Hoolock tianxing, Peng-Fei Fan, Kai He, Xing Chen, Alejandra Ortiz, Bin Zhang, Chao Zhao, Yun-Qiao, Hai-Bo Zhang, Clare Kimock, Wen-Zhi Wang, Colin Groves, Samuel T. Turvey, Christian Roos, Kristofer M. Helgen, Kristofer M. Helgen & Xue-Long Jiang, 2017
|
publication ID |
https://doi.org/ 10.1002/ajp.22631 |
|
persistent identifier |
https://treatment.plazi.org/id/03A487BD-FF91-0265-FCA0-F8BCFAA994F2 |
|
treatment provided by |
Plazi |
|
scientific name |
Hoolock tianxing |
| status |
sp. nov. |
Hoolock tianxing View in CoL sp. nov.
Hylobates hoolock leuconedys: Groves (1967) : 276 (part).
Skywalker hoolock gibbon () or Gaoligong hoolock gibbon
().
3.4.1 | Holotype
AMNH M-43068 (adult male, skin only; Figure 3 View FIGURE 3 ), collected by Roy Chapman Andrews and Yvette Borup Andrews on April 5, 1917 during the American Museum of Natural History's Asiatic Zoological Expedition ( Allen, 1938).
3.4.2 | Type locality
Ho-mu-shu (=Hongmushu) Pass,Baoshan , Yunnan, China (25.00 N, 98.83 E).
GoogleMaps3.4.3 | Paratypes
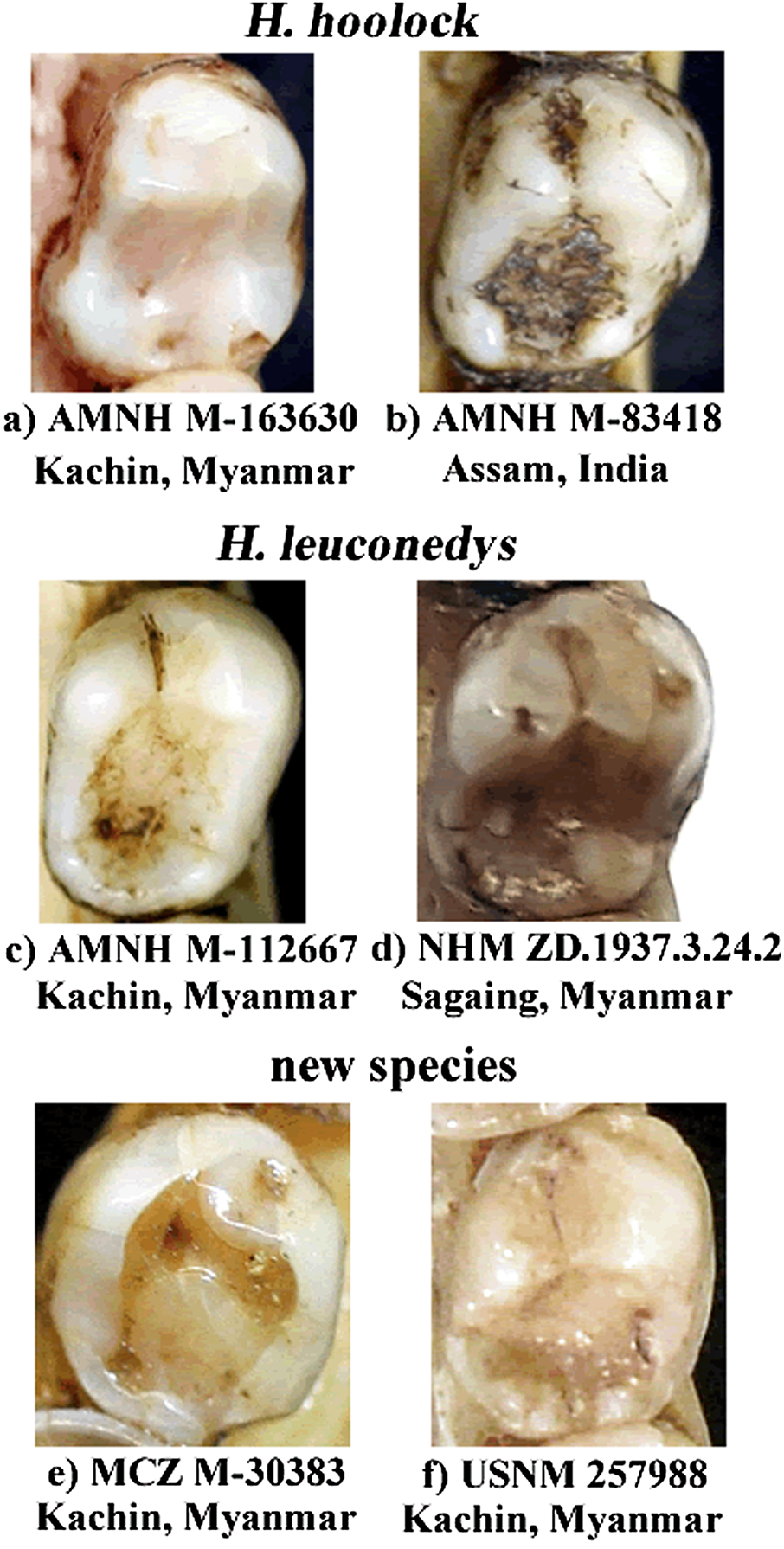
AMNH M-43065 (adult female,skin only;Supplemental Figure S1) and MCZ 26474 (=AMNH M-43067, skin and skull, relocated to MCZ in September 1930), collected at the same locality as the holotype ( Allen, 1938). IOZ 25965 (adult male, skin and skull; Supplemental Figure S3), collected on 4 June, 1965 at Tengchong, Yunnan, China. MCZ 30383 (adult male, skin and skull; Supplemental Figure S3) collected on 15 January, 1932, ca. 40 miles east of Bhamo, northern Myanmar, during the Brooke Dolan expedition.
3.5 | Etymology
Tianxing constitutes the pinyin (standard mainland Chinese phonetic alphabet) transliteration of, meaning heaven's movement or skywalker (xing, movement, can act as either a noun or a verb), a name referring to the unique locomotory mode of gibbons (brachiation; Figure 8 View FIGURE 8 ) and derived from the text of the I Ching, an ancient Chinese work of divination: (“As heaven's movement is ever vigorous, so must the scholarly gentleman (, “ junzi ”) ceaselessly strive for self-improvement”). Gibbons were widely regarded as a symbol of scholar-officials or junzi in ancient China, as the perceived “noble” characteristics of gibbons were considered to accord with the aesthetic taste of both Daoism and traditional Chinese scholars (van Gulik, 1967; Ye & Heule, 2013).
3.6 | Diagnosis
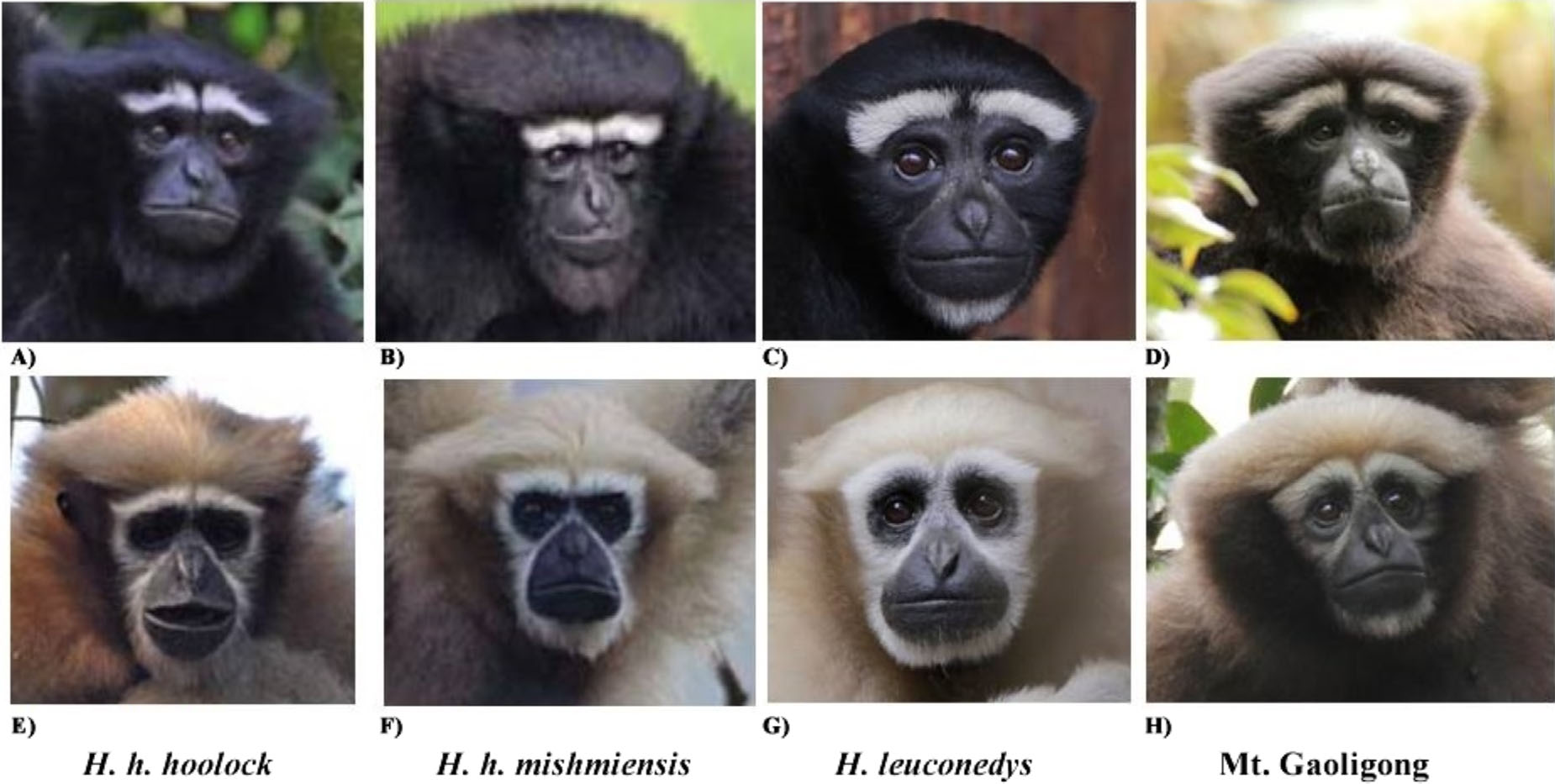
Hoolock tianxing is a hoolock gibbon distinguished from other described hoolock species by a combination of external and dental characters. In males, the ventral pelage is brownish, resembling that of H. leuconedys but differing from H. hoolock . The eyebrows are relatively thinner than in H. hoolock and H. leuconedys , and well-separated, differing from the condition in H. hoolock , where there is only a narrow gap between the eyebrows. White hairs are absent in the suborbital area, differing from H. leuconedys , which has white hairs in the suborbital area. The beards of males are black or brown, differing in color from H. leuconedys , which has a whitish or buffy beard, and not as prominent as in H. hoolock . The black, brown or grayish genital tuft in males differs in color from H. leuconedys , which has a white or silvery tuft. The face rings in females are incomplete, differing from the condition in both H. hoolock and H. leuconedys . The crown outline of the lower p4 is oval, making it distinct from H. leuconedys and H. hoolock individuals from Myanmar and more similar to H. hoolock from Assam.
3.7 | Description
In adult males, the ventral pelage is generally dark brown, and the dorsal pelage has a brownish overlay, especially apparent under bright light (Supplemental Figure S2); eyebrows thin and well-separated; white hairs absent in the suborbital area; beard not conspicuous, black or brown in color, not contrasting with the color of the chest or body; genital tuft prominent, usually black or dark brown in color with a few white hairs present,not contrasting with the color of the groin.In older animals, the genital tuft is fainter and light brownish in color (Supplemental Figure S4). In adult females, pelage color is generally yellowish, but varies with age (yellowish white to reddish blonde); eye rings incomplete; white hair typically not present on the lateral orbital region, or if present, not as conspicuous as on the brows on the lateral orbital region; white hair sometimes also not present on the suborbital region (Supplemental Figure S5). Juveniles do not have white hair on the chin or under the eyes; eyebrows are not always well-separated. Lower p4 is generally mesiodistally short and oval-shaped, with the talonid and trigonid of equal buccolingual width. Distal cusps are present, but not well-developed ( Figure 5 View FIGURE 5 e and f).
3.8 | Distribution
Between the Irrawaddy-Nmai Hka River and the Salween River in China and Myanmar. The Dulongjiang valley, the upper tributary of the Nmai Hka River, may serve as a dispersal barrier for hoolocks. Wild individuals are confirmed to occur on Mt. Gaoligong, and historical museum specimens are also known from further south at Gokteik, Shan State, northern Myanmar. Geissmann et al. (2013) estimated that a healthy population with ca. 5 0,0 0 0 individuals of eastern hoolock live in Shan State subtropical forests, and ca. 16,000 individuals live in montane rainforest in Kayah-Kayin (see below).
3.9 | Comments
Although Groves (1967) suggested that the color of the hands and feet is lighter than the body color in H. leuconedys , we found no difference in coloration between the hands, feet, or bodies in examined individuals of either H. leuconedys or H. tianxing . The two specimens in our study sample from Gokteik, Shan State, Myanmar (USNM 257988 and ZD.1933.7.29.15), which represents the southernmost record of H. tianxing , show minor morphological differences from individuals from Mt. Gaoligong; the male specimen is very similar to the holotype of H. tianxing , but the female possesses more white hair on the suborbital region than individuals from Mt. Gaoligong. Gokteik is 300 km southwest of Mt. Gaoligong, indicating that these observed differences may represent allopatric differentiation between hoolock populations in this region. However, more specimens from Shan, Kayah, and Kayin States need to be examined to assess whether this apparent variation is a genuine population-level characteristic.
Hoolocks no longer survive at the type locality of H. tianxing . The nearest well-documented population occurs at Nankang (N24° 49 ′, E98°46 ′, H: 1800–2300 m a.s.l.), 20 km away from Hongmushu in the southern part of Gaoligong National Nature Reserve. The vegetation in this region consists of humid montane evergreen broad-leaved forest dominated by species of Lauraceae , Fagaceae , Theaceae , and Magnoliaceae . Mean annual temperature in this 4 |
DISCUSSION
4.1 | Con fi dence of the molecular results region between October 2010 and September 2011 was 13.3°C; the lowest recorded mean monthly temperature was 6.4°C in January 2011, and the highest was 20.3°C in August 2010 ( Fan et al., 2013). Annual rainfall was 1801.4 mm during this period; rainfall was greater than 200 mm in each rainy season month from May to October, except in September 2011 (198.1 mm), and was less than 100 mm in each dry season month from November to April ( Fan et al., 2013).
Genomic-scale hybridization capture has been demonstrated to constitute a valid and powerful approach to recover endogenous DNA for ancient and non-invasive sampling, and is extremely useful for conservation of threatened species ( Perry, Marioni, Melsted, & Gilad,2010).In this study,our enrichments were able to recover whole mitogenomes efficiently for 8 of the 11 fecal samples. One potential issue might be so-called nuclear mitochondrial DNA sequences (NUMTs), which commonly exist in primates ( Karanth, 2008); the NUMTs,however,should be at low enough levels to not influence base calling or subsequent assemblage accuracy ( Li, Schroeder, Ko, & Stoneking, 2012).
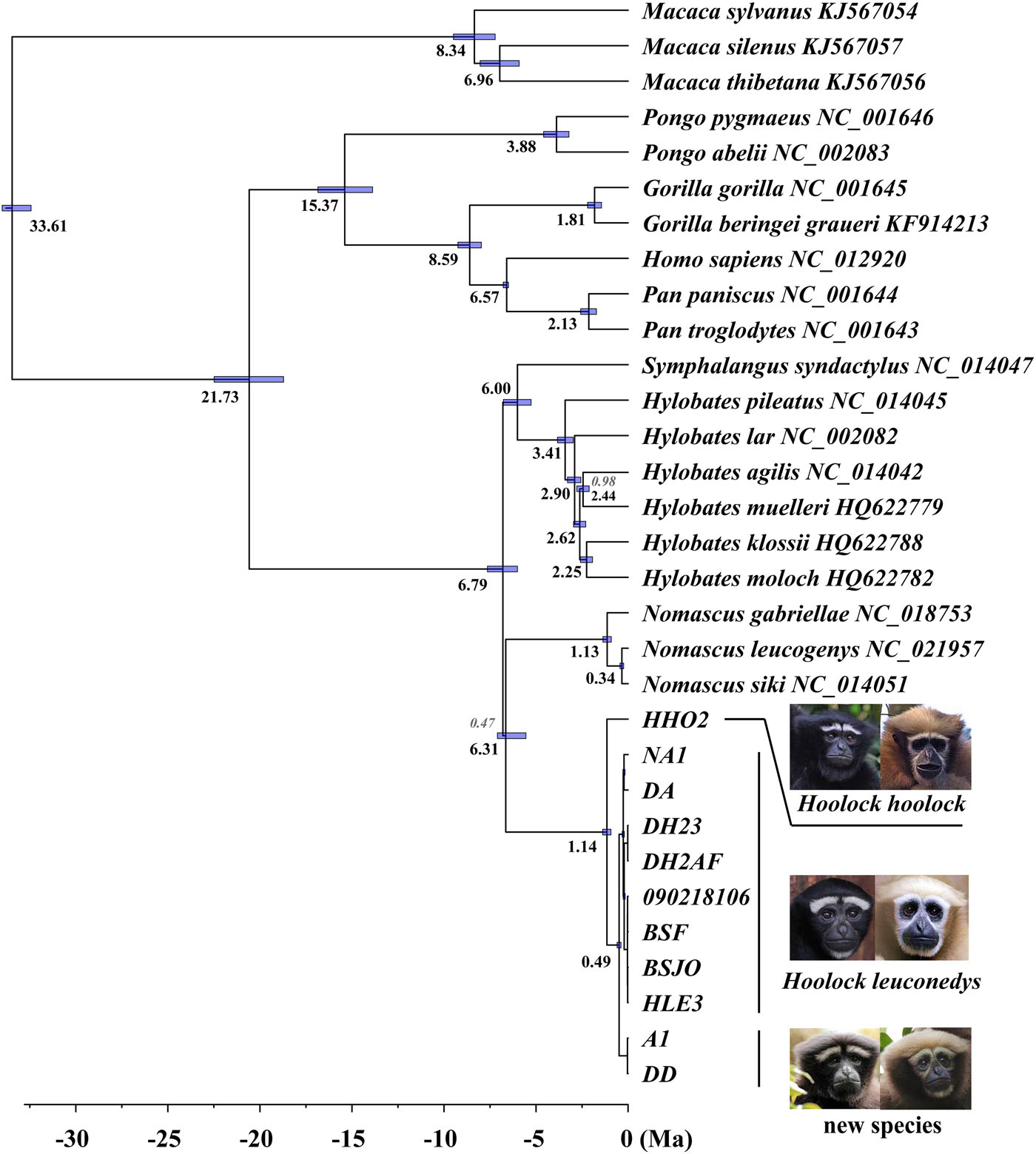
The relationships between hylobatid genera are highly supported in our analyses (PP = 1.0); but they are characterized by short internal branches ( Figure 6 View FIGURE 6 ), a finding similar to previous studies ( Kim et al., 2011; Springer et al., 2012; Thinh, Mootnick, Geissmann et al., 2010). This finding also matches the conclusions of the recent study using whole gibbon genome sequences by Carbone et al. (2014), who suggested a near-instantaneous diversification among the living hylobatid genera.
According to our mitogenomic analyses, the MRCA of living gibbons lived around 6.79 Ma, which is slightly older than the estimate of 5 Ma based on nuclear genome data Carbone et al. (2014). Our result is very similar to the gibbon MRCA age estimate given by Springer et al. (2012). The timing of divergence between H. hoolock and taxa previously classified as H. leuconedys was around 1.14 Ma (1.38–0.93 Ma), overlapping with the estimates given in two previous studies (1.42 [1.90–0.97] in Thinh, Mootnick, Geissmann et al. [2010]; 1.96 [4.4–0.22] in Springer et al. [2012]).
4.2 | Support for the new taxon
Groves (1967) recognized H. hoolock and H. leuconedys based on characters shown by a series of skulls, as well as on four soft tissue characters, namely the color of the preputial tuft, the shape of the eyebrow streaks, the color of the suborbital hair,and the color of the chin hair. His taxonomic assessments were further confirmed by molecular phylogenetic analyses ( Thinh,Mootnick,Geissmann et al.,2010).Here,we found that these characters are equally prominent and distinguishable between H. leuconedys sensu stricto and H. tianxing . Skull shape in hominoids is generally conserved while the shape of postcanine teeth is usually variable ( Uchida, 1996). Nevertheless, the 87–93% correct assignments of individual specimens using each of the morphometric and GM data in the DFAs support the occurrence of morphological differentiation. Similarly, the differentiation of the lower p4 is not clearcut by itself. However, clear morphological differentiation between populations is apparent when considering these characteristics together.
Morphological discrimination is also congruent with divergence of the mitochondrial genomes. We acknowledge that mitogenomic gene trees can differ from nuclear genomic trees, as seen in primates for example in recent analysis of the “odd-nosed” Asian colobines ( Liedigk et al., 2012). However, the two clades representing H. leuconedys and H. tianxing are each strongly supported as monophyletic in our analysis, and diverged in the middle Pleistocene (ca. 0.49 Ma), suggesting long-term matrilineal isolation. In addition, the K2P distance between H. leuconedys and H. tianxing (1.2%) is similar to the differentiation observed between other gibbon sister species, for example, between Nomascus annamensis and N. gabriellae (1.26%) and between N. leucogenys and N. siki (1.0%) ( Thinh, Mootnick, Thanh, Nadler, & Roos, 2010). Similarly, the estimated divergence time between H. leuconedys and H. tianxing is similar to or greater than estimated divergence times between other primate species in Asia ( Table 2). All of these taxa are recognized as full species in the most recent taxonomic review of the world's primates ( Mittermeier et al., 2013).
TABLE 2 Estimated divergence times between gibbon and other Asian primate sister species based on mitochondrial data
Mt.Gaoligong, situated along the border of China and Myanmar,is a hotspot of new species discovery, with recent discoveries including other species of primates ( Geissmann et al., 2011), as well as other vertebrates, for example, amphibians ( Yang, Wang, & Chan, 2016; Yang, Wang, Chen, & Rao, 2016). This high discovery rate at least partly reflects the fact that these mountains have been difficult to access in the past, so that few expeditions have been carried out, and subsequently most animal groups have never been studied in detail. Most of these new species are locally endemic; Mt. Gaoligong is the westernmost part of the Hengduan Mountain Chain, which was formed during the uplift of the Himalayas ( Zhong & Ding,1996), and is geographically isolated from the other mountains in southwestern China by the Salween River valley. The “sky-island” topography and associated unfavorable valley habitats are likely to have driven extensive physical isolation, allopatric speciation, and high endemism in vertebrate populations ( He & Jiang, 2014). Our description of H. tianxing provides further evidence for the unique local fauna of Mt. Gaoligong, and it is very likely that new species are still to be described in other taxonomic groups, many of which remain understudied and need to be re-examined.
Groves (1967) and Choudhury (2013) noticed morphological differences in hoolocks from the east and west of the Irrawaddy River. Groves (1967) also reported that three of the 22 H. leuconedys specimens he examined did not show white chins,and eight specimens did not have white hair under the eyes. However, he hesitated to erect any further hoolock taxa because at the time “too few specimens of either sex are available from the east of the Irrawaddy River to determine whether further splitting may be required.” Following our analysis of a further eight historical specimens and 14 wild animals from the east of the river, we support the suggestion that the Irrawaddy-Nmai Hka River is likely to act as a barrier for different hoolock taxa, on the basis of external and craniodental morphological differences and the divergence of mitochondrial genomes.
4.3 | Individuals or specimens of particular significance
Based on a studbook of captive hoolock gibbons compiled in 2011, we identified a hybrid hoolock family in Kunming Zoo (Hehe♂ × Maomao♀). This pair reproduced five times, and was the most successful captive breeding hoolock pair in any Chinese zoo. Unfortunately, the adult pair and three of their five offspring (KNHMZ 2007090801, KNHMZ 2007082102, and another juvenile) died in 2007, possibly due to a flu-like infection, although two male offspring (Dandan and Xiaobao) still survive. The male had the typical white beard of H. leuconedys (YQL, personal observation), but a photograph of the adult female shows the typical morphology of H. tianxing . Their three male offspring (Xiaobao, Dandan, and KNHMZ 2007090801; Supplemental Figure S6a and b) all have white hair on their chins and genital tufts, but do not have white hair under their eyes, and their white beards are not as conspicuous as in typical H. leuconedys males (Supplemental Figure S2h–j). Genetic analysis of maternally inherited mitochondrial sequence data places them in the H. tianxing clade ( Figure 7 View FIGURE 7 ). We conclude that these offspring are H. leuconedys × H. tianxing hybrids. Xiaobao is now paired with a morphologically typical H. leuconedys female (Baimei) in Kunming Zoo; mitochondrial genetic analysis of the offspring of this pair (Jiaojiao and Yuanyuan) places them as expected in the H. leuconedys clade ( Figure 7 View FIGURE 7 ).
One skin specimen of an adult female (KIZ LS970114) was placed in the H. leuconedys clade in our phylogenetic analysis ( Figure 7 View FIGURE 7 ). Morphologically, this specimen resembles H. leuconedys in having thick white hair between its eyes ( Figure 2 View FIGURE 2 c); it was, however, reportedly collected from Tengchong County, Yunnan, which is within the geographic distribution of H. tianxing . Its original collection record contains no further information on either the collector, collection date, and skull or body measurements. We consider it is highly possible that this specimen in fact originated in Myanmar, and was bought in Tengchong.
4.4 | Conservation implications
The eastern hoolock , based on an assessment comprising populations of both H. leuconedys and H. tianxing , is currently listed as Vulnerable on the IUCN Red List ( Brockelman & Geissmann, 2008), because a large population of 310,000–370,000 individuals (estimated based on very limited field surveys) has been reported from Myanmar ( Geissmann et al., 2013). As the hoolock population on the east bank of the Irrawaddy River represents a new species, its formal conservation status must also be re-evaluated. According to the most recent available survey data from 2008 and 2009, the total population size of H. tianxing in China is less than 200 individuals, and the population is highly fragmented across different forest areas ( Fan et al., 2011). Illegal hunting, habitat destruction, degradation and fragmentation, and the stochastic effects of small population size and isolation all threaten the future of H. tianxing in China ( Fan et al., 2011; Fan, 2016). Based on average group density and area of suitable habitat, Geissmann et al. (2013) estimated the total population of hoolocks in Kachin State to be 240,000–290,000 individuals.This estimate is likely to include both H. tianxing and H. leuconedys , although hoolocks have a limited distribution on the east bank of the Nmai Hka River, suggesting that most of the Myanmar hoolocks in this estimate are likely to be H. leuconedys . Three infants or small juvenile hoolocks have been confiscated by the Chinese border police in the last 2 years, and one small juvenile hoolock from Myanmar was raised as a pet by a woman in Dulongjiang, Yunnan; all these individuals were H. leuconedys . The population of H. tianxing in Kachin State is therefore likely to be very small if it even still survives.A larger population of H. tianxing might still survive in the southern part of its proposed range; Geissmann et al. (2013) estimated that approximately 50,000 hoolocks occur in the subtropical forest of Shan State and 16,000 individuals occur in the montane rainforest of Kayah and Kayin States. These populations are distributed on the east bank of the Irrawaddy River, and therefore are likely to represent H. tianxing . These populations, however, face a series of threats including hunting, illegal trade, and rapid habitat loss ( Geissmann et al., 2013). It is difficult to evaluate the conservation status of H. tianxing without more robust information on the status of these poorly known populations, but we propose that H. tianxing should probably be assessed as Endangered on the IUCN Red List, under criterion A4acd ( IUCN, 2001).
Only 21 captive hoolock individuals are recorded in Chinese zoos in the hoolock studbook (Yang, 2011). We surveyed 22 captive hoolocks in China during this study, most of which were, however, not listed in the studbook.We found that only two of these individuals can be assigned to H. tianxing . Although it is likely that other captive hoolocks that we did not survey may also be H. tianxing individuals,the total number of captive individuals of this species must be very small, and we know of no captive H. tianxing females in China.
Only two pairs of either eastern hoolock species are known to have bred in China before 2011: in Kunming Zoo and Beijing Zoo (Yang, unpublished). The pair in Kunming Zoo died in 2007, and the other pair and another adult female in Beijing Zoo died in 2005. Kunming Zoo currently has a new hoolock pair (Xiaobao♂ × Baimei♀), which has bred successfully three times, and another hoolock pair in Dehong Wildlife Rescue Center (DH3♂ × DH2♀) gave birth in 2015; as discussed above, however, Xiaobao is a H. leuconedys × H. tianxing hybrid, whereas Baimei, DH3, and DH2 are all H. leuconedys individuals. Further investigation of hoolocks currently held in Chinese captive facilities, together with accurate species identification of captive hoolock individuals, is necessary in order to establish a national conservation breeding program for H. leuconedys , and to evaluate whether a similar conservation breeding program is feasible for H. tianxing .
TABLE 2 Estimated divergence times between gibbon and other Asian primate sister species based on mitochondrial data Sepcies group Estimated divergence time (Ma) References
| Sepcies group | Estimated divergence time (Ma) | References |
|---|---|---|
| Hoolock tianxing and H. leuconedys | 0.49 | This study |
| Nomascus leucogenys and N. siki | 0.55 | Thinh, Mootnick, Geissmann et al. (2010) |
| 0.34 | This study | |
| Trachypithecus francoisi and T. leucocephalus | 0.27–0.46 | Liu et al. (2013) |
| Trachypithecus francoisi and T. poliocephalus | 0.25–0.50 | Liu et al. (2013) |
| Trachypithecus obscurus and T. phayrei | 0.36 | He et al. (2012) |
| Trachypithecus cristatus and T. germaini | 0.55 | He et al. (2012) |
| Rhinopithecus bieti and R. strykeri | 0.24 | Liedigk et al. (2012) |
| 0.30 | Zhou et al. (2014) | |
| Pygathrix cinerea and P. nemaeus | 0.23 | Liedigk et al. (2012) |
| AMNH |
USA, New York, New York, American Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.