Tenagodus barbadensis, Bieler, 2004
|
publication ID |
https://doi.org/ 10.1111/j.1096-3642.2003.00104.x |
|
publication LSID |
lsid:zoobank.org:pub:8773D6CE-6F0B-4F28-AA6D-4CE788B4C6C3 |
|
persistent identifier |
https://treatment.plazi.org/id/039F87F5-7041-231B-ADA4-FBDCCCFFFB66 |
|
treatment provided by |
Diego |
|
scientific name |
Tenagodus barbadensis |
| status |
|
(SYNONYM TENAGODIDAE GILL, 1871 )
GENUS TENAGODUS GUETTARD, 1770 View in CoL
Type species by subsequent designation ( Adams & Adams, 1853 -4: 360–361): Serpula anguina Linnaeus, 1758 (Indo-Pacific). Linnaeus’ (1758: 787) Serpula anguina was based on more than one species, and the Linnaean collection contains several taxa under this name, without indication of type specimen ( Hanley, 1855: 448). Linnaeus had adopted the species name from the nonbinominal ‘ Solen anguinus ’ of Rumphius (1705: 125) and referred to illustrations in that work. The specimen illustrated by Rumphius (1705: pl. 41, fig. H) from Ambon, Moluccas, Indonesia, as cited by Linnaeus, is here selected as lectotype. Siliquaria Bruguière, 1789 , is an objective synonym of Tenagodus , with Serpula anguina Linnaeus, 1758 , as type species by subsequent monotypy ( Lamarck, 1799: 79). The complex synonymies at the generic and familial levels were discussed by Bieler (1992).
TENAGODUS MODESTUS ( DALL, 1881) View in CoL
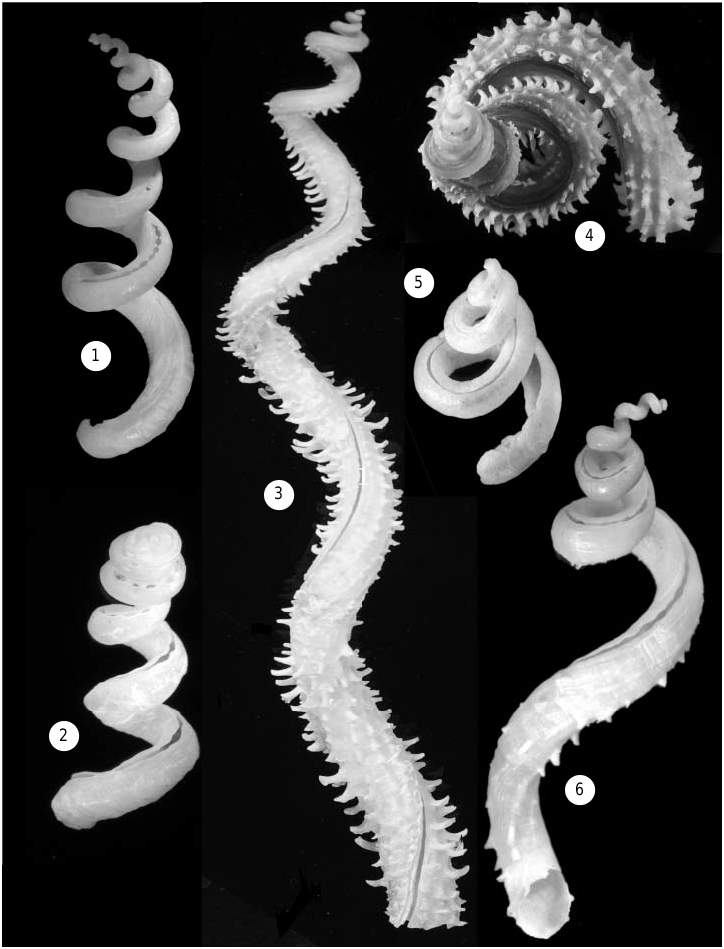
( FIGS 1, 2 View Figures 1–6 , 7–9 View Figures 7–14 , 26–28, 31–33 View Figures 26–31 View Figures 32–35 , 36, 37 View Figures 36–37 , 41–43 View Figures 41–43 )
Siliquaria modesta Dall, 1881: 39 View in CoL . Tryon, 1886: 191. Paetel, 1888: 499. Agassiz, 1888: 71, fig. 296. Dall, 1889a: 260, pl. 26, fig. 4. Dall, 1889b: 144, pl. 26, fig. 4. Dall, 1896: 25 [‘The first reported since the original types’]. Maury, 1922: 104. Abbott, 1974: 96, fig. 926 (from Dall, 1889a,b). Rios, 1985: 47, pl. 18, fig. 210. Rios, 1994: 66, pl. 22, fig. 253. Díaz & Puyana, 1994: 140, pl. 44, fig. 481. Pointier & Lamy, 1998: 48. Hartman & Hubbard, 1999: 1 ff.
Siliquaria View in CoL ? modesta: Mikkelsen, 1981: 47 View in CoL .
Tenagodus modestus: Bieler, 1990: 21 View in CoL . Leal, 1991: 69. Pansini et al., 1999: 429.
Type material: Dall (1881: 39) described this species based on R/V Blake material ‘found in all depths from 80 to 800 fathoms.’ He did not formally select a type specimen but added, ‘The specimen from which the description was taken lived in 220 fathoms (Station 20)’, a locality off Bahia Honda, Cuba. Eight years later, Dall (1889a: 260, pl. 26, fig. 4) listed individual stations, including number 20, and illustrated a specimen without indicating its provenance. Agassiz (1888: 71, fig. 296) illustrated another specimen from an unspecified Blake station. A comparison of the original material in the USNM and MCZ collections showed Dall’s (1889a) figure to be based on a specimen from the originally cited Blake station 20. The 22.8 mm specimen ( MCZ 341075; Fig. 2 View Figures 1–6 ) is here selected as lectotype, with three paralectotypes remaining as MCZ 7419. The type locality is here thus restricted to US Coast Survey Steamer Blake station 20, off Bahia Honda, Cuba, Blake Expedition 1877–78, 220 fathoms (402 m), 23∞02¢30≤N, 83∞11¢00≤W.
Specimens examined: See Appendix 1.
Description
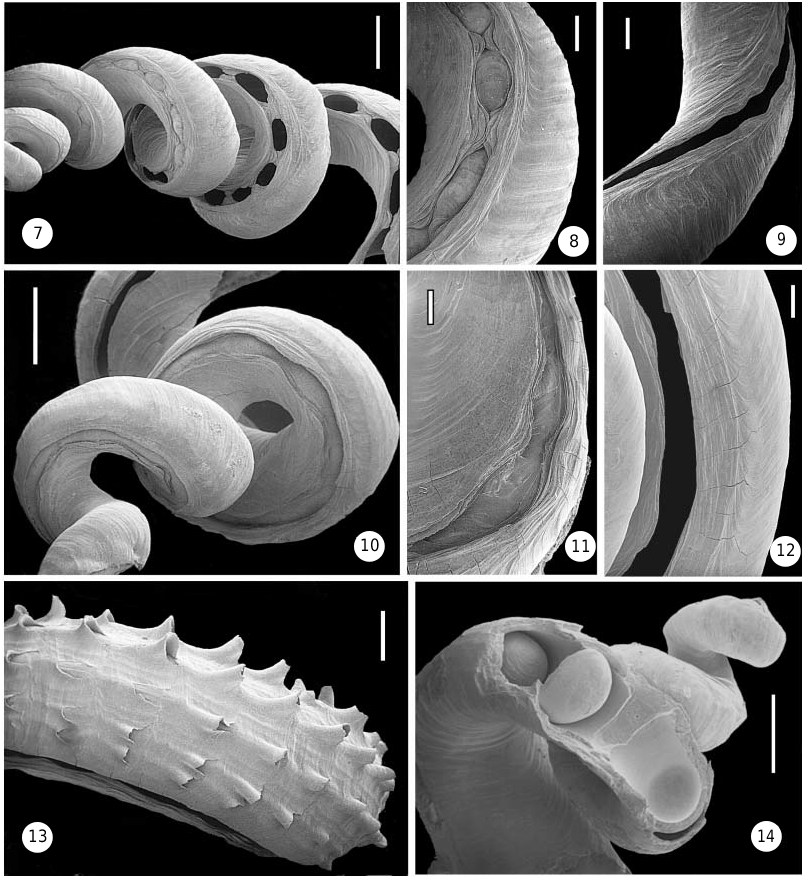
Shell (teleoconch) ( Figs 1, 2 View Figures 1–6 , 7–9 View Figures 7–14 , 42, 43 View Figures 41–43 ). Relatively large, very loosely coiled; specimens usually less than 8 cm in total length, with inner apertural diameter of 4–6 mm, but occasionally reaching a length of 15 cm ( USNM 86776, Trinidad). Sculpture consisting entirely of incremental growth marks, without spiral ribs or striae. Earliest whorls often internally closed off by concave calcareous septa (as shown in Fig. 14 View Figures 7–14 for a different species). Early part of open shell slit (corresponding to region of posterior mantle cavity) narrowed by lateral ingrowths to a – usually very regular – series of holes ( Fig. 7 View Figures 7–14 ); the most recent (body whorl) slit left open with slightly undulating margins ( Fig. 9 View Figures 7–14 ). Slit of earlier whorls (now in the region of digestive gland and gonad) filled-in with shell material that often protrudes outward through the holes ( Figs 7, 8 View Figures 7–14 ). Fresh specimens have somewhat glossy shell surface, particularly on early whorls; later whorls often chalky externally. Colour off-white, sometimes with light tan mottling.
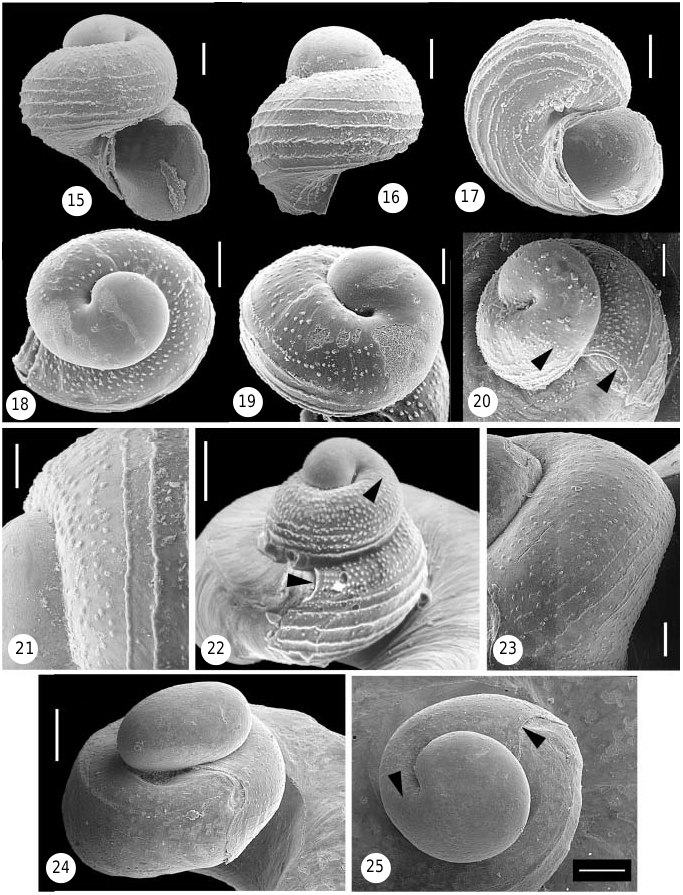
Protoconch ( Figs 15–21 View Figures 15–25 ). Fragile, easily decollated and rarely retained in museum specimens [in fact, Dall’s (1889a: 260) original description of live material maintained that it ‘shows no sign of a regularly formed spiral nucleus’], approximately 212– 240 Mm high and 200–216 Mm wide, with 2 whorls. First whorl represents smooth, bulging embryonic shell (protoconch I, hereafter PC-I), about 129– 133 Mm in diameter, with circular aperture; second whorl formed by helically coiled, broadly rounded, sculptured larval shell (protoconch II, hereafter PC-II). Sculpture consisting of about 5 very irregular rows of distinctly demarcated pustules on upper part of whorl, followed below by about 7 finely granulated spiral striae (which appear as rows of fused pustules). Areas between striae and region around open larval umbilicus with more or less distinct irregular rows of finer pustules. Aperture strongly sinuous, deeply embayed on upper whorl surface at point of contact with teleoconch slit area. Translucent white, lower half of PC-II brown.
Operculum ( Figs 26–28 View Figures 26–31 , 36, 37 View Figures 36–37 ). Large (2.5–2.8 mm in diameter) in relation to body width, dome-shaped, multispiral, tightly fitting inside shell tube, composed of 20–30 spiral layers of corneous lamella. Edge of each layer beset with rounded, thorn-like spines. Lamella not continuous internally: core filled with triangular compartments, about 6 per whorl, spirally arranged around a central axis.
Anatomy ( Figs 31–33 View Figures 26–31 View Figures 32–35 , 36, 37 View Figures 36–37 ; for measurements, n = 12).
Head-foot: Body long and slender, spirally coiled, forming approximately 3 whorls, 2 of which occupied by mantle cavity; overall body length of preserved animal (excluding operculum) 35–42 mm. Mantle cavity up to 30 mm long; body width in mid-mantle region approximately 1.3 mm. Foot forming a round column bearing the large operculum; small (0.6 ¥ 1.0 mm), glandular, transversely furrowed area of foot (the ‘sole’) in front of the mouth. Head small in comparison to width of body; snout short, bluntly rounded, with mouth a vertical slit at its tip. Two cephalic tentacles about as long as snout, with black eyes at their outer bases. Mantle margin simple but with small weak papillae on inner side. Head-foot and area between mantle papillae with brownish pigment (black before preservation?). No pedal tentacles or pedal mucous gland.
Pallial cavity: Corresponding to shell fissure, dorsal right side of mantle with slit, longitudinally opening mantle between rectum and pallial gonoduct. Ctenidium extending as far as mantle slit, its filament lengths gradually decreasing posteriorly; curved, rodshaped gill filaments long (to 1.8 mm), narrow (about 30 ¥ 80 Mm in cross section), and very flexible ( Fig. 31 View Figures 26–31 ). In addition to rows of relatively short frontal and abfrontal cilia, a row of very long (up to 40 Mm) lateral cilia on either side; thickened, club-shaped tip ciliated (details not observed due to poor preservation). Row of filaments extends across mantle cavity top right side of animal. Osphradium restricted to anterior part of mantle cavity, beginning next to and shortly behind anteriormost gill filaments; about 2.4 mm in total length, compressed-S-shaped, with posterior leg the longest; its sensory zone with weak meanders. Endostyle forming a narrow glandular tract between gill axis and osphradium, along entire length of gill. Sharply demarcated food groove on dorsum of animal, with thick, tall ridges; groove extending straight in about mid-line from posterior end of mantle cavity to neck region and then looping around right side of head, ending with spoon-like process at mouth opening ( Fig. 37 View Figures 36–37 , sp.).
Alimentary system: Mouth opening flanked by pair of slender jaws made up of platelets in palisade-like fashion. Simple oesophagus (without dorso-ventral division or crops) with two salivary glands, the latter somewhat increasing in bulk posteriorly and ending well in front of concentrated nerve ring, passing straight to stomach as a simple tube. Digestive gland a single lobe. Simple intestine loops forward and continues as short rectum (about 2.4 mm within mantle cavity), with anal opening in posterior part of mantle cavity; weak ciliated groove leading from anus forward to mantle cavity. Stomach with style sac and gastric shield. Radula small ( Figs 32, 33 View Figures 32–35 ; length 0.94–1.00, width 0.10–0.12 mm), taenioglossate, with 52–54 rows, transparent; rachidian with strong, finger-like projecting main cusp and 8–12 short flanking cusps on either side, smooth basal platform, no lateral or basal projections. Lateral tooth with slender, triangular main cusp and 8–12 short inner flanking cusps and about 20 outer flanking cusps. Marginal teeth hooklike, bluntly tipped; cutting edge of inner marginal tooth finely cuspidate on either side, of outer marginal tooth smooth.
Reproductive system: All sexable animals (5) female. Ovipositor ( Fig. 37 View Figures 36–37 , ov) with central groove anterior and to the right of head.
Habits and habitat
According to Dall (1881: 39, original description): ‘Found at all depths from 80 to 800 fathoms [146– 1463 m], but not in less than 80 fathoms. The specimen from which the description was taken lived in 220 fathoms’. Hartman & Hubbard (1999) described a large aggregation in a sponge ( Thrombus ) at a depth of 21.5 m ( Trinidad). Live material in the present study came from between 56 and 73 m, while shells were recorded from between 18 and 1472 m. Tenagodus modestus was originally described from live material ( Dall, 1881: 39), but no reference was made to its anatomy or even to the fact that it was collected in or with a sponge. Specimens in the present study were found completely embedded in a colony of an unidentified species of Thrombus (Porifera: Demospongiae: Choristida: Thrombidae ; S. Pomponi, pers. comm.) at c. 56 m. In situ , only the apertural openings were visible from the outside. The density of individuals inside the sponge was very high, with about 54 shell apertures emerging from a surface area of 5 ¥ 5 cm. This translated into 5400 snails per surface square metre of the sponge colony (see also Figs 42, 43 View Figures 41–43 ). There was relatively little sponge tissue between the gastropods, explaining the intertwined and contorted condition of shells as a result of competition for space.
Geographical distribution
Widely distributed in deeper waters of the western Atlantic, ranging from Bermuda, Florida, Bahamas to Brazil (Bahia to Espírito Santo, all seamounts; Rios, 1994), the Gulf of Mexico and the Caribbean Sea.
Taxonomic remarks
Tenagodus modestus View in CoL was once considered to be ‘the young’ of T. squamatus ( Abbott, 1954: 145) View in CoL . Many collection specimens labelled ‘ modestus View in CoL ’ were found to be juvenile or strongly eroded (and thus smooth-shelled) T. squamatus View in CoL . The latter species has fluted spines on its shell, does not have its shell slit narrowed to form a regular series of holes, and its protoconch is very different (see below).
TENAGODUS SQUAMATUS ( DE BLAINVILLE, 1827) View in CoL
( FIGS 3–5 View Figures 1–6 , 10–13 View Figures 7–14 , 22 View Figures 15–25 , 29, 30 View Figures 26–31 , 35 View Figures 32–35 , 38–40 View Figures 38–40 )
Siliquaria squamata de Blainville, 1827: 213 View in CoL . Chenu, 1842 -43: 3, pl. 2, fig. 12. Mörch, 1861: 414. Paetel, 1869: 56. Dall, 1889a: 260. Dall, 1889b: 144. Nutting, 1920: pl. 40, fig. 2. Maury, 1922: 103–104. Peile, 1926: 78. Merrill & Petit, 1965: 60. Gould, 1966: 1–11, figs 1-3, 6 (photographs of protoconchs reversed). Abbott, 1968: 84, fig. 4. Rios 1970: 40, pl. 8, fig. Abbott, 1974: 96, fig. 925. Rios, 1975: 46, pl. 13, fig. 171. Emerson & Jacobson, 1976: 74, pl. 6, colour fig. 1. Abbott & Dance, 1982: 61, fig. Rios, 1985: 46, pl. 18, fig. 209. De Jong & Coomans, 1988: 40. Leal, 1989: 8, fig. 9 (SEM photomicrograph of protoconch and juvenile teleoconch). Harasewych, 1989: 48, pl. 32. Lipe & Abbott, 1991: 52, fig. on 53. Rosenberg, 1992: 52–53, fig.? Rios, 1994: 66, pl. 22, fig. 252. Díaz & Puyana, 1994: 140, pl. 44, fig. 482.
Siliquaria View in CoL squammata [sic], Chenu, 1842 -43: figure caption, pl. 2, fig. 12 (erroneously giving ‘ nobis ’, i.e. Chenu, as authority). Chenu, 1859: 322, 309 (from Chenu, 1842 -43).
Tenagoda [sic] squamata, Adams & Adams, 1853 -54: 361.
Tenagodus squamata, Mörch, 1861: 414 View in CoL .
Tenagodus squamatus, Mörch, 1865: 99 View in CoL . Mörch, 1877: 110. Abbott, 1954: 145, pl. 21, fig. G. Parker & Curray, 1956: 2434. Bieler, 1990: 21. Leal, 1991: 68, pl. 6, figs I, J. Bandel & Kowalke, 1997: 263. Turgeon et al., 1998: 69, 213. Pansini et al., 1999: 429. Redfern, 2001: 23, pl. 12, fig. 95A, B.
Tenagodus (Pyxipoma) anguillae Mörch, 1861: 410 View in CoL , 412. Mörch, 1877: 110.
Tenagodus (Agathirses) squamata, Mörch, 1861: 411 View in CoL . Siliquaria (Tenagodus) View in CoL aquillae [sic] Mörch, Sowerby in Reeve, 1876: last page (‘species not known’).
Tenagodus ruber (Schumacher) View in CoL , Mörch, 1877: 109 [ Bermuda and St. Thomas] [non Anguinaria rubra Schumacher, 1817 View in CoL ].
Siliquaria (Agathirses) View in CoL anguina (Linnaeus), Tryon, 1886) [in part]: 190, pl. 58, fig. 25 (after Chenu, 1842 - 43) [non Serpula anguina Linnaeus (1758) ].
Siliquaria View in CoL anguina Vr. squamata, Paetel, 1888: 499 View in CoL .
Siliquaria anguillae, Paetel, 1888: 499 . Menzies et al., 1966: 408 [as S. angullae ], 414, 428. Morris, 1973: 145, pl. 41, fig. 9. Wolfe & Wolfe, 1970: 14. Porter, 1974: 15. Lipka, 1974: 148, 168, fig. 13. Warmke & Abbott, 1975: 67, pl. 12 fig. g.
Siliquaria (Tenagodus) View in CoL ruber Schumacher, Heilprin, 1889: 172 [ Bermuda, following Mörch].
Siliquaria View in CoL ruber Schumacher, Dall, 1889a: 259 View in CoL .
‘ Tenagodus View in CoL , or Siliquaria View in CoL ruber View in CoL ’ Verrill, 1905: 139 [ Bermuda].
Siliquaria rubra, Peile, 1926: 78 [ Bermuda, following Verrill].
Tenagodus (Agathirsus) squamatus, Haas, 1941: 171 View in CoL . Siliquaria View in CoL angullae [sic] Mörch, Abbott, 1974: 96 (in synonymy). Rios, 1985: 46 (in synonymy). Rios, 1994: 66 (in synonymy).
Type material: De Blainville’s original material of Siliquaria squamata has not been located. A lectotype for Tenagodus anguillae Mörch, 1861 , is here designated ( Anguilla, Leeward Islands; before 1848; ex coll. Hornbeck; ZMK; Fig. 5 View Figures 1–6 ), from a lot that also contains 1 paralectotype and 1 loose operculum.
Specimens examined: See Appendix 2.
Description
Shell (teleoconch) ( Figs 3-5 View Figures 1–6 , 10–13 View Figures 7–14 ). Large and very loosely coiled; some specimens exceed a total length of 17 cm and inner apertural diameter of 10 mm. Juvenile shell with little sculpture except growth marks; shell later develops faint longitudinal lines becoming rows of spines. Smaller specimens with seven, larger lines with 9–12 longitudinal rows of spines (occasionally more numerous, sometimes with finer ridges interspersed, especially near shell slit); spines strongest on underside of coils, and nearly absent in what would be the umbilicus in a regularly coiled shell. Spines curved and fluted tube-like, with open side facing aperture. Later part of shell has healed breakages throughout. Earliest whorls often internally closed off by concave calcareous septa (as shown in Fig. 14 View Figures 7–14 for a different species). Early part of open shell slit (corresponding to region of posterior mantle cavity) narrowed by lateral ingrowths, but not normally forming complete holes ( Fig. 11 View Figures 7–14 ); the most recent (body whorl) slit barely restricted, with near-even margins ( Fig. 12 View Figures 7–14 ). Slit of earlier whorls (now in the region of digestive gland and gonad) filled-in with shell material ( Figs 10, 11 View Figures 7–14 ). Fresh specimens with glossy shell surface; strongly eroded specimens often without spines, but with longitudinal sculpture still discernible. Shell light tan to reddish brown, darker near shell slit (older specimens usually faded to yellowishwhite).
Protoconch ( Fig. 22 View Figures 15–25 ). Extremely fragile and easily decollated, rarely retained in museum specimens [in fact, Dall’s (1889a: 260) original description maintained ‘The most perfect spires I have seen showed no sign of a regularly spiral nucleus’. Data presented here obtained from descriptions and images in Gould (1966; note that all protoconch images in that work are reversed), and Leal (1991; pers. comm.)]. Approximately 0.26–0.29 mm high and 0.26–0.27 mm wide (based on 10 specimens studied by Gould, 1966: 6), of two and a quarter whorls. The first part represents smooth, bulging, almost planispiral embryonic shell with circular aperture; the following whorl formed by helically coiled, broadly rounded, sculptured larval shell. Sculpture consisting of 5–8 rows of small pustules on upper part of whorl, followed below by finely granulated spiral striae (which appear as rows of fused pustules); contact between the two types of ornament at the whorl periphery relatively sharp. Aperture strongly sinuous, deeply embayed on upper whorl surface at point of contact with teleoconch slit area. Embryonic shell whitish brown, larval shell light golden brown.
Operculum ( Figs 29, 30 View Figures 26–31 , 38 View Figures 38–40 ). Very large (5.0–6.6 mm in diameter) in relation to body width, cylindrical, multispiral, tightly fitting inside shell tube; composed of numerous spiral layers of corneous lamellae, the earlier ones usually decollate and only 8–12 remaining attached to the animal (height of operculum then 6.8– 12 mm). Edge of each layer with flat spine-like projections ( Fig. 30 View Figures 26–31 ); lamella not continuous internally: core filled with triangular compartments, about 6 per whorl, spirally arranged around a central axis ( Fig. 29 View Figures 26–31 ).
Anatomy ( Figs 34, 35 View Figures 32–35 , 38-40 View Figures 38–40 ; for measurements, n = 3).
Head-foot: Body long and slender, spirally coiled, approximately 3 whorls, 2 of which occupied by mantle cavity; overall body length (excluding operculum) 55–67 mm. Mantle cavity to 52 mm long; body width in mid-mantle region approximately 1.3 mm. Foot forming a round column bearing the very large operculum; small (3.5 ¥ 1.5 mm), glandular, transversely furrowed area of foot (the ‘sole’) in front of the mouth, with tip of the sole always pointed. Head very small in comparison to width of body; snout short, bluntly rounded, with mouth a vertical slit at its tip. Two cephalic tentacles about as long as snout (1.0– 1.3 mm), with black eyes on their outer bases. Mantle margin smooth, but with fine papillae in gill region (when animal retracted). Head and anterior foot region salmon-coloured to rust-red; uppermost skin layer with fine brown-black pigment (fading to lightbrown when ethanol preserved), especially on snout, tentacle and metapodium. Numerous white granules embedded in head-foot tissue. No pedal tentacles or pedal mucous gland.
Pallial cavity: Corresponding to shell fissure, dorsal right side of mantle with slit, longitudinally opening mantle between rectum and pallial gonoduct; slit usually lined on outside with yellow to tan. Ctenidium extending as far as mantle slit, its filament lengths gradually decreasing posteriorly; 25–31 filaments per mm. Curved, rod-shaped gill filaments very long (6.8– 7.5 mm), narrow and very flexible (details of ciliation not observed due to poor preservation); row of filaments extends across mantle cavity top right side of animal. Osphradium restricted to anterior part of mantle cavity, beginning next to and shortly behind anteriormost gill filaments; short, 5.3–7.8 mm in total length, compressed S-shaped, with anterior leg somewhat longer, its sensory zone with weak meanders. Endostyle forming a narrow glandular tract along entire length of gill.
Alimentary system: As in T. modestus , anal opening of short rectum (7.9–9.6 mm in mantle cavity) likewise in posterior part of mantle cavity; weak ciliated groove leading from anus forward to mantle cavity. Radula small ( Figs 34, 35 View Figures 32–35 ), length about 1.36 mm, width 0.18 mm), taenioglossate, with 48–52 rows, transparent brown; rachidian with strong, slender triangular main cusp and 8–12 short flanking cusps on either side, smooth basal platform, no lateral or basal projections. Lateral tooth with slender, triangular main cusp and 7–12 short inner flanking cusps and about 25 outer flanking cusps. Marginal teeth hook-like, bluntly tipped; cutting edge of inner marginal tooth finely cuspidate on either side (up to 30 cusps on outer side), of outer marginal tooth smooth.
Reproductive system: Sexable animals (2) female, with developing eggs in gonad.
Habits and habitat
Tenagodus squamatus View in CoL is usually found associated with the sponges Spongosorites ruetzleri ( Soest & Stentoft, 1988) View in CoL and S. siliquaria Soest & Stentoft (1988) View in CoL . In the original descriptions of S. ruetzleri View in CoL (as? Halichondria View in CoL ) and S. siliquaria, Soest & Stentoft, 1988: 92–93 View in CoL ) noted the association of ‘vermetids’ with the former and members of the genus Siliquaria View in CoL [= Tenagodus View in CoL ] with the latter. Shells in the present study came from depths ranging between 13 and 366 m, with live records from between 51 and 213 m. Abbott (1974: 96) and Rios (1994: 66) reported maximum depths of more than 700 m for the siliquariid.
Geographical distribution
Widely distributed in the deeper waters of the western Atlantic, from Bermuda, the Carolinas (e.g. Merrill & Petit, 1965), Florida, Bahamas, to Brazil (Amapá to Espírito Santo, all Seamounts; Rios, 1994), the Gulf of Mexico and the Caribbean Sea.
Taxonomic remarks
In teleoconch characters, Tenagodus squamatus is very similar to the type species of Tenagodus , T. anguinus ( Linnaeus, 1758; originally described as Serpula ) of the Indian Ocean. Tryon (1886: 190) even synonymized the two. The anatomy of the type species remains unstudied. T. barbadensis sp. nov. is also very similar in teleoconch characters, but differs greatly in its larval shell morphology (see below).
Tenagodus squamatus View in CoL specimens from Bermuda are frequently dark reddish brown in colour and have been called ‘ Tenagodus ruber ( Schumacher, 1817) View in CoL ’. Verrill (1905) thought that it was this form that gives the reddish tint to the ‘pink beaches’ of Bermuda, which in fact is caused by the abundant shells of the foraminiferan Homotrema rubrum (Lamarck, 1816) View in CoL . Schumacher (1817) gave a short description for his Anguinaria rubra View in CoL and therein referred to ‘ Serpula anguina ’ sensu Martini (1769: 50 , pl. 2, figs 13, 14), non Linnaeus, 1758. Martini’s original figures show several different forms. Mörch (1861: 403) excluded Martini’s references and restricted Schumacher’s Tenagodus ruber View in CoL to a purple-shelled form with the type locality ‘Moluccas’, but later ( Mörch 1877: 110) used the name again for material from Bermuda and St. Thomas. Mörch’s earlier (1861) decision is followed here, applying Schumacher’s A. rubra View in CoL to an Indo- Pacific species.
The type material of Tenagodus anguillae Mörch, 1861 View in CoL , consists of two incomplete, eroded juvenile shells without protoconch (11.6 and 10.7 mm), and one damaged, incomplete operculum. The somewhat larger shell is here selected as lectotype (ZMK; Fig. 5 View Figures 1–6 ). The specimens agree in their morphology with beachworn juvenile T. squamatus View in CoL and the nominal species is thus placed in its synonymy. Siliquaria View in CoL ‘ aquillae ’ and S. ‘ angullae ’ of authors are subsequent misspellings without nomenclatural status. North Carolina records of ‘ Siliquaria anguillae ’ are here interpreted as having been based on eroded (and thus smooth) shells of Tenagodus squamatus View in CoL , although it is possible that T. modestus View in CoL specimens might have been commingled.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Family |
|
|
Genus |
Tenagodus barbadensis
| Bieler, Rüdiger 2004 |
Tenagodus modestus
| Pansini M & Cattaneo-Vietti R & Schiaparelli S 1999: 429 |
| Leal JH 1991: 69 |
Siliquaria
| Mikkelsen P 1981: 47 |
Tenagodus (Agathirsus) squamatus, Haas, 1941: 171
| Rios E de 1994: 66 |
| Rios E de 1985: 46 |
| Abbott RT 1974: 96 |
| Haas F 1941: 171 |
Siliquaria rubra
| Peile AJ 1926: 78 |
Tenagodus
| Verrill AE 1905: 139 |
Siliquaria (Tenagodus)
| Heilprin A 1889: 172 |
Siliquaria
| Dall WH 1889: 259 |
Siliquaria
| Paetel F 1888: 499 |
Siliquaria anguillae, Paetel, 1888: 499
| Warmke GL & Abbott RT 1975: 67 |
| Porter HJ 1974: 15 |
| Lipka DA 1974: 148 |
| Morris PA 1973: 145 |
| Wolfe D & Wolfe N 1970: 14 |
| Menzies RJ & Pilkey OH & Blackwelder BW & Dexter D & Huling P & Mccloskey L 1966: 408 |
| Paetel F 1888: 499 |
Siliquaria modesta
| Hartman WD & Hubbard R 1999: 1 |
| Pointier J-P & Lamy D 1998: 48 |
| Rios E de 1994: 66 |
| Diaz JM & Puyana HM 1994: 140 |
| Rios E de 1985: 47 |
| Abbott RT 1974: 96 |
| Maury CJ 1922: 104 |
| Dall WH 1896: 25 |
| Dall WH 1889: 260 |
| Dall WH 1889: 144 |
| Paetel F 1888: 499 |
| Agassiz A 1888: 71 |
| Tryon GW 1886: 191 |
| Dall WH 1881: 39 |
Tenagodus ruber
| Morch OAL 1877: 109 |
Tenagodus squamatus, Mörch, 1865: 99
| Redfern C 2001: 23 |
| Pansini M & Cattaneo-Vietti R & Schiaparelli S 1999: 429 |
| Turgeon DD & Quinn JF Jr & Bogan E & Coan EV & Hochberg FG & Lyons WG & Mikkelsen PM & Neves RJ & Roper CFE & Rosenber G & Roth B & Scheltema A & Thompson FG & Vecchione M & Williams JD 1998: 69 |
| Bandel K & Kowalke T 1997: 263 |
| Leal JH 1991: 68 |
| Parker RH & Curray JR 1956: 2434 |
| Abbott RT 1954: 145 |
| Morch OAL 1877: 110 |
| Morch OAL 1865: 99 |
Tenagodus squamata, Mörch, 1861: 414
| Morch OAL 1861: 414 |
Tenagodus (Pyxipoma) anguillae Mörch, 1861: 410
| Morch OAL 1877: 110 |
| Morch OAL 1861: 410 |
Tenagodus (Agathirses) squamata, Mörch, 1861: 411
| Morch OAL 1861: 411 |
Siliquaria squamata
| Rios E de 1994: 66 |
| Diaz JM & Puyana HM 1994: 140 |
| Rosenberg G 1992: 52 |
| Lipe RE & Abbott RT 1991: 52 |
| Leal JH 1989: 8 |
| Harasewych MG 1989: 48 |
| De Jong KM & Coomans HE 1988: 40 |
| Rios E de 1985: 46 |
| Abbott RT & Dance SP 1982: 61 |
| Emerson WK & Jacobson MK 1976: 74 |
| Rios E de 1975: 46 |
| Abbott RT 1974: 96 |
| Rios E de 1970: 40 |
| Abbott RT 1968: 84 |
| Gould SJ 1966: 1 |
| Merrill AS & Petit RE 1965: 60 |
| Peile AJ 1926: 78 |
| Maury CJ 1922: 103 |
| Dall WH 1889: 260 |
| Dall WH 1889: 144 |
| Paetel F 1869: 56 |
| Morch OAL 1861: 414 |
| de Blainville H 1827: 213 |