Adelophryne gutturosa Hoogmoed and Lescure, 1984
|
publication ID |
https://doi.org/ 10.5281/zenodo.274492 |
|
DOI |
https://doi.org/10.5281/zenodo.5613156 |
|
persistent identifier |
https://treatment.plazi.org/id/03A3E47E-FFFA-794C-FAA1-B5AD0A0AE683 |
|
treatment provided by |
Plazi |
|
scientific name |
Adelophryne gutturosa Hoogmoed and Lescure, 1984 |
| status |
|
Adelophryne gutturosa Hoogmoed and Lescure, 1984 View in CoL
Adelophryne gutturosa Hoogmoed and Lescure, 1984: 101 View in CoL ; Ayarzagüena and Diego-Aransay, 1985: 159; Hoogmoed, Borges and Cascon 1994: 272; Barrio-Amorós 2004: 19; Señaris and MacCulloch 2005: 21.
Adelophryne gutturosa View in CoL was first described by Hoogmoed and Lescure (1984) from specimens from Mount Roraima, Guyana, and Amapá, Brazil. Ayarzagüena and Diego-Aransay (1985) reported a single specimen, a gravid female of 16 mm SVL, from La Escalera, Venezuela. Hoogmoed et al. (1994) provided an additional description based on two new specimens from Amapá. Fifteen specimens of A. gutturosa View in CoL were recently collected from Guyana; two on Mount Wokomung at the same location as the holotype of A. patamona View in CoL , one at Mount Ayanganna (05° 24' N, 0 59° 57' W, 870 m) 10 from Kaieteur National Park (05° 10' N, 059° 29' W, 400–550 m), two from the vicinity of the Meamu River in the northern Pakaraima region (06° 16' N, 0 60° 30' W, 664 m) and two from La Laja, Sierra de Lema, Bolívar, Venezuela (06° 03' N, 0 61° 29' W, 450 m). The specimens from Wokomung are a female of 13 mm SVL and a male of 13.5 mm SVL. The specimen from Ayanganna is a male of 14 mm SVL. The specimens from Kaieteur National Park are six males of 13.3–14.7 mm SVL and four females of 14.6–15.0 mm SVL and the specimens from Meamu are two females of 15.3 and 16.0 mm SVL. The specimens from La Laja are a male of 15 mm and a juvenile of 13 mm SVL. Advertisement calls were recorded at Kaieteur National Park between 2–7 December 2005 and at two additional sites on the headwaters of the Meamu River: 0 6° 10' N, 0 60° 26' W, 703 m, on 28 December 2004 and 0 6° 12' N, 0 60° 29' W, 781 m on 2 September 2004.
These 17 specimens expand the existing descriptions of A. gutturosa View in CoL as follows: head width 90% head length; shanks 45–52% SVL; palpebrum not reticulated, a black band along its upper rim.
The specimens from Ayanganna, Wokomung, Kaieteur, Meamu and La Laja differ from the previous descriptions of A. gutturosa as follows (previously described specimens in parentheses): distance from eye to tip of snout variable, 75–85% eye diameter in specimens from Ayanganna, Wokomung, Meamu and La Laja, 100–120% eye diameter in specimens from Kaieteur (equal); upper eyelid width variable, 70–100% interorbital distance (50%); teeth 7–8 in females, 8 in males at Ayanganna, Wokomung and Meamu, 4 in females, 3– 4 in males at Kaieteur (2, 4–6). In some specimens from Kaieteur and La Laja the skin on the dorsum is shagreened (smooth), and the temporal region and flanks are granular. These differences are within the range of interpopulation variation.
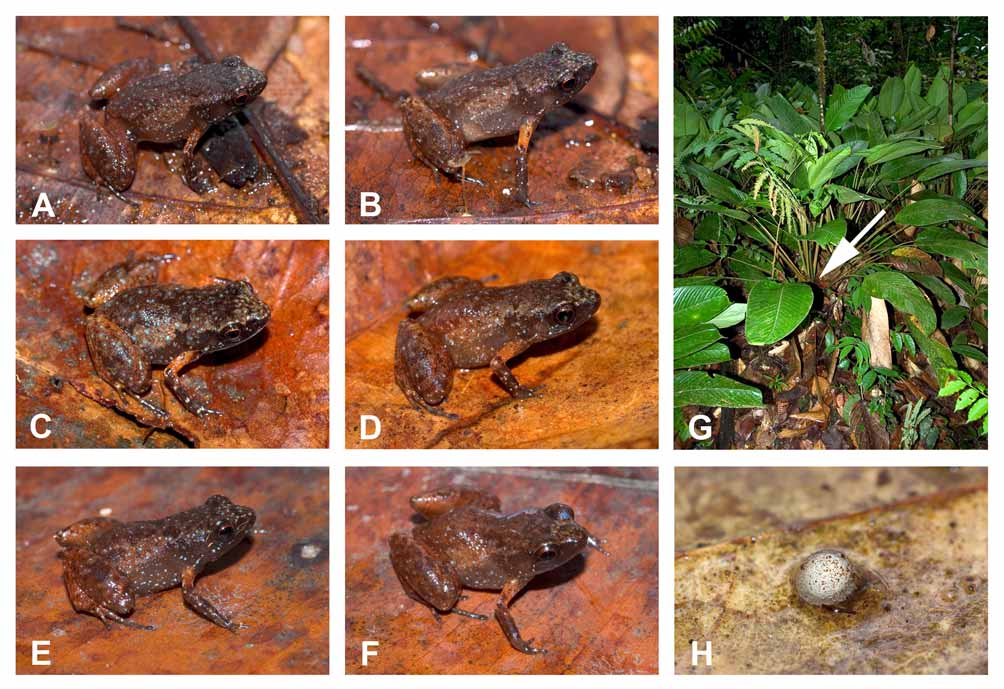
Colour in life. Considerable variation in colouration is evident, both within and among populations. Dorsal ground colour medium brown or grey. A middorsal black “)(“, black canthal and supratympanic bars and other scattered black marks may be present; numerous irregular sky blue or white dots on dorsum and flanks; dorsal surfaces of hindlimbs and forearms medium brown or grey, with or without black marks or crossbands and small irregular sky blue or white dots; upper arm orange ( Fig. 9 View FIGURE 9 A–F). In the two specimens from Meamu the flanks and upper surfaces of thighs are whitish with dark stippling and no crossbands. Venter brown or grey with small irregular sky blue or white dots; iris copper, with a red ring around pupil ( Fig. 9 View FIGURE 9 A–F). In preservative the orange and sky blue colours become white, otherwise there is little change.
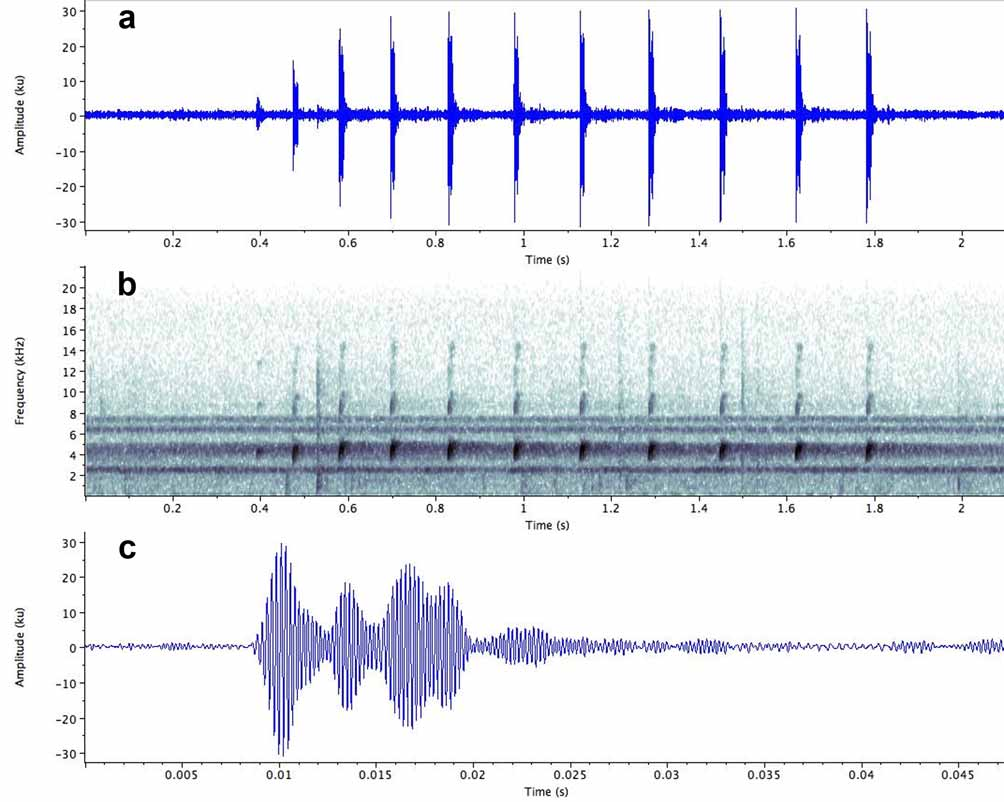
Vocalisation ( Fig. 10 View FIGURE 10 ; Tables 3–4 View TABLE 3 View TABLE 4 ). The following description is based on a sample of 29 advertisement calls from 12 males at the three localities.
Temporal structure. The advertisement call consists of a group of short notes produced in quick succession, with the interval between notes increasing progressively from the beginning to the end of the call ( Fig. 10 View FIGURE 10 ). Consequently, calls containing only a few notes have a higher note repetition rate than those containing many notes, since in the latter the notes are on average more widely spaced. Thus a 2-note call from Kaieteur National Park (specimen IRSNB 13784) has a duration of 0.058s and a note repetition rate of 22.22 notes/s; a 7-note call from the same locality (specimen IRSNB 13791) has a duration of 0.85s and a note repetition rate of 7.26 notes/s, and a 15-note call from Meamu has a duration of 2.39s and a note repetition rate of 6.2 notes/ s. Call duration varies considerably, both between, and within individuals participating in the same chorus and may reflect social context (eg. territorial encounters or proximity to a female). At Kaieteur, a male situated close to a female produced 2-note calls continuously.
Each note contains 4–5 high amplitude pulses which are followed by up to 7 low amplitude pulses ( Fig.10 View FIGURE 10 c). The pulses vary in duration and are unevenly spaced: some are clearly separated from adjacent pulses, while others merge. Pulse rate therefore varies within and between notes and is unlikely to play a role in mate recognition.
Spectral structure. Three to four harmonics are developed, with the fundamental frequency dominating (range: 3896 – 4979 Hz; mean: 4242 Hz). The distribution of sound energy decreases progressively through the 2nd, 3rd and 4th harmonic. The frequency rises slightly from the beginning to the end of the note.
Vocalisation parameters of three Adelophryne species are compared in Table 4 View TABLE 4 .
Natural history. Specimens were collected in forest, in late afternoon or early evening. Specimens from Ayanganna, Wokomung and Meamu were collected in leaf litter. Most specimens from Kaieteur were closely associated with formations of Monotagma spicatum (Marantaceae) , locally named Dalibana ( Fig. 9 View FIGURE 9 G). A few individuals were observed sheltering at the base of other vegetation, although M. spicatum appears to be the preferred habitat. Males were usually found calling from the base of the plant or hidden among the rootlets; some were observed calling among dead leaves near the base of the plant. Most of the females were collected close to calling males. Small size and cryptic colouration make A. gutturosa difficult to locate, even when calling. When disturbed the frogs move slowly or remain motionless among rootlets or in leaf litter. If disturbed while away from shelter, they can rapidly jump considerable distances.
A few ants were found in the stomachs of two specimens.
Once we became familiar with the call of A. gutturosa , we found that this species is locally abundant. Choruses were heard between 0300–0600 and 1800–1930 in several locations in Kaieteur and Meamu. In some localities such as the Elinkwa River in Kaieteur National Park, and La Laja, A. gutturosa is the most common frog heard in late afternoon, early evening or during rains.
On 2 December 2005 at 1540 a male A. gutturosa (IRSNB 13784) was located by call and a female (IRSNB 13785) was observed, immobile, in close proximity. Both specimens were collected and kept overnight in a plastic container with some dead leaves. The next morning a single large egg of 4.63 mm external diameter was found among the dead leaves ( Fig. 9 View FIGURE 9 H). The large size of the egg, 31% of the female’s SVL, supports the hypothesis of direct development in this species ( Ayarzagüena and Diego-Aransay 1985). The short wet season in Guyana begins in December; Ayarzagüena & Diego-Aransay (1985) and Hoogmoed et al. (1994) theorized that oviposition takes place at the beginning of the rainy season (to minimize desiccation of terrestrial eggs) It is likely that eggs are laid among plant roots.
Our observations indicate that males exhibit site fidelity, positioning themselves as close as 2 m from adjacent calling males. Males rarely call simultaneously, although the call of one male usually prompts an adjacent male to call and there is a slight overlap between the two calls. Calls are emitted in successive waves; due to the nature of the call, this creates a very powerful “cascading chorus”.
Distribution and relationships. Adelophryne gutturosa is a widespread species, whose range extends from Amapá, Brazil, to Bolívar, Venezuela, a distance of some 1500 km. Elevation ranges from 110 and 300 m in Amapá ( Hoogmoed et al. 1994), through 400–550 m at Kaieteur, 450 m at La Laja, 664 m at Meamu, 870 m at Ayanganna, 900 m at Roraima ( Hoogmoed & Lescure 1984), 950 m at Auyán ( Señaris & Ayarzagüena 2006), approximately 1100 m at La Escalera ( Ayarzagüena & Diego-Aransay 1985), 1234 m at Wokomung, to 1400 m at Maringma. This broad geographical and elevational range invites further study into the possible presence of cryptic species within A. gutturosa .
Although they may be locally abundant, the small size and secretive habits of Adelophryne make discovery difficult. There are numerous large gaps in the known distribution of Adelophryne ( Hoogmoed et al. 1994) . Calls could be used to verify the presence of these frogs during biodiversity surveys.
Several characters such as number of phalanges, digital discs and pads, skin texture and colour pattern have been used to distinguish among species of Adelophryne and among genera of small eleutherodactylids ( Hoogmoed & Lescure 1984). Nonetheless, understanding of the phylogeny of these poorly known animals will require considerable further sampling. Relationships of this group were discussed by Lynch (1986). As pointed out by Heyer (1977) and Da Silva et al. (2007), many of the characters present in these taxa may be due to convergence, and may not reflect relationships.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Adelophryne gutturosa Hoogmoed and Lescure, 1984
| Macculloch, Ross D., Lathrop, Amy, Kok, Philippe J. R., Minter, Leslie R., Khan, Samir Z. & Barrio-Amorós, César L. 2008 |
Adelophryne gutturosa
| Senaris 2005: 21 |
| Barrio-Amoros 2004: 19 |
| Hoogmoed 1994: 272 |
| Ayarzaguena 1985: 159 |
| Hoogmoed 1984: 101 |