Atalotegaeus Luxton, 1988
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5365.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:1DC72714-D0E8-49D8-821D-03C6B2A7AE80 |
|
DOI |
https://doi.org/10.5281/zenodo.10248601 |
|
persistent identifier |
https://treatment.plazi.org/id/03A2C77C-467F-FFD3-C79C-B77D17C3DA74 |
|
treatment provided by |
Plazi |
|
scientific name |
Atalotegaeus Luxton, 1988 |
| status |
|
Atalotegaeus Luxton, 1988 View in CoL
Atalotegaeus Luxton, 1988a View in CoL . p. 82.
Type species: Eutegaeus mensarosi J. and P. Balogh, 1983a, p. 83.
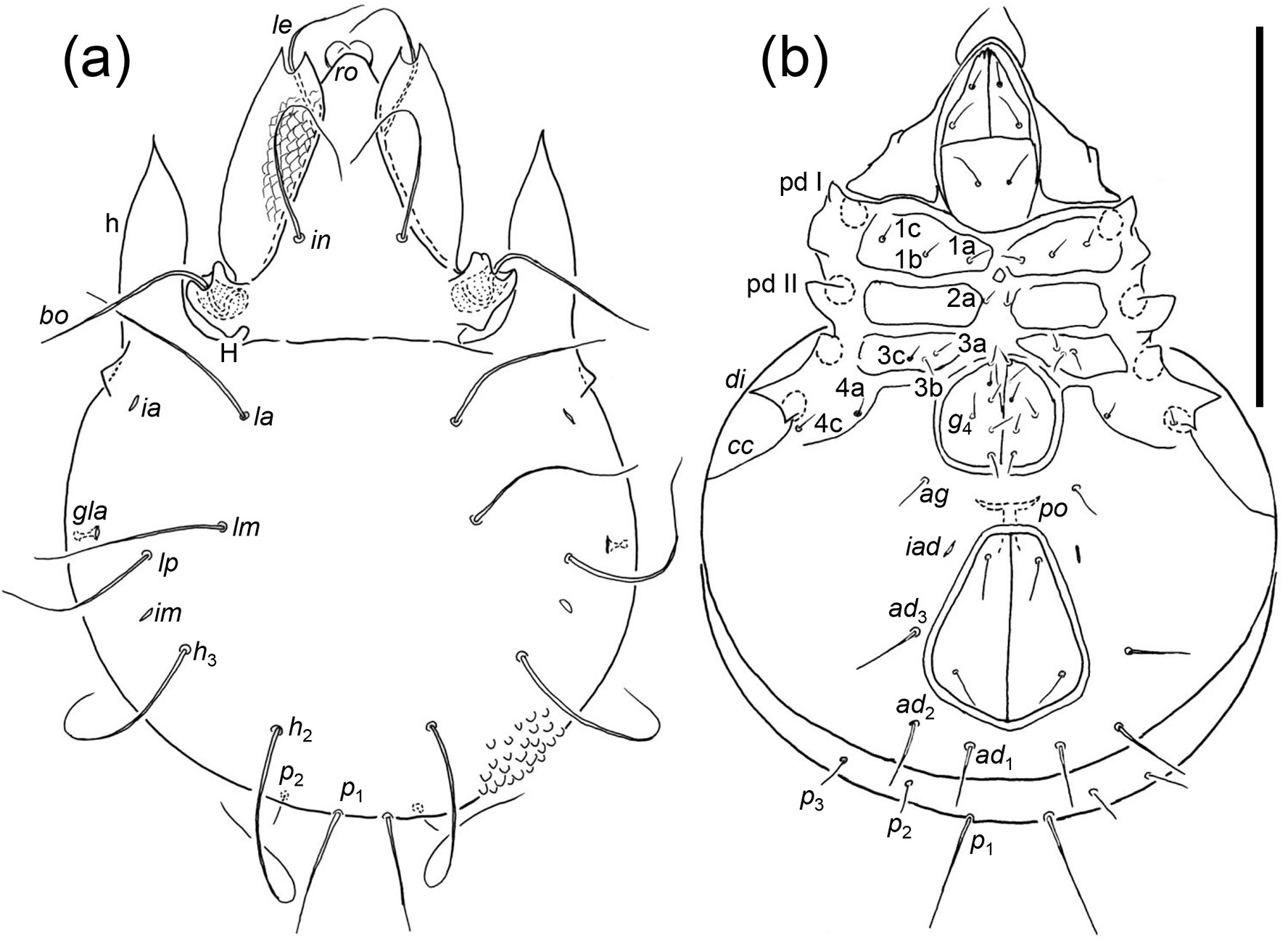
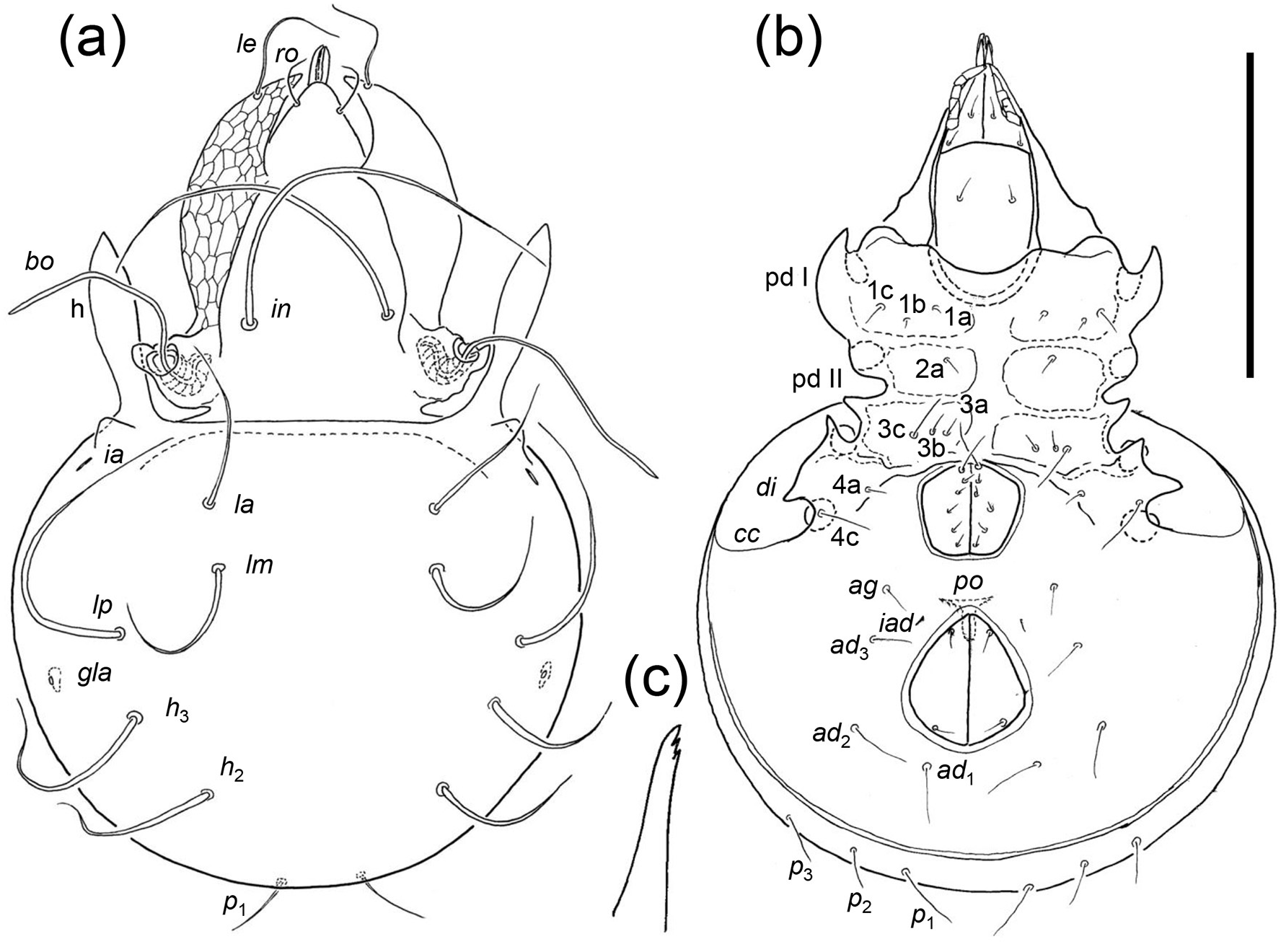
Diagnosis. the following diagnosis is modified from that of Luxton (1988a) and includes new character states for immatures. Adults: medium sized oribatid mites (600–700 μm); rostrum broadly conical; lamellae delicate and transparent, lamellar cusps about one-third of lamellar length, extending just beyond rostrum, concave medially, with single tooth apically, lamellar setae typically positioned sub-apically (apically in A. monteithi comb. nov.); lamellae separate medially, translamella incomplete or absent, lateral margin strongly convex, covering lateral margins of prodorsum. Interlamellar setae long, overlapping, recurved or flagelliform. Posterior margin of prodorsum generally lacking condyles, though anterior condyle of enantiophysis H present in A. monteithi ; humeral processes long, narrow and delicate, originating on anterior margin of notogaster, reaching to point level with middle of lamella, extremely narrow and waisted basally. With rounded or pointed projection at base of humeral process. Seven pairs of notogastral setae (lm absent) or eight pairs; l series in centrodorsal position, recurved. With five or six pairs of genital setae, penultimate pair displaced laterally. Perigenital carina and enantiophyses E4 absent. Pre-anal organ T-shaped. Chelicerae chelate-dentate and of normal proportions or narrow and elongated. Immatures: margin of prodorsum with two pairs of incisions between bothridia and apex of lamellae. Gastronotal setae emerging from tubercles, not scales, except tritonymphal seta c 3 emerging from a flat extension of the humeral region that is rounded anteriorly and pointed posteriorly; deutonymphal and protonymphal seta c 3 emerging from tubercle. Tritonymph with transverse groove posterior of dorsosejugal scissure.
Remarks. J. and P. Balogh (1983a) described Eutegaeus mensarosi from Australia which was recombined with the newly-established genus, Atalotegaeus , by Luxton (1988a) who differentiated it from other genera of Eutegaeidae by its ‘peloptoid’ (or pelopsiform) chelicerae and narrow subcapitulum. Some Atalotegaeus spp. have chelate-dentate chelicerae of normal proportions (e.g. A. crobylus sp. nov.), whereas others have modified, slim chelicerae (cf. below) but they are not truly pelopsiform in that the basal part is not considerably broader than the rest of the chelicera as in Eupelops acromios ( Hermann, 1804) (cf. Grandjean, 1936, Fig. 11b View FIGURE 11 therein). These chelicerae are in fact more like those of Allosuctobelba and other Suctobelbidae (cf. Grandjean, 1951): slim and tapering, or ‘attenuate-edentate’ ( Norton and Behan-Pelletier (2009, Fig. 15.8.K View FIGURE 15 therein). The subcapitulum is unlike that of the peloptoid form found in the Phenopelopidae in which the rutella are considerably narrowed apically, lack teeth, and the genae are fused with the mentum and, hence, lack the labiogenal articulation. Some Atalotegaeus spp. have a long, narrow subcapitulum and a slim, but toothed, rutellum ( Fig. 15b View FIGURE 15 ) but the labiogenal articulation is present and the apices of the genae are toothed. For A. mensarosi , J. and P. Balogh (1983a, p. 82) stated ‘mouth parts suctorial’ but without specifying which parts. Accordingly, I consider the character states of the mouthparts alone are insufficient to differentiate Atalotegaeus from other Eutegaeidae . Rather, the thin, narrow humeral processes ( Luxton, 1988a, p. 82 described them as ‘delicate and transparent’), expanded lamella with a long, incurved tooth and the sub-apical emergence of the lamellar setae are more consistent character states for the adults. Also, there are morphological differences between immatures of Eutegaeus and Atalotegaeus , as detailed in the generic definitions above, most notably the tritonymphal seta c 3 of Atalotegaeus emerging from a flat, humeral extension, pointed posteriorly, and from a tubercle in the deutonymph and protonymph, whereas in Eutegaeus c 3 emerges from a scale in all the nymphal stages. These character states are consistent among immatures of the two genera, as detailed below.
Ermilov (2021) described adults and immatures of Eutegaeus aysenensis and E. queulatensis from Chile. The adults have an incurved lamellar tooth with a sub-apical lamellar seta and thin humeral processes, typical of species of Atalotegaeus . The notogastral setae of the nymphs emerge from tubercles, as in the tritonymph of A. mensarosi (cf. below) and lack elongate, hemi-semicircular scales found in immatures of all Eutegaeus spp. described to date ( E. woiwurrung , E. nothofagi , E. parapapuensis Ermilov, 2020 and E. paralagrecai Ermilov, 2020 ). Accordingly, Eutegaeus aysenensis and E. queulatensis are hereby recombined as Atalotegaeus aysenensis (Ermilov, 2021) comb. nov. and Atalotegaeus queulatensis (Ermilov, 2021) comb. nov. These, with the new species described below and the recombination of N. monteithi to Atalotegaeus , increases the total number of described species of Atalotegaeus to five.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Atalotegaeus Luxton, 1988
| Colloff, Matthew J. 2023 |
Atalotegaeus
| Luxton 1988 |