Marmosops carri ( Allen and Chapman, 1897 )
|
publication ID |
https://doi.org/ 10.1206/0003-0090-402.1.1 |
|
DOI |
https://doi.org/10.5281/zenodo.4612714 |
|
persistent identifier |
https://treatment.plazi.org/id/03A68972-980C-FFF3-049F-7469D1F8F965 |
|
treatment provided by |
Felipe |
|
scientific name |
Marmosops carri ( Allen and Chapman, 1897 ) |
| status |
|
Marmosops carri ( Allen and Chapman, 1897)
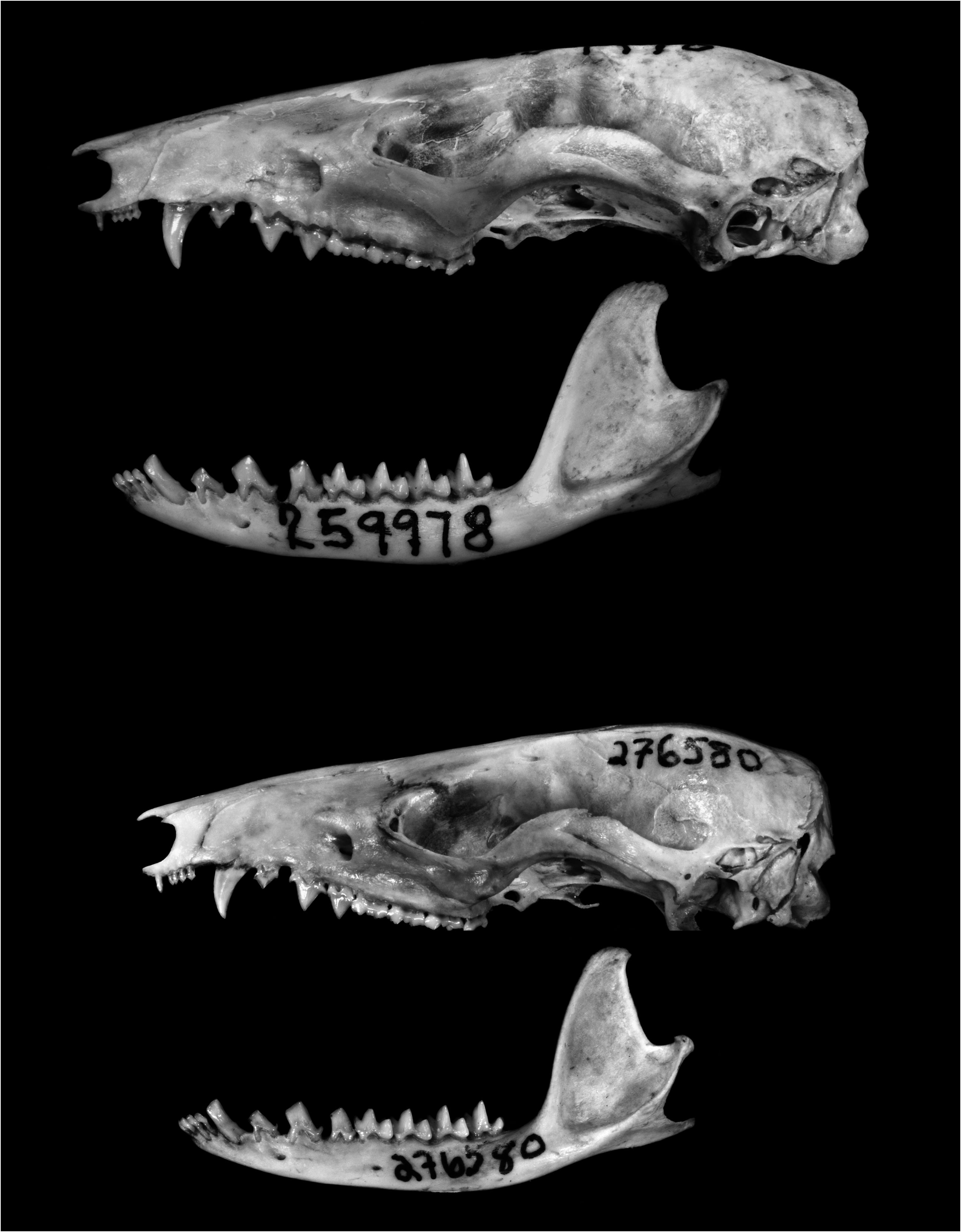
Figures 6C View FIG , 7B View FIG , 8B View FIG , 15 View FIG , 16 View FIG
Thylamys carri Allen and Chapman, 1897: 27 (original description).
Didelphys (Marmosa) carri: Trouessart, 1905: 856 (name combination).
Marmosa (Thylamys) carri: Cabrera, 1919: 40 (name combination).
Marmosops fuscatus: Gardner, 1993: 19 View in CoL View Cited Treatment ; part ( carri treated as a subjective junior synonym of fuscatus Thomas, 1896 View in CoL ) Marmosops fuscatus carri: Gardner View in CoL and Creigh- ton, 2008: 66 (name combination).
Marmosops fuscatus fuscatus: Gardner and Creighton, 2008: 66 View in CoL (name combination); part (Venezuelan material of carri misiden- tified as M. f. fuscatus View in CoL ).
TYPE MATERIAL: The holotype (by original designation) consists of the skin and skull of an adult male ( AMNH 7314 About AMNH /5922, original number 731) collected by Frank M. Chapman at Caparo (fig. 17: locality 97), Trinidad, on 20 March 1894. Two other specimens (paratypes) were also part of Allen and Chapman’s type series: one is AMNH 7313 About AMNH /5921 (a young adult female), and the other was AMNH 7315 About AMNH /5923 (said to be an adult male; not examined and presumed lost) .
DISTRIBUTION, HABITATS, AND SYMPATRY: Marmosops carri is known from Trinidad, Tobago, and Venezuela (fig. 17). 4 On the Venezuelan mainland, examined specimens have been collected in premontane and montane rain forest along the northern coastal cordilleras (e.g., at Hotel Humboldt; Handley, 1976: 79) and the Cordillera de Trujillo (e.g., at Hacienda Misisí; Handley, 1976: 71) between about 1000 m and 2300 m (but see Remarks). On Trinidad, however, it is definitely known to occur near sea level (e.g., at Rio Grande Forest; Appendix 1: locality 100), presumably in lowland rain forest. Although the known range of M. carri overlaps with that of M. ojastii (a member of the Bishopi Group; see below) and might be expected to contact that of M. fuscatus , these species have apparently not been collected sympatrically.
4 Our material of Marmosops carri from Tobago, the first to be reported from that island, was originally identified as such by Darrin P. Lunde.
DESCRIPTION: Body pelage grayish brown (near Fuscous or Hair Brown) middorsally, sometimes indistinctly paler laterally, and about 7–9 mm long at midback; ventral pelage superficially whitish, but hairs of throat, chest, and abdomen uniformly gray based in most specimens (a few have patches or narrow streaks of self-white fur on the throat, chest, groin, or along the insides of the thighs; e.g., AMNH 144832, USNM 370037). Manus covered dorsally with uniformly pale hairs (the metacarpals not contrasting sharply in color with the digits); lateral carpal tubercles bladelike in all examined adult males. Mammae 3–1–3 = 7 in all examined females with visible teats. Tail longer than combined length of head and body (mean LT/HBL × 100 = 120%); dorsal caudal surface dark, but indistinctly paler distally in some specimens; ventral caudal surface distinctly paler than dorsal surface.
Nasal bones long (extending well behind the lacrimals) and uniformly narrow, without conspicuous lateral expansion at the maxillary-frontal suture. Interorbital region broad, with rounded supraorbital margins in females and young adult males, but relatively narrower and tending to develop squared supraorbital margins in large adult males; postorbital processes absent or indistinct. Lacrimal foramina usually visible in lateral view; zygomatic process of the squamosal broadly overlapped dorsally by the jugal. Palatine fenestrae present or absent (when present, palatine fenestrae usually consist of several small irregular perforations, but in a few specimens they are large). Dorsolateral margin of ethmoid foramen usually formed by the orbitosphenoid.
Upper canine (C1) sexually dimorphic (taller and unicuspid in males, shorter and with one or two accessory cusps in females). 5 Upper third molar (M3) anterolabial cingulum discontinuous with preprotocrista (anterior cingulum incomplete). Lower canine (c1) sexually dimorphic (erect, without accessory cusps, and taller than p 1 in males versus procumbent, with posterior accessory cusp, and subequal in height to p 1 in females); c1 anterolingual accessory cusp usually absent in both sexes. Entoconid of m1 apparently subequal to m2 paraconid (but few specimens with unworn molars examined for this trait); unworn m4 talonid with three distinct cusps.
COMPARISONS: Marmosops carri is externally similar to M. fuscatus (with which it was formerly synonymized; see Remarks) but has somewhat paler dorsal fur, averages larger in all external measurements (tables 5, 6), and has a relatively longer tail (e.g., LT/HBL ×100 = 122%
5 All examined females have posterior accessory C1 cusps, and insular populations (from Trinidad and Tobago) usually also have small anterior accessory cusps (e.g., AMNH 5921, 234951, 234954, 234956, 259979). By contrast, females from mainland populations usually lack anterior accessory cusps on C1 (e.g., AMNH 144832; USNM 370030, 517256).
in male carri versus 110% in male fuscatus ). Marmosops carri averages larger than M. fuscatus in all measured craniodental dimensions (except nasal breadth, NB) but especially in three dental dimensions (MTR, LM, M1–3) that exhibit nonoverlapping variation. In other (qualitative) aspects of craniodental morphology the two species cannot be consistently distinguished, although there are modal differences in some characters. For example, the dorsolat- eral margin of the ethmoid foramen is usually formed by the orbitosphenoid in M. carri , whereas this foramen is bordered dorsolaterally by the frontal in M. fuscatus . Handley and Gordon (1979) implied that carri and fuscatus (which they regarded as no more than subspecifically distinct) differ in nasal morphologythe nasals were said to be more expanded posteriorly in fuscatus than in carri —but we did not observe a consistent taxonomic difference in our comparisons of representative specimens of these taxa.
Marmosops carri is substantially larger than M. handleyi in all external measurements, especially in three dimensions (HBL, LT, and Ear) that exhibit nonoverlapping variation in samesex sample comparisons. In qualitative external comparisons, these species principally differ in coloration: whereas M. carri has grayish-brown dorsal body pelage and entirely white forefeet, M. handleyi has dark-brown dorsal pelage and dark metacarpals that contrast in color with its whitish manual digits. Skulls and dentitions of M. carri are considerably larger than those of M. handleyi , exhibiting nonoverlapping variation in same-sex comparisons of eight dimensions (CBL, ZB, PL, PB, MTR, LM, M1–3, and WM3). Overall, the skull of M. carri is more heavily built (especially in males) by comparison with the more delicate cranial construction of M. handleyi . In qualitative aspects of craniodental morphology, M. carri principally differs from M. handleyi by its uniformly narrow nasals (the nasals are posteriorly expanded in M. handleyi ); unicuspid male C1 (this tooth has a posterior accessory cusp in male M. handleyi ), and absence of a lingual accessory cusp on c 1 in most specimens of both sexes (a lingual accessory cusp on c1 is present in both sexes of M. handleyi ).
Marmosops carri is much larger than M. invictus , with no overlapping variation in same-sex comparisons of most measured dimensions. These species also differ strikingly in dorsal pelage coloration (much paler in M. carri than in M. invictus ), coloration of the forefeet (entirely whitish in M. carri , whereas the metacarpals are dark in M. invictus ), and tail markings (the tail is much more distinctly bicolored in M. carri ). Qualitative craniodental comparisons reveal several additional contrasting differences. Among others, M. carri has uniformly narrow nasals, whereas the nasals are laterally expanded near the maxillary-frontal suture in M. invictus ; at least the ventral lacrimal foramen is consistently visible in lateral view in M. carri , whereas this foramen is concealed inside the orbit in M. invictus ; palatine fenestrae are often present in M. carri , whereas palatine perforations are consistently absent in M. invictus ); c1 is a tall unicuspid tooth in male M. carri , whereas this tooth is short and premolariform in male M. invictus ; and the m1 entoconid is subequal in height to the adjacent m2 paraconid in M. carri , whereas the m1 entoconid is shorter than the m2 paraconid in M. invictus .
REMARKS: Marmosops carri exhibits noteworthy sexual size dimorphism, with adult males averaging larger than adult females in most measured dimensions, especially those prone to strong positive allometry (e.g., zygomatic breadth; tables 5, 6). The sexes additionally differ in interorbital and canine morphology as described above. Maturational changes in the interorbital morphology of adult males appear to result from enlargement of the temporalis musculature, which is accommodated by an increasingly well-defined postorbital constriction and by supraorbital remodeling.
This species was long regarded (e.g., by Tate, 1933) as endemic to Trinidad, but Handley and Gordon (1979) correctly observed that specimens from the coastal cordilleras on the adjacent Venezuelan mainland are morphologically indistinguishable from Trinidadian material. Handley and Gordon recognized that specimens referable to Marmosops carri are distinctively larger than topotypical material of Marmosops fuscatus (from the Mérida Andes), but they judged these taxa to be conspecific and used the older name ( fuscatus ) for both.
Although Marmosops carri and M. fuscatus are morphologically similar (in particular, these are the only species of Sciophanes that exhibit large size and uniformly narrow nasals), they form distinct cytochrome- b haplogroups ( Díaz-Nieto et al., 2016a), and measured specimens have nonoverlapping molar dimensions: LM = 7.0–7.7 mm in M. carri (N = 28) versus 6.0–6.7 mm in M. fuscatus (N = 8). The specimen from Trujillo (USNM 372934) that Handley and Gordon (1979) believed to be intermediate to M. carri and M. fuscatus has large molars (LM = 7.0 mm) and belongs to the same haplogroup that occurs on Trinidad (Díaz- Nieto et al., 2016a: fig. S1); therefore, we refer this specimen (and others collected in the Cordillera de Trujillo) to M. carri .
We have not examined any voucher material from two ecological studies of mouse opossums identified as Marmosops fuscatus , one by O’Connell (1979) and the other by Cordero (2001). O’Connell’s study was carried out at Parque Nacional Guatopo in the interior coastal range of northern Venezuela (ca. 10°05′ N, 66°30′ W; on the border between the states of Miranda and Guárico), where “ M. fuscatus ” was said to occur only at higher elevations, presumably in premontane rain forest. Cordero’s study was carried out at a field station of the Universidad Simón Rodríguez, close to the Caribbean coast in northern Venezuela (10°20′ N, 66°15′ W; in Miranda), where “ M. fuscatus ” was trapped in lowland rain forest near sea level. Based on their geographic locations (fig. 17), we expect that the species observed in both of these studies was M. carri . Also unexamined by us are a substantial number of Venezuelan specimens identified as M. fuscatus (see Pérez-Hernández, 1989), many of which seem likely to represent M. carri . A careful review of this and subsequently collected in-country material should contribute to a more accurate assessment of the geographic ranges of these taxa, and we encourage additional sequencing efforts to test the correlation between haplotype membership and morphometric divergence upon which our hypothesis of full species status is largely based.
SPECIMENS EXAMINED (N = 70): Trinidad and Tobago— Tobago, near Charlotteville ( AMNH 259970, 259978, 259979); Trinidad, Caparo ( AMNH 7313/5921, 7314/5922), Cedros ( AMNH 234951), Cumaca ( AMNH 169759, 169760), Rio Grande Forest ( AMNH 186437, 186438, 188353), St. Augustine ( AMNH 150007), Turure Forest ( AMNH 214439, 214441, 234952– 234956, 234959, 234974). Venezuela — Aragua, Rancho Grande ( AMNH 144832; USNM 517256–517260); Carabobo, 4 km NW Montalbán ( USNM 418515, 443731, 443783–443789, 496520, 496521), Cumbre de Valencia ( AMNH 31531); Distrito Federal, Los Venados ( USNM 370022–370024); Miranda / Vargas, Hotel Humboldt ( USNM 370027–370031, 370033–370039, 370041, 370042, 371300, 496517); Monagas, San Agustín ( USNM 406925–406932, 4069324); Trujillo, 13–14 km E Trujillo ( KU 120254, USNM 372933, 372934).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Marmosops carri ( Allen and Chapman, 1897 )
| Díaz-Nieto, Juan F. & Voss, Robert S. 2016 |
Marmosa (Thylamys) carri: Cabrera, 1919: 40
| Cabrera, A. 1919: 40 |
Didelphys (Marmosa) carri:
| Trouessart, E. - L. 1905: 856 |
Thylamys carri
| Allen, J. A. & F. M. Chapman 1897: 27 |