Paraeurybata, Marshall, 2019
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4624.4.8 |
|
publication LSID |
lsid:zoobank.org:pub:B7944A5F-6549-40D5-854B-F958C716BD94 |
|
DOI |
https://doi.org/10.5281/zenodo.5927523 |
|
persistent identifier |
https://treatment.plazi.org/id/039D87C4-E232-FFE0-FF23-1B66DCB757A4 |
|
treatment provided by |
Plazi |
|
scientific name |
Paraeurybata |
| status |
gen. nov. |
Paraeurybata View in CoL new genus
Type species: Calobata taeniata Macquart, 1851 , here designated.
Included species: Calobata taeniata .
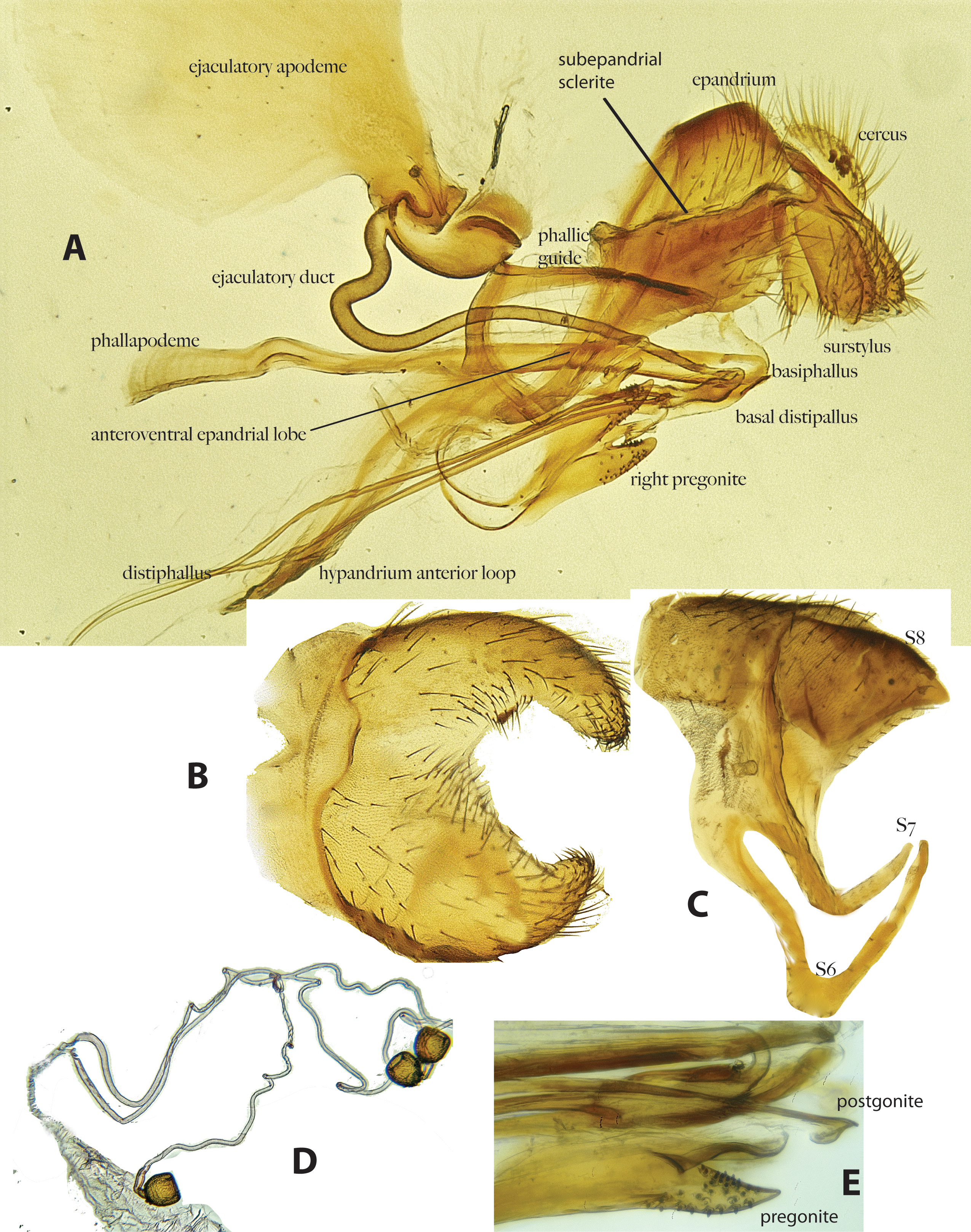
Diagnosis: Eurybatinae with a darkly infuscated wing crossed by narrow clear discal band. Head chaetotaxy complete, with large and equal postocellar, inner and outer vertical and upper and lower fronto-orbital setae ( Fig. 1F View FIGURE 1 ). Pleuron with a prominent black-margined white longitudinal band. Hypandrium narrow, looped anteriorly; distiphallus with very short basal part, an indistinct phallic bulb and longitudinally divided distal part; ejaculatory duct strikingly thick.
Description: (abdominal characters only; see also the full redescription of external head and abdominal features of “ Cothornobata taeniata ” in Barraclough 1992 ):
Female abdomen: Pleural membrane of segments 2–5 with dorsal brown patch contiguous with tergite, otherwise white ( Figs 1 View FIGURE 1 A-B). Oviscape orange. Spermathecal ducts with long common stem arising from tapered bursa copulatrix and dividing apically into slender, extremely long ducts ( Fig. 2D View FIGURE 2 ); ducts uniform except for sclerotized stem at base of each spermatheca; spermathecae acorn-shaped, small.
Male abdomen: Pleuron as in female, but with brown areas more extensive, occurring on segments 1–6 ( Fig. 1E View FIGURE 1 ). Sternite 5 (genital fork) bulbous, with 2 shallowly separated inflated, rounded lobes (apices collapsed on all available dried specimens) ( Fig. 2B View FIGURE 2 ); inner surfaces with only small setulae. Sternite 6 narrow, but deeply V-shaped ( Fig. 2C View FIGURE 2 ). Pregonite large and prominent, basally with a narrow loop-like connection to hypandrium and apically bilobed with lower lobe longer and tapered, with numerous short stout setae ( Fig. 2A,E View FIGURE 2 ); postgonite much smaller, with rounded preapical ventral lobe. Phallapodeme sinuate (with a distinct kink) anteriorly. Basiphallus elongate and slender, extending beyond ejaculatory duct; basal part of distiphallus very short and bulbous, ending in indistinct phallic bulb; distiphallus beyond phallic bulb divided into 2 lateral strips that taper to whip-like apex after passing through narrow, loop-like hypandrium; posterior arms of hypandrium articulating with very long and slender anteroventral epandrial corner ventrally, and continuous with phallic guide (phallic plate) dorsally ( Fig. 2A View FIGURE 2 ). Ejaculatory apodeme enormous, filling most of abdomen ( Fig. 2A View FIGURE 2 ). Ejaculatory duct unusually broad. Surstylus large, bulbous, long setose and apically membranous ventrally, narrow at base with sclerotized inner basal margin continuous with subepandrial sclerite. Cercus simple, long setose.
Comments: Specimens of P. taeniata in the Natural History Museum, London were labelled as “ Trepidaroides?” by Verbeke in 1963, who first recognized the affinity of this species to the Eurybatinae. McAlpine (1975) suggested that it belonged in the otherwise Australian-Oriental genus Cothornobata and Barraclough (1992) formally transferred it to Cothornobata . McAlpine (1998) characterized Cothornobata as a “… diverse and widely distributed genus, which is poorly studied morphologically and may not be monophyletic”. Li et al. (2015) concurred that Cothornobata as a whole is weakly supported and revised a northern Oriental clade, including the type species of the genus, as Cothornobata s.s. That clade, diagnosed by an elongate genital fork with relatively strong and hirsute tubercles on inner surface, a distiphallus with trifurcate terminal filaments and a broad, plate-like hypandrium, has since held up to scrutiny with both morphological and molecular characters, but the non-Oriental “ Cothornobata ” fall into two widely separated lineages of which neither belongs in Cothornobata . One of these lineages includes only P. taeniata , the only Afrotropical eurybatine.
New collections from Mauritius have allowed reconsideration of the generic status of P. taeniata and the relative status of the Mauritian and Réunion populations. The new specimens differ in colour from Réunion specimens, especially in the dark mid femur with a distinct preapical yellow ring. This initially suggested that two species were involved, but sequencing of newly collected specimens from both Réunion and Mauritius suggests that the different populations are very close and that they are appropriately treated under the same species name. More significantly, the new sequence data allowed inclusion of Paraeurybata taeniata in a dataset of several hundred micropezids sequenced for CO1 (Marshall, unpublished) and a dataset of nine genera of Eurybatinae sequenced for multiple genes (16s, 28s, CAD, COI and EF-1α; Nur Md Yusof unpublished doctoral thesis, University of Guelph). None of the molecular data suggested that Paraeurybata was either within or closely related to Cothornobata , instead indicating that the sister group is either ( Cothornobata s.s. + Eurybata ) or just Eurybata . New morphological data (previously unstudied details of the male terminalia) also indicate that P. taeniata is not closely related to Cothornobata and support the decision to treat it as generically distinct from other eurybatines. The slender, looped hypandrium, the split and elongate distiphallus with neither a distinct phallic bulb nor distal filaments, the thick ejaculatory duct and the short and inflated genital fork render this species markedly different from both Cothornobata and Eurybata .
The new specimens of Paraeurybata taeniata used for this work are important not only for allowing reconsideration of the generic status of the species and for expediting the careful comparison of Réunion and Mauritian populations, but also because they came with the first information on the biology of the species. It was photographed ovipositing in large, damp, shaded mossy logs. This is significant because it provides a possible explanation for the apparent rarity of the species and its apparent restriction to a few protected patches of forest.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.