating that this paralectotype is not part of the species described here but, instead, is a misidentification of P. martensi . Semper (1882: 272) also mentioned some material (likely only one specimen) collected in Brisbane (Queensland, Australia) which could not be located. While Queensland is part of the geographic distribution of the species described here, that material from Brisbane could belong to P. luteus . Finally, Semper (1882: 272) mentioned two specimens from “des Wiener Museums” (the Vienna Museum) but from an unknown locality. Due to the presence of at least two species of Platevindex in the type material (certainly P. coriaceus and P. martensi , and possibly P. luteus ; see Remarks for more details), it is necessary to designate a lectotype to clarify the application of the name P. coriaceus .
Onchidella condoriana . The radula, the penial complex and most of the posterior (female) reproductive parts were all previously removed and are missing from the holotype. Some parts remain though (digestive gland, intestine, oviduct of the posterior reproductive parts). It is unclear how many specimens were used by Rochebrune (1882) for the original description, but only one specimen is present at the MNHN and it is regarded as the holotype (there is no evidence suggesting that Rochebrune examined more than one specimen).
Oncis semperi . Both syntypes were previously emptied of all their internal organs, likely by Plate himself. Two stomachs and some pieces of digestive glands remain in the jar but the type of intestinal loops could not be checked. Plate’s description of intestinal loops of type I ( Plate 1893) is inconsistent with P. coriaceus but it was most likely a mistake (see our remarks below). The designation of a lectotype would be pointless because the two syntypes are externally similar and it is unclear whether Plate’s description was mostly based on the largest specimen or both specimens.
Other material
BRUNEI • 1 spec. (30/27 [1031] mm); Pulau Kaingara; 04°57.020′ N, 115°01.785′ E; 28 Jul. 2011; station 33; open mangrove with Rhizophora trees and logs, by the river; BDMNH GoogleMaps • 1 spec. (25/20 [1029] mm); near Batu Marang; 04°59.131′ N, 115°01.820′ E; 29 Jul. 2011; station 34; old mangrove with tall Rhizophora trees with high roots and Thalassina mounds; BDMNH. GoogleMaps
INDONESIA – Sumatra • 1 spec. (38/29 [1772] mm); Pulau Sinaboi; 02°18.145′ N, 100°59.309′ E; 8 Oct. 2012; station 73; some small Avicennia and Rhizophora near shore; UMIZ 00075 GoogleMaps • 2 specs (30/19 [1735] and 24/20 [1731] mm); Kualapenet ; 05°16.275′ S, 105°51.287′ E; 17 Oct. 2012; station 77; narrow band of mangrove between ocean and fish ponds; UMIZ 00076 GoogleMaps . – Sulawesi • 1 spec. (49/34 [2190] mm); Wawontulap; 01°19.275′ N, 124°31.053′ E; 11 Mar. 2013; station 86; Rhizophora mangrove with some Avicennia and dead logs; UMIZ 00078 . GoogleMaps – Lombok • 2 specs (35/23 [3000] and 29/18 [3001] mm); Kayangan Bay , Bajo Village ; 08°29.491′ S, 116°39.719′ E; 28 Mar. 2014; station 151; mangrove of small, sparse Avicennia trees, and a few older trees; UMIZ 00079 GoogleMaps .
MALAYSIA – Peninsular Malaysia • 1 spec. (30/26 [941] mm); Sungai Ular ; 03°56.512′ N, 103°22.320′ E; 14 Jul. 2011; station 20; dense forest of mostly Rhizophora , higher intertidal; USMMC 00025 GoogleMaps • 3 specs (35/21 [937], 28/21 [940] and 25/29 [918] mm); Merbok ; 05°39.035′ N, 100°25.782′ E; 18 Jul. 2011; station 21; deep Rhizophora forest with old, tall trees, hard mud, many small creeks and dead logs; USMMC 00026 GoogleMaps • 1 spec. (24/18 [935] mm); Matang , close to Crocodile River; 04°49.097′ N, 100°37.37′ E; 19 Jul. 2011; station 28; old and open Rhizophora forest with tall trees, hard mud, creeks, and many dead logs; USMMC 00027 GoogleMaps .
PHILIPPINES – Luzon • 3 specs (44/26 [3193], 31/17 [3170] and 21/14 [3194] mm); Nasugbu, Batangas; 14°10.714′ N, 120°36.817′ E; 6 Jul. 2014; station 182; dense forest of Avicennia and Rhizophora ; PNM 041236 GoogleMaps . – Bohol • 3 specs (30/29 [3247], 28/18 [3245] and 26/15 [3248] mm); Inabanga; 10°00.389′ N, 124°03.522′ E; 12 Jul. 2014; station 186; rehabilitated fish ponds with mostly young Rhizophora ; PNM 041237 GoogleMaps • 2 specs (33/17 [3256] and 27/19 [3257] mm); Inabanga; 10°04.432′ N, 124°04.691′ E; 13 Jul. 2014; station 188; old forest of mostly Avicennia with many dead logs; PNM 041238 GoogleMaps • 2 specs (37/25 [3293] and 34/24 [3290] mm); Mabini; 09°51.532′ N, 124°31.685′ E; 17 Jul. 2014; station 194; narrow mangrove on the edge of fish ponds, tall Rhizophora and Avicennia , many dead logs; PNM 041239 GoogleMaps • 1 spec. (34/22 [3356] mm); Mabini; 09°51.402′ N, 124°30.982′ E; 18 Jul. 2014; station 195; narrow Rhizophora and Avicennia mangrove by the sea with fish ponds built on landward side, cement ditches between the mangrove patches and the ponds; PNM 041240 GoogleMaps .
SINGAPORE • 1 spec. (28/20 [998] mm); Lim Chu Kang ; 01°26.785′ N, 103°42.531′ E; 2 Apr. 2010; station 7; mud outside mangrove on sun-exposed mudflat; ZRC.MOL.10475 GoogleMaps .
VIETNAM • 1 spec. (32/15 [5625] mm); Can Gio mangrove forest ; 10°24.430′ N, 106°53.878′ E; 11 Jul. 2015; station 222; Avicennia and Rhizophora mangrove patch by road; ITBZC IM 00012 GoogleMaps • 2 specs (38/16 [5629] and 27/23 [5657] mm); Can Gio mangrove forest ; 10°27.804′ N, 106°53.289′ E; 16 Jul. 2015; station 228; open forest of tall Rhizophora , hard mud; ITBZC IM 00014. GoogleMaps
Description
Color and morphology of live animals ( Fig. 11 View Fig )
Live animals are not usually covered with mud, and their natural color can be observed without washing. The dorsal notum is typically dark brown, dark grey or black and often has some lighter brown longitudinal bands or mottling. The dorsal surface is typically bumpy, granular and not smooth. The color of the hyponotum in unit #1 (from Peninsular Malaysia to China and Sulawesi) is grey or blue-grey, or occasionally light grey ( Fig. 11 View Fig J–K), while in unit #2 ( Philippines) it is generally dark grey or dark blue ( Fig. 11 View Fig D–E). The margin of the hyponotum may be bright white, which is especially common in unit #2. No specimens or pictures of the hyponotum were available for unit #3 from China or Japan (only a photo of the dorsal notum was published by Takagi et al. 2019). The foot is light yellow in units #1 and #2. The ocular tentacles are frequently retracted when animals are observed in the field, but extended ocular tentacles are brown and short (up to 4 mm long). Inferior to the ocular tentacles, superior to the mouth, the head bears a pair of oral lobes. On each oral lobe, there is an elongated bump or protuberance, likely with sensitive receptors.
Dorsal gills are absent. Papillae with dorsal eyes are present. The exact number of papillae bearing dorsal eyes is variable (between 20 and 68 in unit #1 and between 17 and 45 in unit #2), with the largest animals bearing the largest numbers of eyes. The number of eyes on each papilla is one. The dorsal eyes are distributed across the notum but are absent on the margin (are never <2 mm from the notum edge).
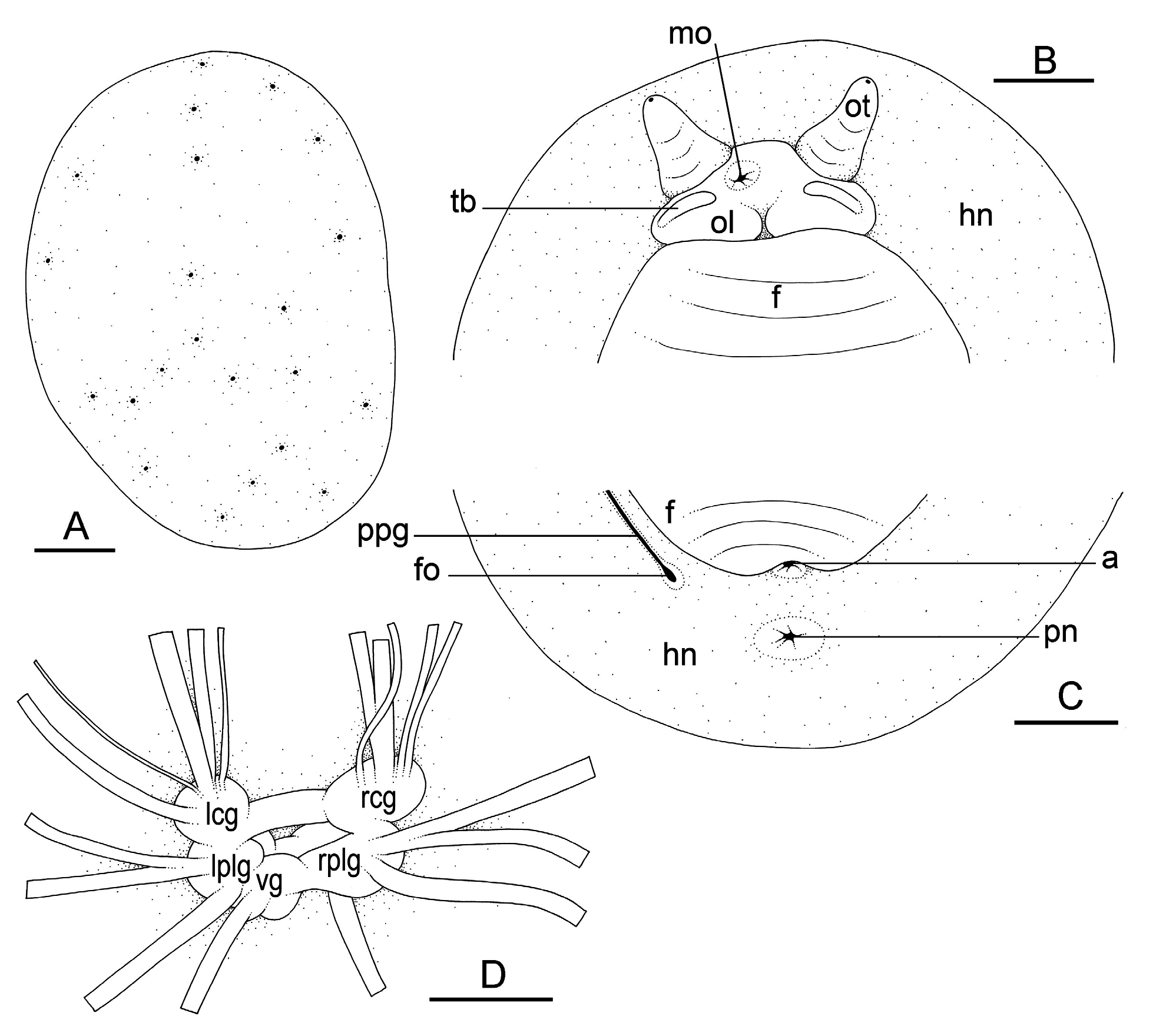
External morphology ( Fig. 12 View Fig A–C)
The notum is oval and the body is dorso-ventrally flattened ( Fig. 12A View Fig ). The foot is narrow in proportion to the hyponotum and is approximately ¼ to ⅓ of the total width. The anus is posterior, median, close to the edge of the pedal sole. The pneumostome is median and is near the anus (closer to the anus than to the posterior margin of the hyponotum) ( Fig. 12C View Fig ). On the right side (to the left in ventral view), a peripodial groove is present at the junction between the pedal sole and the hyponotum, running longitudinally from the buccal area to the posterior end and ending with the female opening. The female opening is approximately 1 to 3 mm from the anus in most individuals but may be up to 6 mm away in large specimens (observed in unit #1). The male aperture is below the ocular tentacles, almost centered between them, but slightly to the right side ( Fig. 12B View Fig ).
Visceral cavity and pallial complex
The heart is enclosed in the pericardium, on the right side of the visceral cavity, slightly posterior to the middle. The large, anterior ventricle becomes a large aorta that branches into smaller vessels delivering blood to the visceral organs. The auricle, significantly smaller than the ventricle, is posterior. The pericardium communicates through a small hole with the right portion of the renal-pulmonary complex. The kidney is intricately attached to the pulmonary cavity, which is slightly asymmetrical, the right part being slightly larger than the left part.
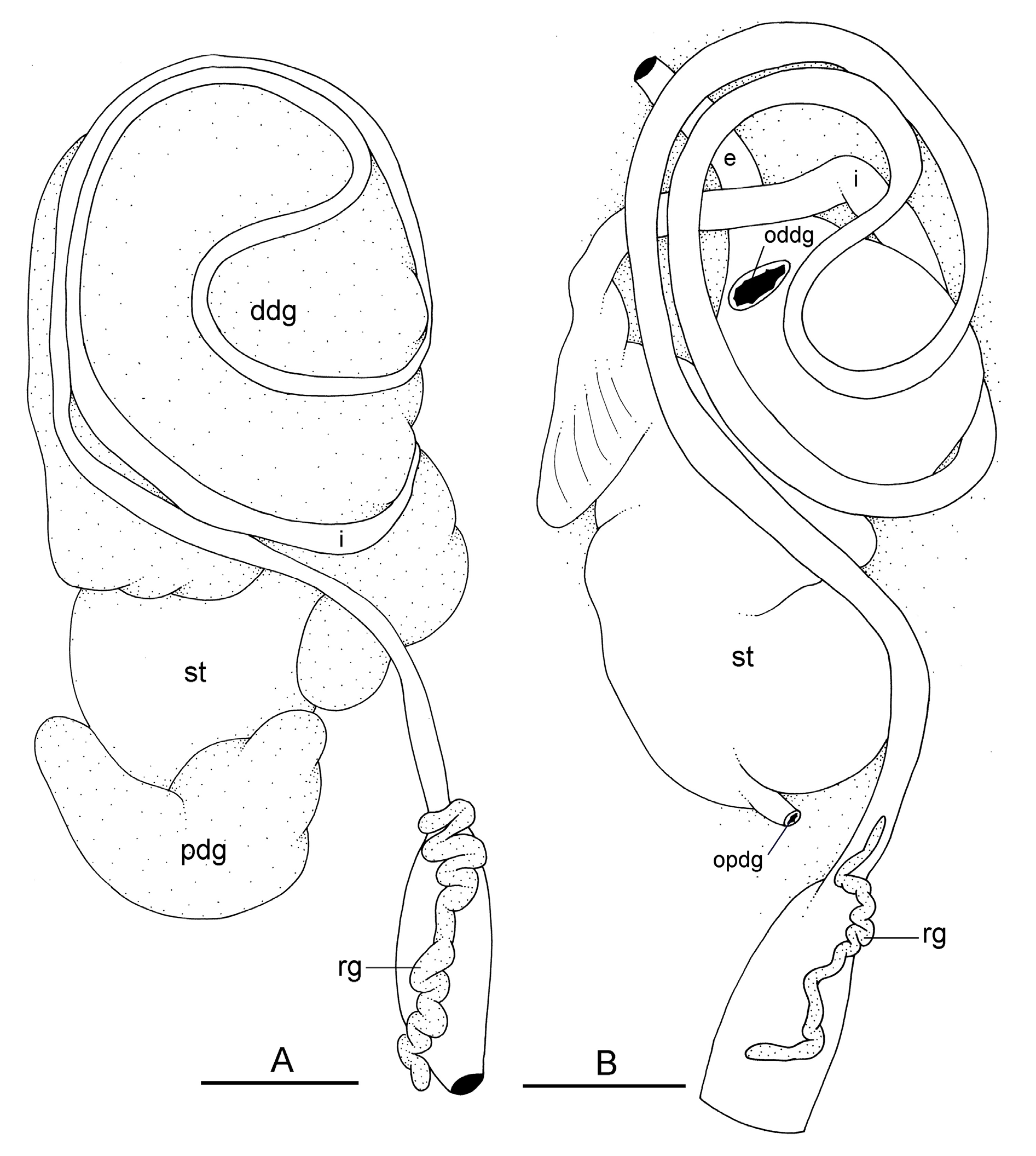
Digestive system ( Figs 2B, H View Fig , 13–17A View Fig View Fig View Fig View Fig View Fig )
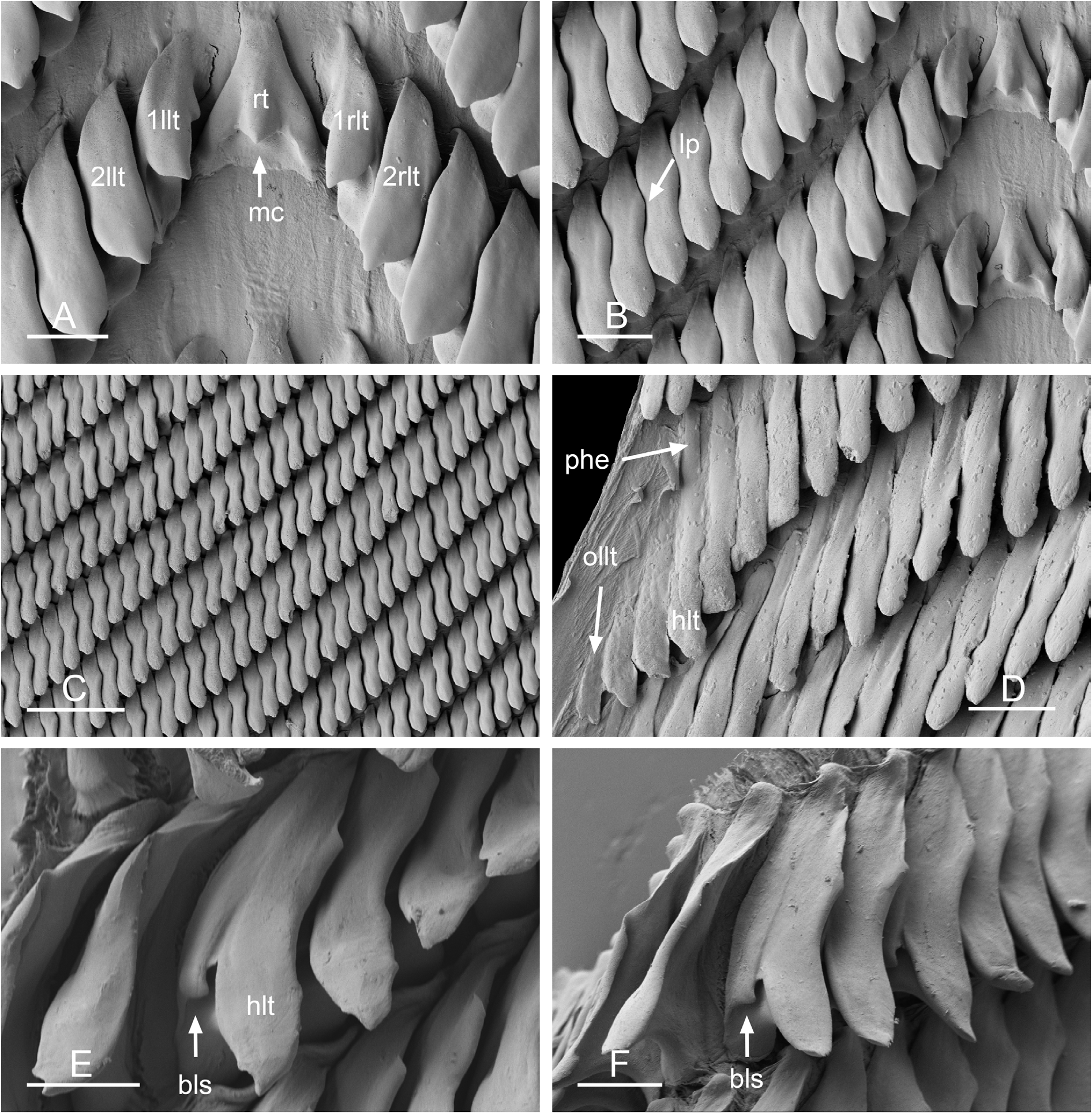
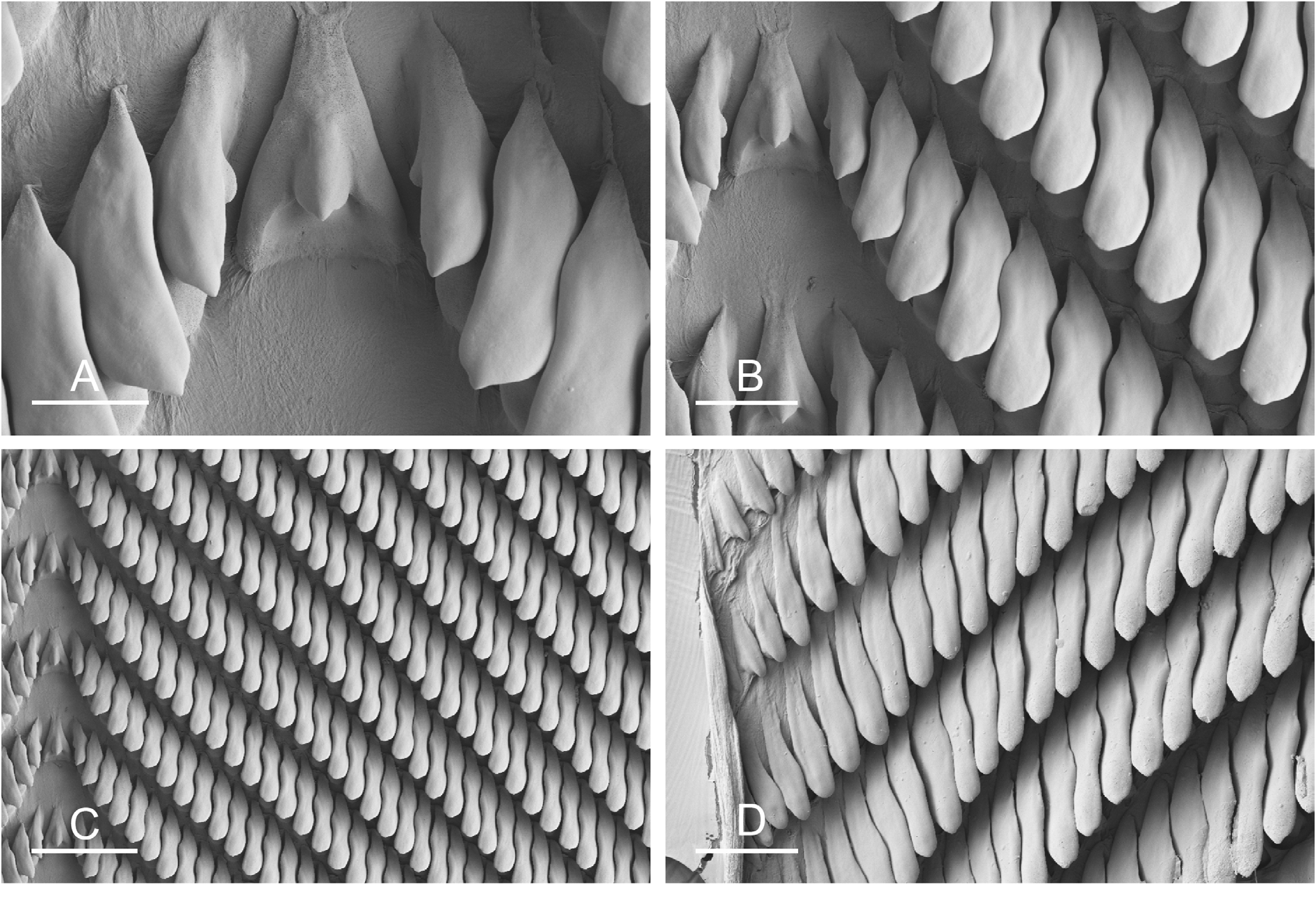
There is no jaw. The left and right salivary glands have numerous branches and join the buccal mass dorsally on either side of the esophagus. The radula is located between two large posterolateral muscular masses. Radulae measure up to 6.1 mm in length (unit #1) and 4.7 mm (unit #2). Each radular row contains a central rachidian tooth and two, left and right, half rows of lateral teeth. Examples of radular formulae are presented in Table 5 View Table 5 . The half rows of lateral teeth form an angle of 45° with the rachidian axis. The rachidian teeth are unicuspid: a median cusp is always present; there are no distinct lateral cusps ( Figs 13A View Fig , 14A View Fig ). The lateral aspect of the base of the rachidian teeth is straight (neither concave nor convex). The length of the cusp of each rachidian tooth is approximately 15–35 µm, significantly smaller than that of the lateral teeth. The hook of the lateral teeth is extended posteriorly by a tail-like structure attaching to the radular membrane and making the hook look longer. The tail-like structure (posterior hook extension, Fig. 13D View Fig ) is most noticeable in the outermost lateral teeth. The lateral teeth are unicuspid with a flattened hook. The tip of the hook may be rounded or pointed, which varies between individuals and even within a particular radula. Lateral teeth are approximately 35 to 75 µm long, and their length gradually increases along the half row, excluding the few innermost lateral teeth and outermost lateral teeth which are significantly smaller ( Fig. 13B, D View Fig ). The lateral teeth bear an outer pointed spine on the lateral expansion of the base. In most cases, the basal lateral spine cannot be observed because it is hidden below the hook of the adjacent lateral tooth. It can only be observed when the teeth are not too close together or when the teeth are turned in an unusual position ( Fig. 13 View Fig E–F). The inner lateral aspect of the hook of the lateral teeth is not straight. It is marked by a moderate protuberance placed over the inner adjacent tooth. That protuberance is slight and rounded (not pointed) and makes the inner aspect of the hook look wavy. The protuberance diminishes in the outermost teeth, and, as a result, their lateral aspect is almost straight.
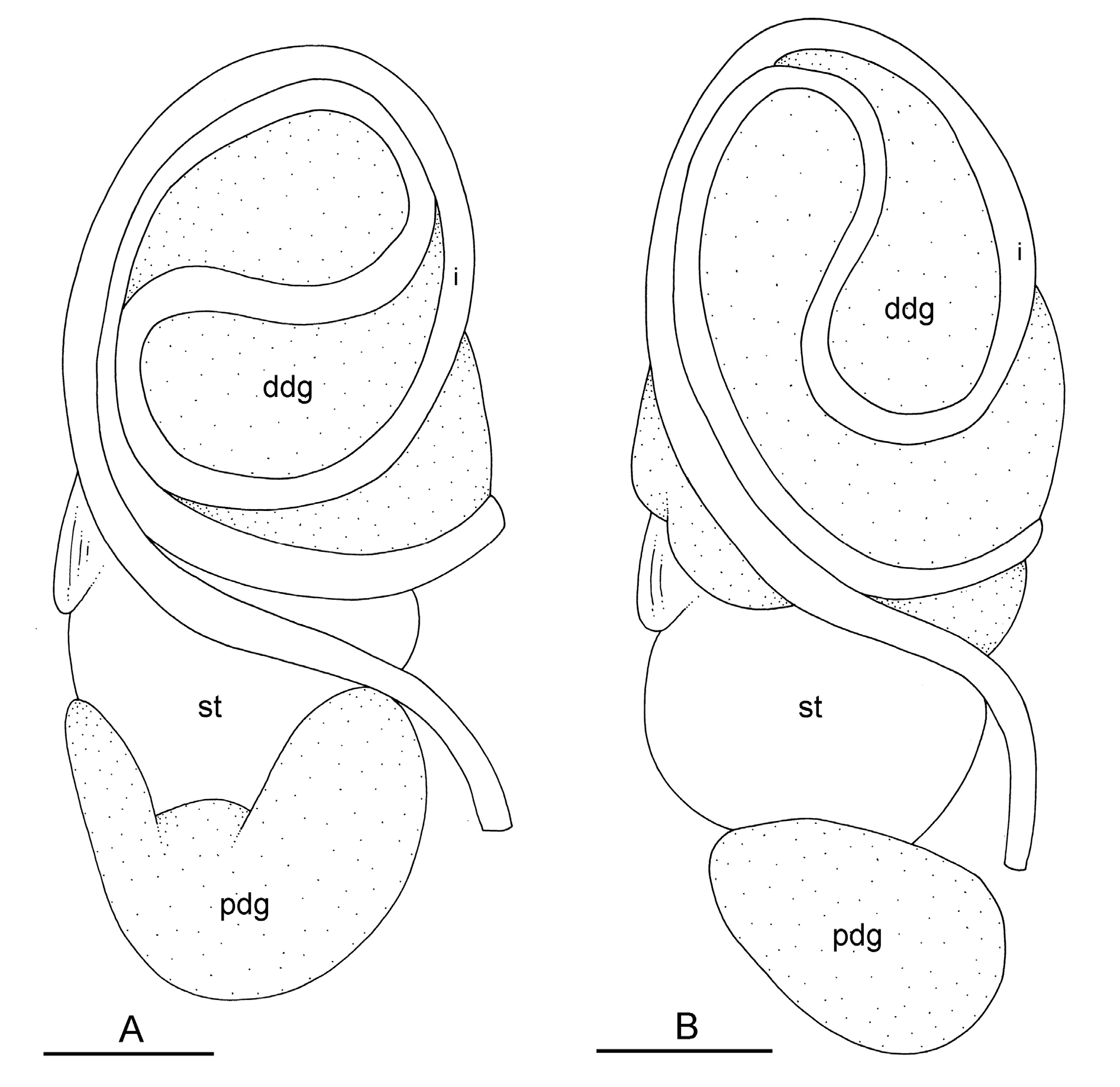
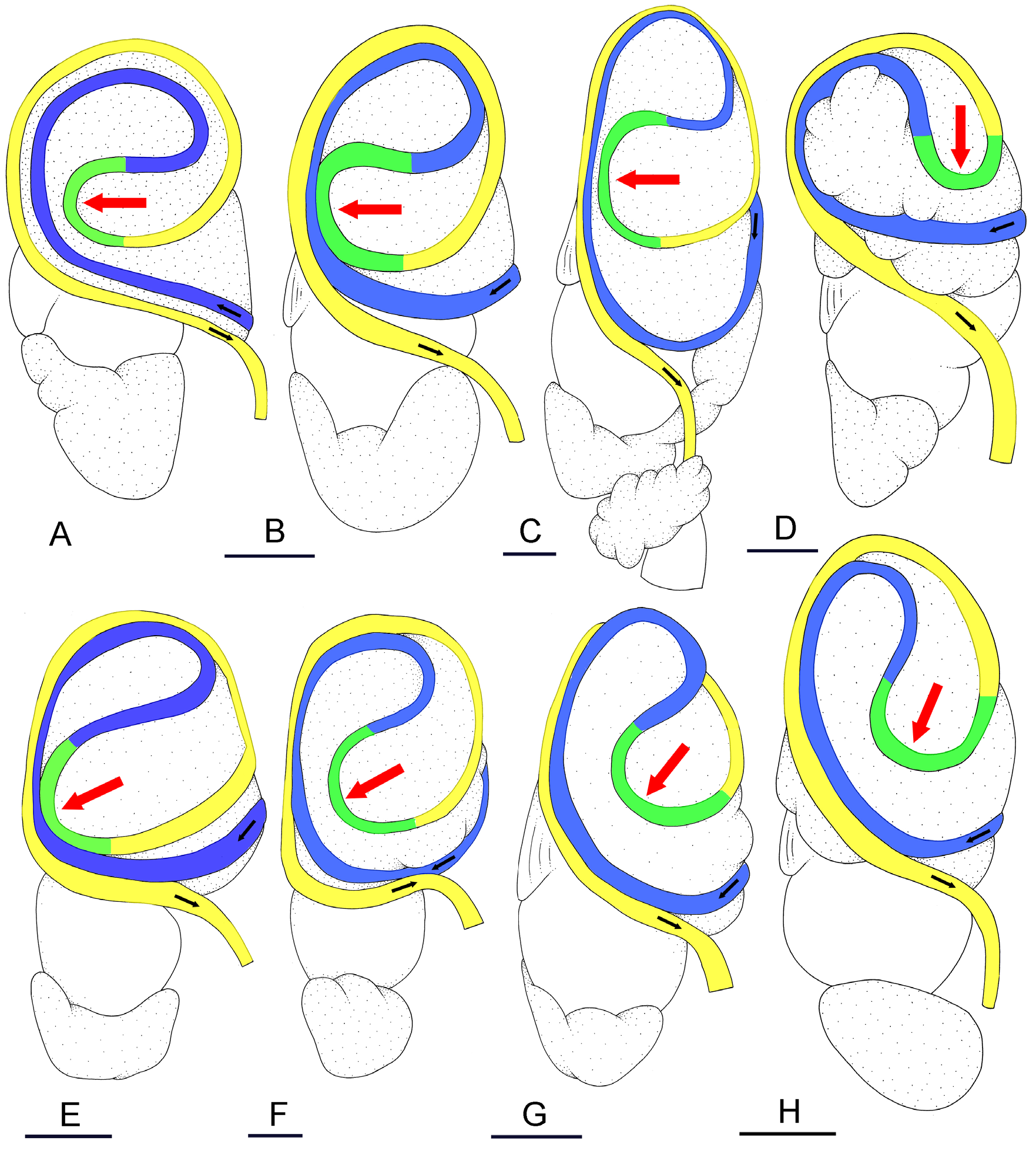
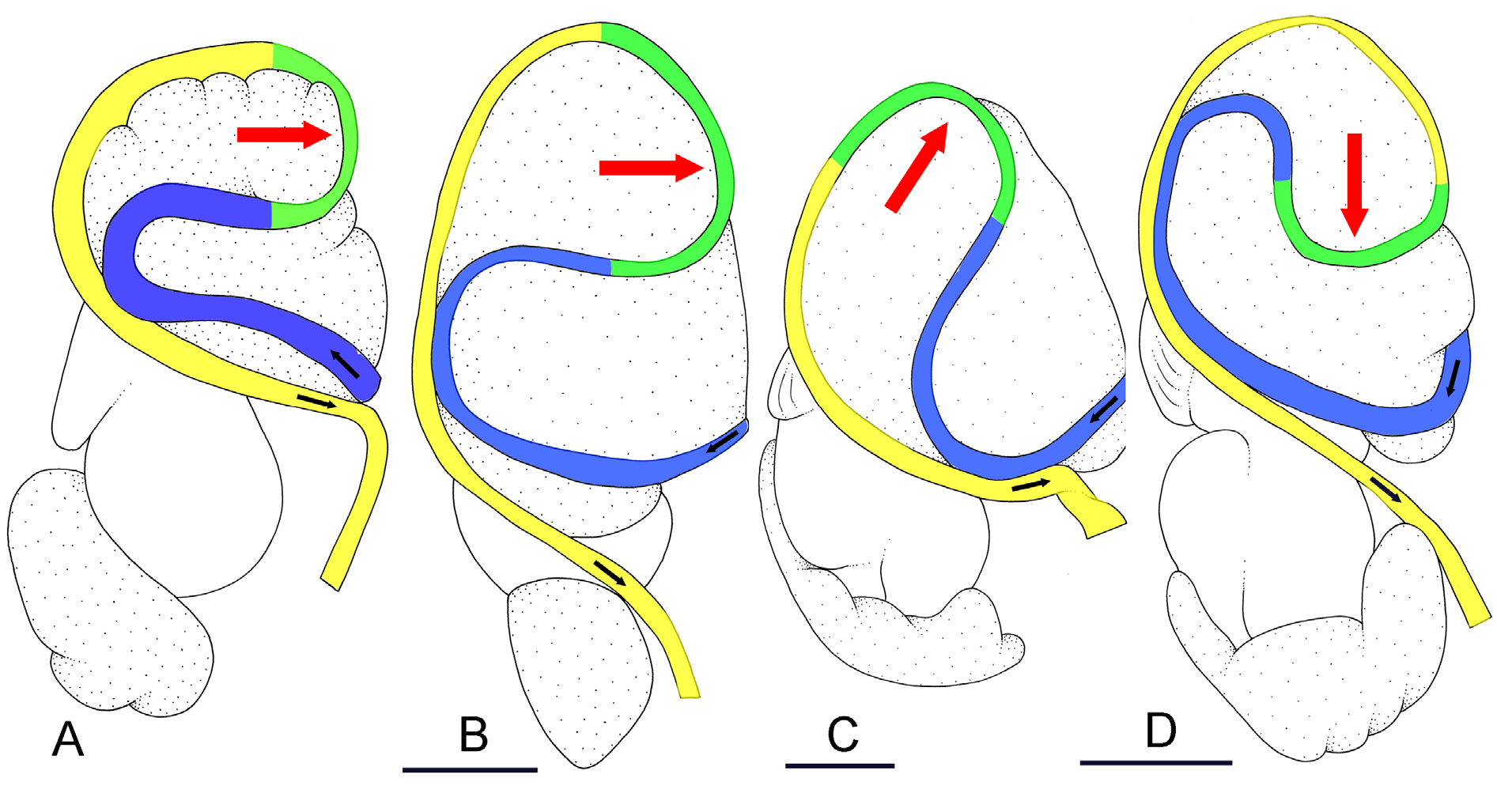
The esophagus, narrow and straight, enters the stomach anteriorly. The stomach is located on the left, dorsal side of the visceral mass. In dorsal view, only a portion of the stomach can be seen because it is partly covered by the lobes of the digestive gland. The dorsal lobe is mainly on the right. The left, lateral lobe is mainly ventral. The posterior lobe covers the posterior aspect of the stomach. The stomach is a U-shaped sac divided into four chambers ( Fig. 15 View Fig C–D). The first chamber, just distal to the esophagus, is delimited by a thin layer of tissue, and receives the ducts of the dorsal and left lateral lobes of the digestive gland. The second chamber is delimited by a thick, muscular layer of tissue and receives the duct of the posterior lobe of the digestive gland. In the third chamber of the stomach, thick ridges extend towards the middle of the chamber. The fourth chamber is externally similar to the third chamber but is characterized by much lower and thinner internal ridges. The intestine is long and narrow, with intestinal loops of type II. The transitional loop is oriented between 7 and 9 o’clock in unit #1 ( Fig. 16 View Fig ), and between 6 and 9 o’clock in unit #2 ( Figs 2B, H View Fig , 16–17 View Fig View Fig ). A rectal gland is present.
Nervous system ( Fig. 12D View Fig )
The circum-esophageal nerve ring is post-pharyngeal and pre-esophageal. The cerebral commissure between the paired cerebral ganglia is short but its length varies among individuals. The paired pleural and pedal ganglia are also all distinct. The visceral commissure is short but distinctly present and the visceral ganglion is approximately median. Cerebro-pleural and pleuro-pedal connectives are very short, and pleural and cerebral ganglia touch each other. Nerves from the cerebral ganglia innervate the buccal area and the ocular tentacles, and, on the right side, the penial complex. Nerves from the pedal ganglia innervate the foot. Nerves from the pleural ganglia innervate the lateral and dorsal regions of the mantle. Nerves from the visceral ganglion innervate the visceral organs.
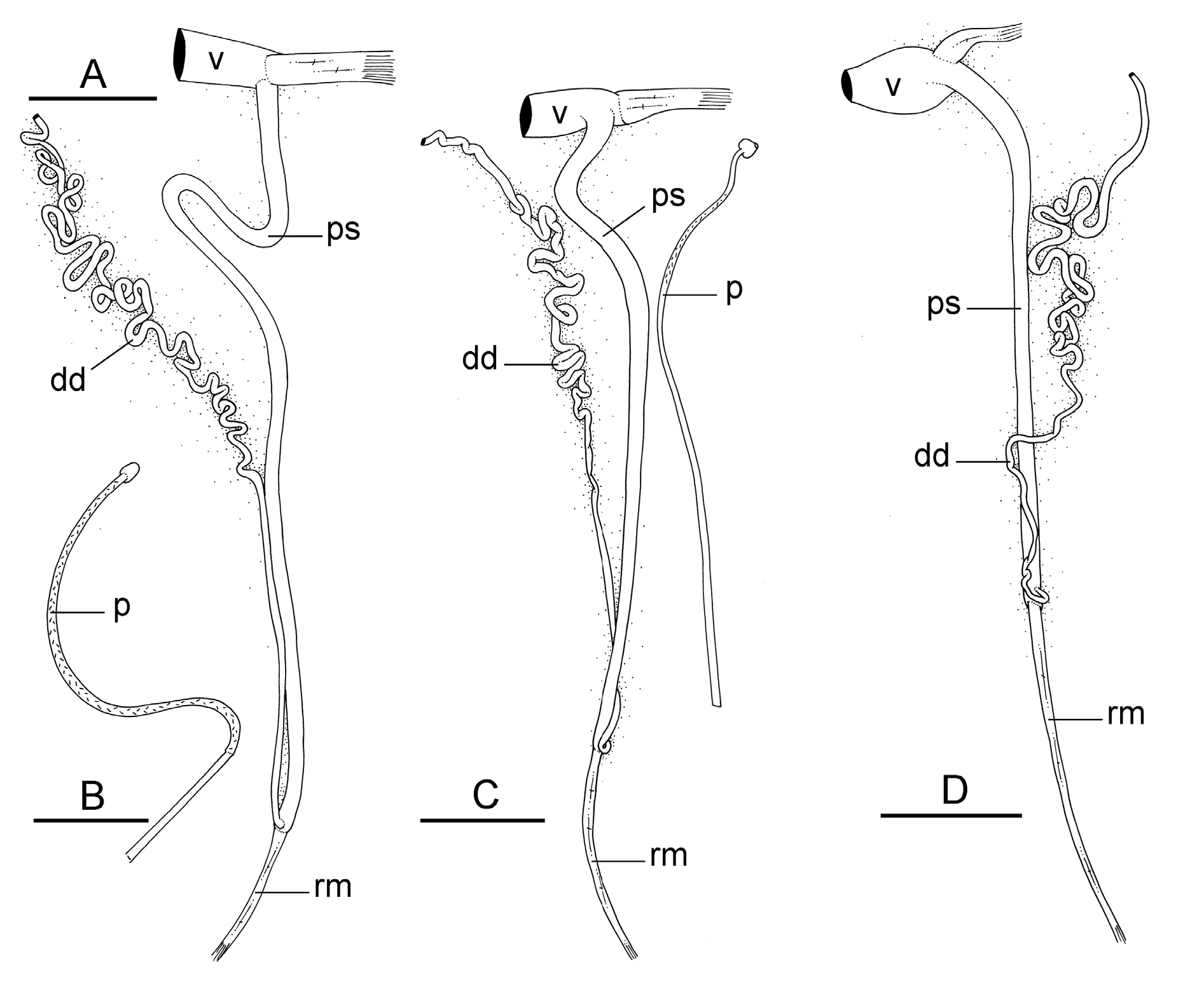
Reproductive system ( Figs 18–19 View Fig View Fig )
Sexual maturity is correlated with animal length. Mature individuals have large, posterior, female (hermaphroditic) organs and fully-developed, anterior, male copulatory parts. Immature individuals (<22 mm long) may have small or simply no posterior female organs, and rudimentary male parts. Hermaphroditic organs are located at the posterior end of the visceral cavity and consist of the female parts mixed with some male parts ( Fig. 18 View Fig ). The hermaphroditic gland is a single mass. A hermaphroditic duct conveys the eggs and the autosperm from the hermaphroditic gland to the fertilization chamber, which connects to a large receptaculum seminis (caecum). The receptaculum seminis is generally elongated but its shape varies and it may be oval-shaped or almost spherical. The female gland mass contains various glands (mucus and albumen) of which the exact connections remain uncertain. The spermoviduct (for autosperm, exosperm and eggs) is embedded within the female gland mass, at least proximally. Distally, the spermoviduct branches into the deferent duct (which conveys the autosperm to the anterior region, running through the body wall) and the oviduct. The latter conveys the eggs up to the female opening and the exosperm from the female opening to the fertilization chamber. The section of the oviduct distal to the spermatheca is long, up to three or four times the length of the proximal section (from the female gland mass to the spermatheca). The oviduct is much wider (at least two or three times wider) than the deferent duct. The latter is closely attached to the surface of the oviduct, and is highly coiled, with U-shaped turns back and forth. The spherical spermatheca connects to the distal portion of the oviduct through a short duct.
The male anterior organs include the penial complex (penis, vestibule, deferent duct, retractor muscle) ( Fig. 19 View Fig ). There is no accessory penial gland. The penial sheath protects the penis for its entire length. The vestibule is continuous with the distal part of the penial sheath. The vestibule is cylindrical. Short muscle fibers connect the distal part of the penial sheath, near the vestibule, to the anterior wall of the visceral cavity. Inside the penial sheath, the penis is a narrow, thin, elongated, hollow tube. Its proximal region is rigid. Its distal region is flexible and bears hooks measuring from 80 to 130 μm ( Fig. 20 View Fig ). The flexible region of the penis with hooks is generally between 4 and 8 mm long and approximately 160 to 190 µm wide. The hooks are inside the tube-like penis when it is inside the penial sheath (but can be seen by transparency) and are outside when the penis is evaginated like the finger of a glove. The penial sheath measures from approximately ¾ the length of the visceral cavity to nearly the entire length of the visceral cavity. The beginning of the retractor muscle marks the separation between the penial sheath and the deferent duct. The retractor muscle inserts at the posterior end of the body cavity near the rectum (in mature specimens). The length of the retractor muscle is variable. In unit #1, the retractor muscle is either shorter than the penial sheath (as short as ½ of its length) to almost equal to it. In unit #2, the retractor muscle is shorter than the penial sheath (between ⅓ and ½ of its length). The deferent duct is highly convoluted, with many loops, though it is less convoluted in immature specimens.
Distinctive diagnostic features ( Table 4 View Table 4 )
Externally, P. coriaceus coriaceus cannot be confused with the only two species of Platevindex which lack dorsal eyes ( P. amboinae and P. latus ). Externally, the blue-grey hyponotum and light yellow foot differentiate P. coriaceus coriaceus from P. martensi (orange foot) and P. aptei sp. nov. (black foot). Platevindex coriaceus coriaceus can generally be distinguished from P. luteus and P. applanatus by the presence of distinctly-raised dorsal papillae in the two latter species (which are absent in P. coriaceus ). Large individuals of P. coriaceus coriaceus (> 40 mm long) can be distinguished from P. luteus , P. applanatus and P. burnupi (<30 mm) by their size. Small individuals of P. coriaceus coriaceus could potentially be confused with individuals of P. burnupi (which, unlike P. luteus and P. applanatus , lack distinctly-raised dorsal papillae). However, note that based on current data, P. burnupi is only found in the western Indian Ocean and does not overlap geographically with P. coriaceus coriaceus . In addition, P. coriaceus coriaceus is distinct from P. luteus , P. applanatus and P. burnupi based on several details of the reproductive and digestive systems ( Table 4 View Table 4 ). For instance, the deferent duct is closely attached to the oviduct in P. coriaceus coriaceus while it is not (or only loosely) attached to it in P. luteus and P. burnupi . Internally, also, the intestinal loops are of type II in P. coriaceus coriaceus and of type I in P. applanatus .
The only species with which Platevindex coriaceus coriaceus overlaps geographically and cannot be distinguished externally is P. tigrinus , due to a similar foot color (variants of grey and yellow) and hyponotum color (variants of blue and grey). Internally, however, P. coriaceus coriaceus can easily be distinguished from P. tigrinus : the retractor muscle of the penis inserts at the posterior end of the visceral cavity in P. coriaceus coriaceus and near the heart in P. tigrinus ; the distal, flexible region of the penis with hooks is> 4 mm long in P. coriaceus coriaceus and <1.5 mm long in P. tigrinus ; in the posterior part of the reproductive system, the deferent duct (closely attached to the oviduct) is tightly convoluted in P. coriaceus coriaceus while it is almost straight in P. tigrinus .
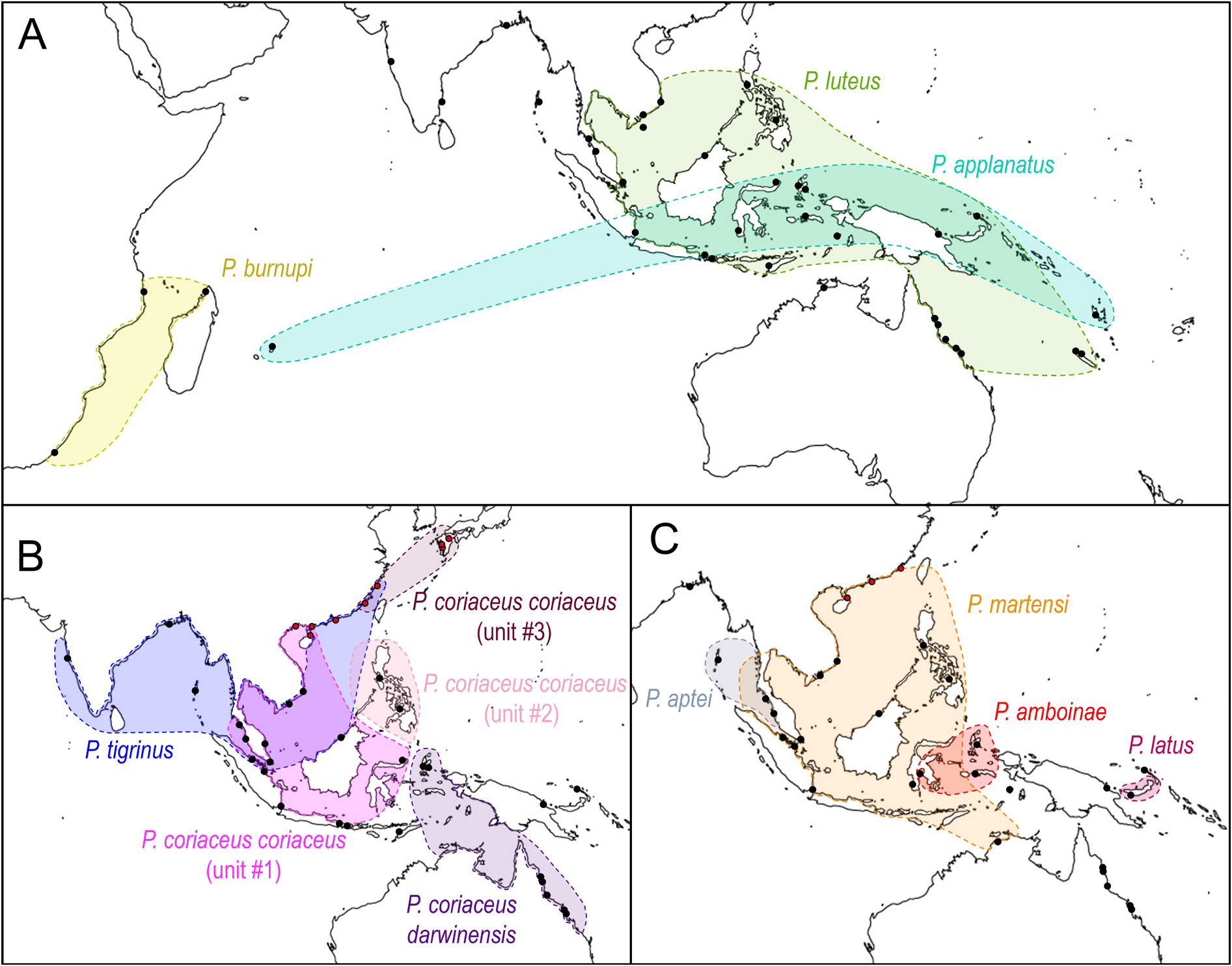
Distribution ( Fig. 10B View Fig )
Mitochondrial unit #1. Brunei. China: Qinzhou and Guangxi ( Sun et al. 2014, misidentified as P. mortoni ). Indonesia: Lombok, Sumatra and Sulawesi. Peninsular Malaysia. Singapore (paralectotypes of P. coriaceus and newly collected specimens). Vietnam (type locality of O. condoriana and newly collected specimens).
Mitochondrial unit #2. Philippines (type locality of O. coriaceum ): Luzon, Bohol, and Mindanao. Fresh specimens were collected from Luzon and Bohol, but not from Mindanao. It could not be determined whether the lectotype selected here was from Manila (Luzon), Bohol, or Zamboanga (Mindanao).
Mitochondrial unit #3. China: Fujian Province ( Sun et al. 2014, misidentified as P. mortoni ). Japan ( Takagi et al. 2019, identified as Platevindex sp. A).
All records are new, except for the localities of some of the type material. Note that the paralectotype (s) of O. coriaceum from Brisbane are not included in the distribution of P. coriaceus coriaceus .
Habitat ( Fig. 21 View Fig )
Platevindex coriaceus coriaceus is found in mangrove forests, on tree roots and trunks, and on dead logs, but not directly on mud. The logs and trees may be in silty mud saturated with water or on harder mud. It does not live on rocky shores. Platevindex coriaceus coriaceus is very common across its range, except in central Indonesia where it seems less common. Few specimens were found in Lombok and Sulawesi and it was not found in Maluku ( Ambon, Seram and Kei Islands).
Remarks
The publication dates of the various sections of the volume on Landmollusken by Carl Semper in the Reisen im Archipel der Philippinen series were clarified by Johnson (1969). The species name Onchidium coriaceum was first published with three figures illustrating the external morphology, dorsal eyes and penial hooks ( Semper 1880: pl. 19, figs 1, 16, pl. 23, fig. 12) and no written description. Because Onchidium coriaceum was published before 1931, ICZN Article 12.2.7 applies and Semper’s figures are regarded as an indication accompanying the new name Onchidium coriaceum . The text of the description and the illustration of the radula (pl. 21, fig. 7) were published in 1882. However, not everything in the written description refers to the subspecies described here (see below for more details). The illustrations of the dorsal surface (pl. 19, fig. 1), the dorsal eyes (pl. 19, fig. 16) and the radular teeth (pl. 21, fig. 7) could apply to any almost any species of Platevindex , so there is no reason to consider that they do not refer to the subspecies described here. The illustration of the male apparatus (pl. 23, fig. 12) could refer to the subspecies described here (if Semper illustrated one of the paralectotypes from Singapore or the Philippines) or P. coriaceus darwinensis subsp. nov. (if Semper illustrated the paralectotype from Brisbane, both subspecies being anatomically cryptic), even though his written description of the insertion of the retractor muscle (near the heart) matches that of P. tigrinus instead of the subspecies described here (insertion near the posterior end of the visceral cavity).
Semper (1880) originally described Onchidium coriaceum based on syntypes from Brisbane ( Australia), Penang ( Malaysia), Singapore, and Manila (Luzon), Bohol and Zamboanga (Mindanao)in the Philippines. Our sampling across the Indo-West Pacific shows that multiple species of Platevindex are present across the region encompassing these countries. A re-examination of the type material and a critical analysis of the original description reveal that, when describing O. coriaceum, Semper used specimens that were part of at least two distinct species.
The lectotype from the Philippines (ZMB/Moll 39028a) designated here clarifies the application of the name Platevindex coriaceus because it was dissected for the present study and it displays all the diagnostic features of the nominotypical subspecies described here. Two paralectotypes (27/26 and 20/ 16 mm) from the Philippines (ZMB/Moll 39028b) are also part of P. coriaceus coriaceus . The other paralectotypes from the Philippines, however, cannot be identified to the species level because the male reproductive parts were previously removed by Semper. Even though they seem to be part of P. coriaceus coriaceus , they potentially could belong to P. luteus or P. martensi , both of which are found in the Philippines.
Semper mentioned that the paralectotype (27/ 25 mm) from Penang (ZMB/Moll 39029) had previously been misidentified as Onchidium typhae (there is only one dorsal eye on each of its papillae while there are 3 or 4 eyes per papilla in O. typhae ). This paralectotype from Penang was dissected (likely by Semper) and its penial complex is missing; however, the deferent duct in its posterior reproductive system is not attached to the oviduct, indicating that this paralectotype is not part of the subspecies described here but is, instead, a misidentification of P. martensi (an undescribed species at that time). Platevindex martensi also differs from P. coriaceus coriaceus with respect to the insertion of the retractor muscle: in the posterior half of the visceral cavity (from ½ to ¾ its length) in P. martensi and near the end of the visceral cavity in P. coriaceus coriaceus . Also, in P. martensi , the length of the flexible portion of the penis with hooks does not exceed 2.5 mm, while it measures from 4 to 8 mm in P. coriaceus coriaceus . Finally, the position of 20 dorsal eyes on the edge of the mantle described by Semper is a character compatible with P. martensi , but not with P. coriaceus coriaceus or any related species within clade A ( Figs 1 View Fig , 5 View Fig ). Labbé (1934) considered that the paralectotype from Penang was part of Onchidium tigrinum rather than O. coriaceum (likely based on Semper’s description because Labbé, who did not comment on the anatomy of that specimen, does not seem to have examined it).
The large (22/ 10 mm) paralectotype from Singapore (ZMB/Moll 39030) was previously dissected and its copulatory apparatus is missing. However, it seems to be part of the subspecies described here because even though its posterior reproductive system is largely destroyed, the highly-coiled deferent duct is attached to the distal part of the oviduct, which is characteristic of P. coriaceus coriaceus . The notum of the small (20/ 17 mm) paralectotype from Singapore (ZMB/Moll 39030) was cut open for the present study. It is partly immature (its posterior reproductive system is not developed) but is definitely part of the subspecies described here because its penis is almost as long as the visceral cavity.
Semper (1882: 272) also mentioned some material (likely only one specimen) collected in Brisbane (Queensland, Australia) which could not be located by us. Queensland is not part of the geographic distribution of P. coriaceus coriaceus , so Semper’s material from Brisbane most likely did not belong to P. coriaceus coriaceus . Instead, it could belong to P. coriaceus darwinensis subsp. nov. or P. luteus . Finally, Semper (1882: 272) mentioned two specimens at the Vienna Museum but from an unknown locality. It is not possible to determine which species of Platevindex Semper observed, which, thanks to the designation of the lectotype from the Philippines, is not an issue.
Semper (1882: 273) noted that the only character which differed between the descriptions of O. coriaceum and O. tigrinum was Stoliczka’s description of a “supplementary albuminous gland” (i.e., accessory penial gland) ( Stoliczka 1869: 107), and indicated that O. coriaceum could be a synonym of Stoliczka’s O. tigrinum . Semper was not able to properly compare the anatomy of the two species without examining Stoliczka’s specimens and stated and that he could not be sure about the synonymy. An accessory penial gland is actually absent in Platevindex tigrinus , which is anatomically and genetically distinct from P. coriaceus coriaceus .
Finally, Semper’s description of the penial morphology in the original description of O. coriaceum needs to be commented on. The length of the flexible distal region of the penis with hooks was described by Semper as being 6 mm long, which is within the range observed here in P. coriaceus coriaceus (4 to 8 mm) but is incompatible with those of P. tigrinus (1 to 1.5 mm) or P. martensi (2 to 2.5 mm). However, Semper also described a retractor muscle inserting “at the height of the heart” (1882: 272, translated from German), which is compatible with P. tigrinus (the retractor muscle inserts near the heart), somewhat compatible with P. martensi (insertion in the posterior half of the visceral cavity), but not with P. coriaceus coriaceus (insertion at the posterior end of the visceral cavity). There are only two possible explanations. First, Semper’s written description of the insertion of the retractor muscle is based on the paralectotype from Penang (which belongs to P. martensi ), or, second, Semper made a mistake.
The description of Onchidella condoriana by Rochebrune did not include any information about the internal anatomy. Externally, O. condoriana was described as “granulous and sprinkled with whitish tubercles and grey underneath” ( Rochebrune 1882: 67, translated from Latin). The holotype (23/ 16 mm) can readily be identified as a Platevindex by the hard, flattened notum, but it was previously dissected by someone who removed the penial complex (it is unclear who dissected it because the species was not mentioned by Plate and neither Hoffmann nor Labbé commented on the internal anatomy). Most of the posterior (female) reproductive system is missing but the oviduct, which remains, is similar to that of the subspecies described here (with the highly-coiled deferent duct attached to it). Thus, the holotype of O. condoriana belongs to P. coriaceus coriaceus and not P. tigrinus (in which the deferent duct is almost straight). Note that P. tigrinus is likely also present in Vietnam because it is known from western India all the way to China, even though we did not find it there. Because the type locality of O. condoriana (Côn Son Island, Côn Ðảo Archipelago, Vietnam) is within the range of mitochondrial unit #1 (from Peninsular Malaysia to China and Sulawesi), the name Platevindex condorianus could potentially apply to it, if it would ever need to be formally named. Platevindex slugs were not found at the type locality of O. condoriana (we explored the Côn Ðảo Archipelago in 2015), but were found in mainland Vietnam, in the Can Gio mangrove forest protected area. All those Platevindex slugs from the Can Gio mangrove forest belonged to P. coriaceus coriaceus . Finally, P. martensi is likely also present in Vietnam ( Fig. 10 View Fig ), but the lack of dorsal eyes at the margin of the notum and the deferent duct being attached to the oviduct indicate that Rochebrune’s type of O. condoriana is not part of P. martensi .
Plate (1893: 190–191) re-described P. coriaceus based on Semper’s original specimens from the Philippines. Semper did not indicate how many individuals he examined from the Philippines, but Plate listed ten specimens. Only seven of Semper’s type specimens were located from the Philippines (ZMB/Moll 39028), suggesting that some specimens may have been previously destroyed. Plate’s description of one single dorsal eye per papilla with papillae irregularly distributed is consistent with a species of Platevindex . He also mentioned new details that were not included in Semper’s original description, such as the granular notum, the male pore being above and to the left of the right oral lobe (i.e., below and to the left of the right oral tentacle) and intestinal loops of type II. The number of radular teeth per half row (120) described by Plate is within the range reported here (from 70 to 120), and it is expected that the number he observed would be at the high end of the range due to the large size of the specimens that he examined (up to 47 mm long).
Plate described Oncis semperi based on two syntypes (22/18 and 22/ 18 mm) from Mindanao (ZMB/Moll 45661). The width of the foot and the position of the male opening confirm that they belong to a species of Platevindex . According to Plate (1893: 193), the penis of Oncis semperi is extremely long (26 mm in a slug that was only 24.5 mm long), inserts at the posterior end of the body cavity, and its distal, flexible region with hooks is 8 mm long. This description is only consistent with P. coriaceus ( Table 4 View Table 4 ). However, Plate’s description of intestinal loops of type I is problematic because it is inconsistent with P. coriaceus . Two explanations are possible. Most likely, Plate’s description of a type I was a mistake (his description of the intestinal loops in P. amboinae was a mistake). Alternatively, one of the syntypes could belong to P. luteus (in which the intestine can be of type I). Because Plate’s description of the copulatory apparatus matches perfectly that of P. coriaceus , P. semperi is regarded as a synonym of P. coriaceus and it is assumed that Plate confused the types of intestinal loops.
Semper (1882: 272) questioned whether Platevindex coriaceus could be a synonym of Onchidium marmoratum Lesson, 1831 , and noted that the original description was insufficient to separate it from O. coriaceum . Bretnall (1919: 323) also listed this potential synonymy with a question mark. The original description of O. marmoratum did not indicate any characters which would distinguish it from P. coriaceus , but the type specimens of O. marmoratum do not share the flattened shape of Platevindex slugs and clearly do not belong to this genus. Onchidium marmoratum was recently transferred to the genus Marmaronchis Dayrat & Goulding, 2018 ( Dayrat et al. 2018). Hoffmann (1928: 88) suggested that O. coriaceum , O. martensi and O. condoriana were all synonyms of Oncis stuxbergi ( Westerlund, 1883) but he did not describe the anatomy of Oncis stuxbergi . Westerlund (1883: 165) also did not describe the internal anatomy of this species (described as Vaginulus stuxbergi ). Thus, neither Westerlund’s description nor Hoffmann’s remarks can be attributed to Platevindex coriaceus . The specific name stuxbergi applies to a species in the genus Onchidium as a result of the designation of a lectotype ( Dayrat et al. 2016: 22). Note that Hoffmann (1928) thought that Onchidium coriaceum was a junior synonym of Oncis stuxbergi because he adopted 1885 as the date of publication for Semper’s monograph in the Reisen im Archipel der Philippinen series (for a collation of Semper’s monograph, see Johnson 1969). Labbé (1934: 235–236), who does not seem to have examined any specimens, followed Hoffmann in considering Oncis coriacea a synonym of Oncis stuxbergi . For unclear reasons, he indicated an insertion of the penial retractor near the heart, which is not consistent with Platevindex coriaceus .
DNA sequences of P. coriaceus from southern China were misidentified as P. mortoni by Sun et al. (2014). Recent COI DNA sequences by Wang et al. (unpublished, GenBank Accession GU166575 View Materials and GU166577 View Materials ) were also identified as P. mortoni , and are shown here to belong to P. coriaceus . Therefore, P. coriaceus is not restricted to southernmost China but is also present in the Fujian Province of China (the province facing Taiwan). Platevindex coriaceus should also be present around Hong Kong, which is south of Fujian province. Hong Kong is the type locality for Platevindex mortoni , a name which is regarded here as a nomen dubium (see our remarks on P. tigrinus and P. luteus ). Additionally, DNA sequences published by Takagi et al. (2019) show that P. coriaceus is present in southern Japan, and is closely related to individuals from northern China, i.e., north of Taiwan ( P. coriaceus coriaceus unit #3). Finally, a voucher specimen identified as Platevindex cf. coriaceus ( Dayrat et al. 2011) in the collection of the Natural History Museum in London (NHMUK 20060274) actually belongs to Platevindex burnupi . Von Martens (1897: 127) indicated that Oncis coriacea was identified from “ Celebes: bei Luwu” [Luwu, Sulawesi, Indonesia] by M. Weber, from “Halmaheira: Dodinga” by himself, as well as from Java and “ Nikobaren, Pulo Pinang und Philippinen ” [ Nicobar Islands; Penang, Malaysia; the Philippines] based on specimens in the Berlin Museum. Von Martens did not provide any details about how the specimens were identified. The specimens from Halmahera, Java, Malaysia, the Philippines and Sulawesi, Indonesia could be either P. coriaceus or P. tigrinus . Platevindex coriaceus was not found in the Andaman Islands, but could be present in the Nicobar Islands.
Table 5. Radular formulae in species of Platevindex Baker, 1938.
| Species | Radular formula | Specimen length (mm) | Catalog Number | DNA extraction number |
|---|---|---|---|---|
| P. coriaceus coriaceus (unit #1) | 82 × (120-1-120) | 49 | UMIZ 00078 | 2190 |
| 70 × (90-1-90) | 28 | ZRC.MOL.10475 | 998 | |
| 70 × (100-1-100) | 38 | ITBZC IM 00014 | 5629 | |
| P. coriaceus coriaceus (unit #2) | 58 × (70-1-70) | 21 | PNM 041236 | 3194 |
| 65 × (80-1-80) | 33 | PNM 041238 | 3256 | |
| 63 × (85-1-85) | 34 | PNM 041240 | 3356 | |
| P. coriaceus darwinensis subsp. nov. | 90 × (115-1-115) | 23 | MTQ stn 123 | #1 |
| 62 × (80-1-80) | 28 | UMIZ 00080 | 5021 | |
| 59 × (70-1-70) | 22 | UMIZ 00081 | 5111 | |
| P. tigrinus | 68 × (75-1-75) | 19 | USMMC 00022 | 955 |
| 67 × (85-1-85) | 26 | USMMC 00023 | 5522 | |
| 62 × (70-1-70) | 23 | BDMNH | 1030 | |
| P. luteus | 47 × (50-1-50) | 22 | MTQ stn 108 | 2597 |
| 41 × (35-1-35) | 28 | UMIZ 00089 | 2837 | |
| 43 × (40-1-40) | 22 | PNM 041246 | 3367 | |
| P. applanatus | 35 × (35-1-35) | 9 | MNHN-IM-2019-1393 | 3152 |
| 30 × (23-1-23) | 7 | MNHN-IM-2019-1394 | 3442 | |
| 30 × (26-1-26) | 6.5 | MNHN-IM-2019-1392 | 3604 | |
| 40 × (35-1-35) | 22 | UMIZ 00101 | 2754 | |
| 46 × (55-1-55) | 18 | UMIZ 00101 | 2755 | |
| 54 × (65-1-65) | 16 | UMIZ 00102 | 2893 | |
| 52 × (60-1-60) | 16 | MNHN-IM-2013-14043 | 5424 | |
| 49 × (60-1-60) | 13 | MNHN-IM-2013-14051 | 5450 | |
| 36 × (35-1-35) | 12 | MNHN-IM-2013-62402 | 5491 | |
| P. burnupi | 56 × (70-1-70) | 32 | ELM W04105 | 6146 |
| 50 × (60-1-60) | 19 | ELM W04109 | 6148 | |
| 50 × (60-1-60) | 20 | ELM W04107 | 6149 | |
| 55 × (65-1-65) | 22 | ELM W04108 | 6153 | |
| 41 × (50-1-50) | 12 | MNHN-IM-2019-1397 | 3602 | |
| 47 × (60-1-60) | 16 | MNHN-IM-2019-1395 | 3603 | |
| P. martensi | 75 × (95-1-95) | 30 | USMMC 00029 | 964 |
| 75 × (100-1-100) | 39 | NTM P.57611 | 1711 | |
| 48 × (65-1-65) | 14 | PNM 041250 | 3336 | |
| P. aptei sp. nov. | 81 × (95-1-95) | 35 | USMMC 00078 | 5955 |
| 76 × (115-1-115) | 42 | USMMC 00078 | 5967 | |
| 77 × (105-1-105) | 37 | USMMC 00078 | 5968 | |
| P. amboinae | 80 × (120-1-120) | 38 | UMIZ 00105 | 2883 |
| 85 × (120-1-120) | 32 | UMIZ 00106 | 5043 | |
| 87 x (110-1-110) | 32 | UMIZ 00106 | 5843 | |
| P. latus | 98 × (145-1-145) | 32 | ZMB/Moll 45656 | N/A (spm #1) |
| 93 × (145-1-145) | 30 | ZMB/Moll 45656 | N/A (spm #2) |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Heterobranchia |
|
Order |
|
|
SuperFamily |
Onchidioidea |
|
Family |
|
|
Genus |
Platevindex coriaceus coriaceus ( Semper, 1880 )
| Goulding, Tricia C., Bourke, Adam J., Comendador, Joseph, Khalil, Munawar, Quang, Ngo Xuan, Tan, Shau Hwai, Tan, Siong Kiat & Dayrat, Benoît 2021 |
Platevindex mortoni
| Sun B. & Chen C. & Shen H. & Zhang K. & Zhou N. & Qian J. 2014: 63 |
Platevindex coriaceus
| Baker H. B. 1938: 88 |
Oncis semperi
| Stantschinsky W. 1907: 395 |
Oncis semperi
| Plate L. H. 1893: 193 |
Oncis coriacea
| Stantschinsky W. 1907: 395 |
| Plate L. H. 1893: 190 |
Onchidella condoriana
| Rochebrune A. T. de 1882: 67 |
Onchidium coriaceum
| Semper 1882: 271-273 |