Selatodryas luteosoma, Herbert, 2017
|
publication ID |
https://doi.org/ 10.5852/ejt.2017.309 |
|
publication LSID |
lsid:zoobank.org:pub:1E8FE779-D6E7-428E-9538-5E5F8ECFB271 |
|
DOI |
https://doi.org/10.5281/zenodo.3846870 |
|
persistent identifier |
https://treatment.plazi.org/id/3527BF02-E261-4D1A-9978-5460F2BDD562 |
|
taxon LSID |
lsid:zoobank.org:act:3527BF02-E261-4D1A-9978-5460F2BDD562 |
|
treatment provided by |
Carolina |
|
scientific name |
Selatodryas luteosoma |
| status |
gen. et sp. nov. |
Selatodryas luteosoma View in CoL gen. et sp. nov.
urn:lsid:zoobank.org:act:3527BF02-E261-4D1A-9978-5460F2BDD562
Figs 15 View Fig , 27–30 View Fig View Fig View Fig View Fig
Diagnosis
Characterised by the relatively small, low-spired, glossy yellowish-brown shell, non-punctate protoconch and columella that runs into the axis of coiling rather than fusing with the parietal region. Distal genitalia with short flagellum, of which f1 comprises only one whorl.
Etymology
From the Latin ‘ luteus ’, yellow, and the Greek ‘ soma ’, body; referring to the yellow coloration of the living animal.
Material examined
Holotype
SOUTH AFRICA: E Cape, Langeni area, Nocu Forest , 31.41547° S, 28.49998° E, ca 1190 m, large block of indigenous forest, alive on understorey herbs ( Begonia and Plectranthus ), D. Herbert and L. Davis leg., st. 06-004, 18 Feb. 2006 ( NMSA W3925/T3873 , dry shell with body in ethanol).
GoogleMapsParatypes (listed north to south, all E Cape)
SOUTH AFRICA: Mount Frere area, just S of Buffalo Nek village, 10 km NW of Mount Frere, 30.854799° S, 28.892971° E, 1466 m, M. and K. Cole leg., 7 Apr. 2015 ( ELM W3857/T161, whole specimen in ethanol); Langeni area, Cwecwe Forest, 31.388900° S, 28.565878° E, 1149 m, M. Cole and V. Ndibo leg., 25 Jan. 2013 ( ELM W3730/T160, one dry shell with body in ethanol); Langeni area, Jenca Valley, 31.36593° S, 28.55727° E, ca 1420 m, small piece of indigenous forest in rocky valley above escarpment, D. Herbert and L. Davis leg., 18 Feb. 2006 ( NMSA W3937/T3876, 2 dry shells with bodies in ethanol); same data as holotype ( NHMUK 20160243, one dry shell; NMSA W9691/T3874, seven dry shells, with seven bodies and one whole specimen in ethanol; W4279/T3875, 6 dry shells).
Other material
SOUTH AFRICA: E Cape, Langeni area, Nocu Forest, 31.4185° S, 28.5037° E, 1090 m, Afromontane Podocarpus forest, in leaf-litter, D. Herbert leg., 13 May 2001 ( NMSA V9083).
Description
SHELL ( Fig. 27 View Fig ). Lenticular; periphery at mid-whorl or slightly below, evenly rounded; H:D 0.61–0.68 (N =6); suture indented, inserting above periphery; very thin and delicate; translucent, more or less uniformly yellowish-brown; apical and basal surfaces both glossy. Protoconch diameter 1.68–1.75 mm (N =5); smooth and glossy, sculptured only by extremely fine, close-set, microscopic scratch-like spiral lines; no evidence of punctation; junction with teleoconch poorly defined. Teleoconch of up to 2.25 whorls; whorls expanding moderately rapidly; spiral sculpture virtually obsolete, later whorls with only the finest traces of microscopic, close-set, wavy, spiral ripples, even weaker on base; teleoconch otherwise only with weak, uneven growth irregularities, most evident below suture. Umbilicus absent, edge of columella lip reflected and slightly thickened with a thin, whitish callus; columella running into axis of coiling rather than fusing with parietal region; aperture roundly lunate. Diameter up to 11.7 mm; holotype, diameter 10.5 mm, height 6.7 mm.
LIVING ANIMAL ( Fig. 28 View Fig ). Head-foot ground colour pale greyish-cream, with pale yellowish pigment granules concentrated in skin tubercles and along pedal margin; optic tentacles and their retractors dark grey to black, tentacle bases speckled with yellowish pigment granules; caudal appendage well developed, dark grey. Body lobes of mantle extensive, pigmentation yellowish-buff and more dense than neck region, shell lobes well developed. Lining of pulmonary cavity with little pigmentation except for an opaque, cream-yellow subsutural band overlying rectal region; this spreading out over viscera posterior to pulmonary cavity, becoming restricted to irregularly shaped blotches on mid-spire viscera; apical viscera with rather more extensive, finely reticulate, cream pigmentation; no dark pigment band overlying primary ureter, kidney region yellowish-buff.
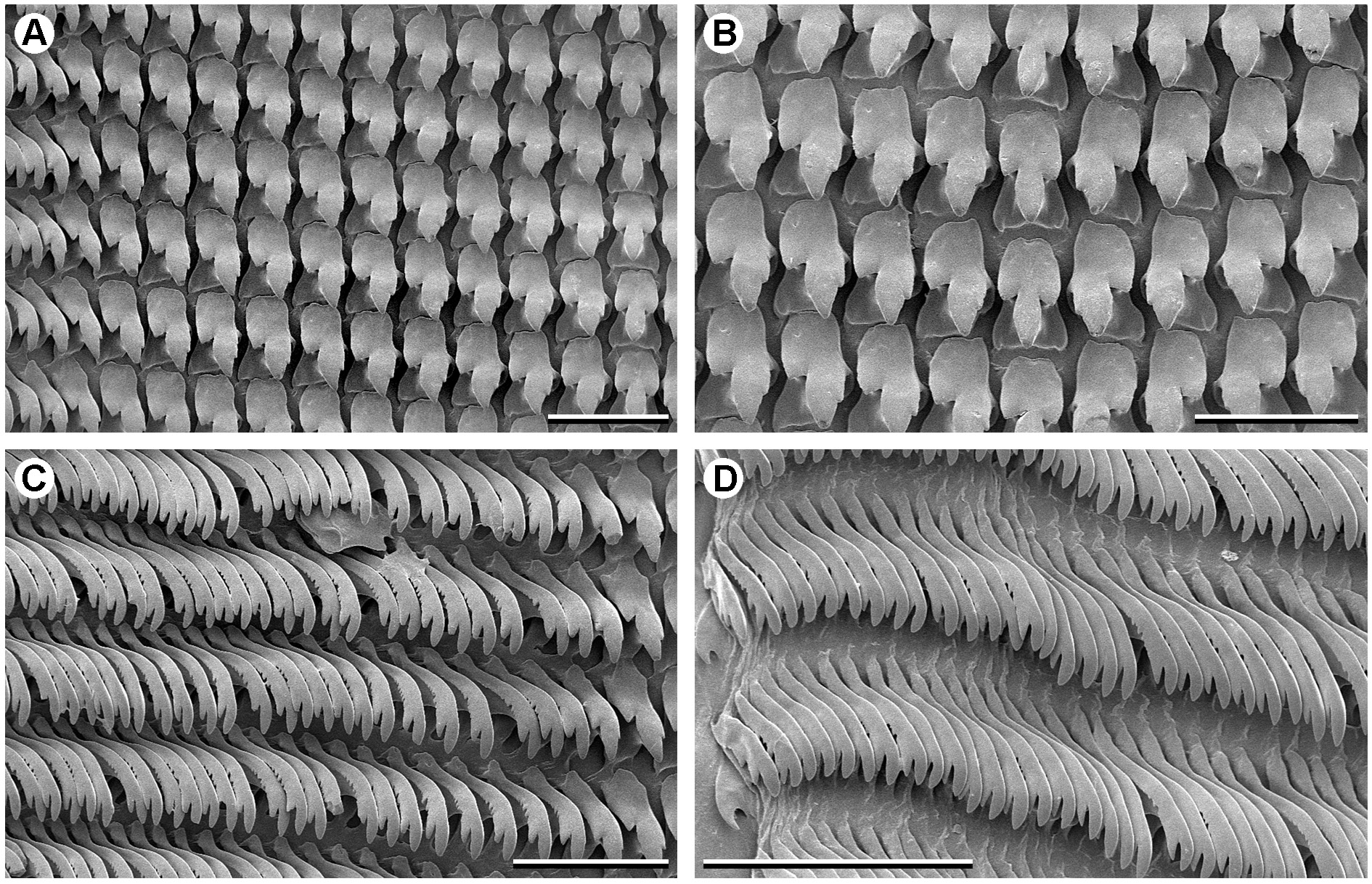
RADULA ( Fig. 29 View Fig ). Formula R+10+(1–2)+(70–80); rachidian tricuspid, anterior edge of shaft base minimally indented in mid-line; laterals essentially bicuspid with a mesocone and strong basal ectocone, but also with a minute endocone on side of mesocone; shaft of laterals roundly quadrate, more or less in line with mesocone (cf. S. roseosoma gen. et sp. nov. above); laterals followed by 1–2 intermediary teeth and then a long series of marginals; marginals curved, with a large terminal cusp and a smaller subterminal one on outer (concave) margin, outer edge of shaft with a series of small serrations; marginals progressively decreasing in size toward edge of radula, but otherwise morphologically similar.
DISTAL GENITALIA ( Fig. 30 View Fig A–C).As in Selatodryas roseosoma gen. et sp. nov., but with characteristic differences relating primarily to flagellum and spermatophore. In S. luteosoma gen. et sp. nov., f1 component of flagellum shorter, comprising only one whorl; f2 similarly short in both species; additionally, flange at base of penis slightly closer to genital atrium. Gametolytic sac frequently containing allospermatophores (up to three; specimens collected in February).
SPERMATOPHORE ( Fig. 30D View Fig ). Similar to that of Selatodryas roseosoma gen. et sp. nov., but tail shorter (tail length approx. 4.5 mm) and coiled into only one revolution; early part of tail with short, branched spines with 3–4 T-shaped tips; spines progressively smaller and with fewer branches toward tail tip, but final 4–5 spines significantly larger, curved, un-branched and their tips pointed rather than T-shaped.
Distribution ( Fig. 15 View Fig )
A narrow-range endemic, known only from the edge of the Great Escarpment in the Mount Frere– Langeni area, north and west of Mthatha, E Cape, South Africa; at altitudes between 1090 m and 1470 m above sea level.
Habitat
Southern Mistbelt Forest ( Mucina & Rutherford 2006); living in leaf-litter and on foliage of understorey herbs and forbs.
Remarks
Selatodryas luteosoma gen. et sp. nov. is clearly close to S. roseosoma gen. et sp. nov. from the neighbouring Prentjiesberg. In terms of shape and sculpture, the shells of the two species are virtually indistinguishable. However, that of S. roseosoma gen. et sp. nov. attains a larger size (diameter up to 15 mm) and usually has a slightly greenish tint when fresh. There are additional differences in body colour, S. luteosoma gen. et sp. nov. having a predominantly yellowish coloration with extensive pigmentation on the spire viscera, whereas S. roseosoma gen. et sp. nov. has a predominantly reddishpink head-foot with little pigmentation on the spire viscera. There are also significant differences in the distal genitalia and spermatophore structure. In S. roseosoma gen. et sp. nov. the basal component of the flagellum (f1) is twisted through approximately three whorls (only one in S. luteosoma gen. et sp. nov.), a difference reflected also in the coiling of the spermatophore tail. The spermatophore spines are also more extensively branched in S. roseosoma gen. et sp. nov. and the un-branched distal spines have T-shaped tips (pointed in S. luteosoma gen. et sp. nov.). The consistency of these characters has been confirmed in several specimens of each species. A further difference between the species is evident in the radula, where the shaft of the lateral teeth is more or less square and in line with the mesocone in S. luteosoma gen. et sp. nov., but in S. roseosoma gen. et sp. nov. the shaft is rectangular and set at a distinct angle relative to the mesocone.
The ranges of the two species are focused on escarpment-edge forest habitats spanning two altitudinal ranges— S. luteosoma gen. et sp. nov. at ca 1100–1470 m in the Langeni–Mount Frere area west and north of Mthatha and S. roseosoma gen. et sp. nov. at ca 1380–1700 m in the Prentjiesberg west of Ugie and Maclear. The two escarpments are separated by only ca 50 km, but this has evidently been sufficient to isolate the populations and promote speciation. The overall similarity of the species suggests that they are phylogenetically close, but the consistent differences, particularly in flagellum and spermatophore structure, provide strong evidence that speciation has occurred and that the two populations are distinct species-level entities, most probably sister species.
In terms of body coloration, S. luteosoma gen. et sp. nov. resembles ‘ Sheldonia ’ transvaalensis (Craven, 1880), particularly with regard to the yellowish-cream pigment blotches on the spire whorls. However, in ‘ S ’. transvaalensis the shell is more globose and has a conventional, rimate umbilicus, albeit narrow. Its penis is also not distally coiled or folded within its sheath, the epiphallic caecum is small and spherical, the flagellum is bifid, and the vagina is comparatively short. As noted by Watson (1934), it is closer to species that I consider referable to Microkerkus (e.g., M. burnupi , M. leucospira and M. pondoensis ).
Like S. luteosoma gen. et sp. nov., the recently described velvet worm, Opisthopatus amaxhosa Daniels, Dambire, Klaus & Sharma, 2016 , is narrowly endemic to the escarpment forests west of Mthatha ( Daniels et al. 2016).
Conservation
As with Selatodryas roseosoma gen. et sp. nov., the limited range of S. luteosoma gen. et sp. nov. renders its conservation a matter of concern. The forests in which it occurs do not fall within formally protected areas, although a degree of oversight may be exercised by the Department of Agriculture, Forestry and Fisheries. Extensive exotic timber plantations surround the remaining pockets of indigenous forest in this area and represent a potential disturbance threat, particularly with regard to invasive alien plants and perhaps also non-native invertebrates dispersed via the sylviculture industry (Herbert 2010). Uncontrolled burning in neighbouring grassland habitats may represent an additional threat.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Heterobranchia |
|
Order |
|
|
Family |
|
|
SubFamily |
Sheldoniinae |
|
Genus |