Labrundinia longipalpis ( Goetghebuer, 1921 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5346.2.3 |
|
publication LSID |
lsid:zoobank.org:pub:10D195DD-0D0B-4C45-A80C-7053DBB58DB1 |
|
DOI |
https://doi.org/10.5281/zenodo.8368415 |
|
persistent identifier |
https://treatment.plazi.org/id/039CAD6D-FFDD-FFC4-48AC-FCE5FBC5F8E4 |
|
treatment provided by |
Plazi |
|
scientific name |
Labrundinia longipalpis ( Goetghebuer, 1921 ) |
| status |
|
Labrundinia longipalpis ( Goetghebuer, 1921) View in CoL View at ENA
Tanypus longipalpis Goetghebuer 1921: 18 View in CoL ; Goetghebuer 1927: 61, description of male.
Pentaneura longipalpis (Goetghebuer) , Edwards 1929: 294, description of male.
Ablabesmyia longipalpis (Goetghebuer) , Goetghebuer 1936: 43, description of male.
Labrundinia longipalpis (Goetghebuer) View in CoL , Fittkau 1962: 376, description of male and pupa.
Labrundinia maculata Roback 1971: 271 View in CoL [= Labrundinia longipalpis View in CoL (Goetghebuer: 1921) syn. n., Silva et al. 2011: 234], description of male and female; Roback (1987: 192), description of immature stages.
Labrundinia longipalpis (Goetghebuer) View in CoL , Silva et al. 2011: 234, redescription of male, female and immature stages.
Type locality: Belgium, Flandres, Broeck d’Overmeire.
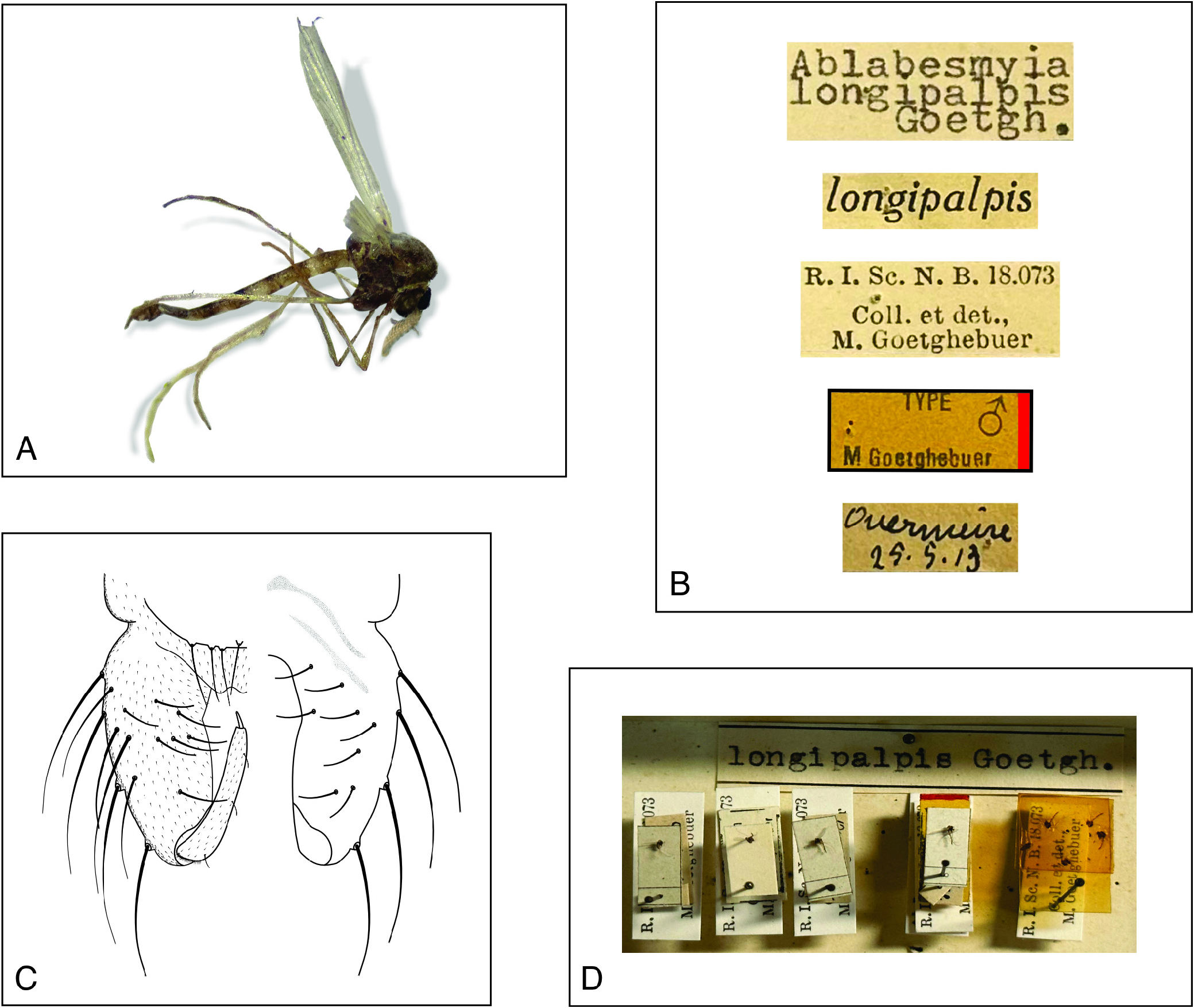
Type material: [ BELGIUM] Lectotype male [by present designation] with labels: (1) original label handwritten in black ink reads Overmeire [= Broeck d’Overmeire], 25-v-1913; (2) yellow printed label [marked by a lateral red ink] reads “Type” ♁ M. Goetghebuer; (3) white printed label reads R. I. Sc. N. B. 18.073, coll. et det., M. Goetghebuer; (4) longipalpis ; (5) typed label reads Ablabesmyia longipalpis Goetgh. ; coll. IRSNB Brussels. Paralectotypes [by present designation]: 3 males same data as lectotype except for the presence of the yellow printed label, coll. IRSNB Brussels.
Additional material: 5 males same data as lectotypes except for previously mounted in balsam under two coverslips on a slide; overcleared; coll. IRSNB Brussels .
Condition of type. Pinned, overall well-preserved. Antenna and right wing missing. Maxillary palps collapsed.
The lectotype ( Figs 1A, D View FIGURES 1 ) and paralectotype specimens ( Fig. 1D View FIGURES 1 ) were designated along with examining additional material ( Fig. 1D View FIGURES 1 ) to validate the diagnostic features for Labrundinia longipalpis . Several distinguishing characteristics contribute to the identification of this species, such as a pale brown tergite I on the abdomen with brown transverse bands on tergites II-VI near the proximal margin for adult males; hypopygium pale brown, sternapodeme with reduced anterior process ( Fig. 1C View FIGURES 1 ). Adult females have brown abdominal segments, with tergites II, IV, and VI being lighter towards the apex. These features were described by Silva et al. (2011). Furthermore, Silva et al. (2014a) observed that the pupal thoracic horn is wedge-shaped, and the preapical groove is indistinct, while the larvae have a weakly developed lateroventral spine group on the head and lack a posteroventral spine group. Finally, the subbasal seta of the posterior parapod has multiple teeth and a basal spine group, while the posterior parapod bifid claw has a U-shaped lower groove, and the lower spur is arched downwards towards the base of the claw, as also described by Silva et al. (2011).
Specimens of Labrundinia longipalpis and L. maculata (Roback) from Europe and North America, respectively, was thoroughly examined by Silva et al. (2011), which led them to conclude that L. maculata is a junior synonym of L. longipalpis . The lack of significant differences between the two species, such as male abdominal coloration or pupal features, supported their conclusion. It’s worth noting that Roback (1971) had already observed the similarity between the male of L. longipalpis and L. maculata , but proposed the new name without further study or supporting argument. However, Silva et al. (2011) did report that the North American larvae had spine-covered head surfaces, potentially indicating adaptations to distinct environmental conditions or ecological niches, which could distinguish them from their European counterparts. These variations in head surfaces may have resulted from variations in environmental factors or selective pressures acting on the populations in different regions. For instance, the North American populations may have evolved to survive in different environments or feed on different prey compared to the European populations, leading to differences in morphology. Although several adult specimens from Europe were examined in this study, no larvae were reared through to the adult stage to confidently associate all life stages ( Silva et al. 2011). However, it is worth noting the record of a second form of Labrundinia with larval lateroventral spine group with several conspicuous spines, similar to the Nearctic L. neopilosella Beck and Beck , in Europe, based on the analysis of Polish sub-fossil chironomids (Larocque-Tobler 2013). Given this new information and the lack of conclusive evidence for separate species status, it is plausible to consider the Palearctic and Nearctic populations as a single species, even considering the discrepancies in their larval morphology.
DNA barcoding analysis
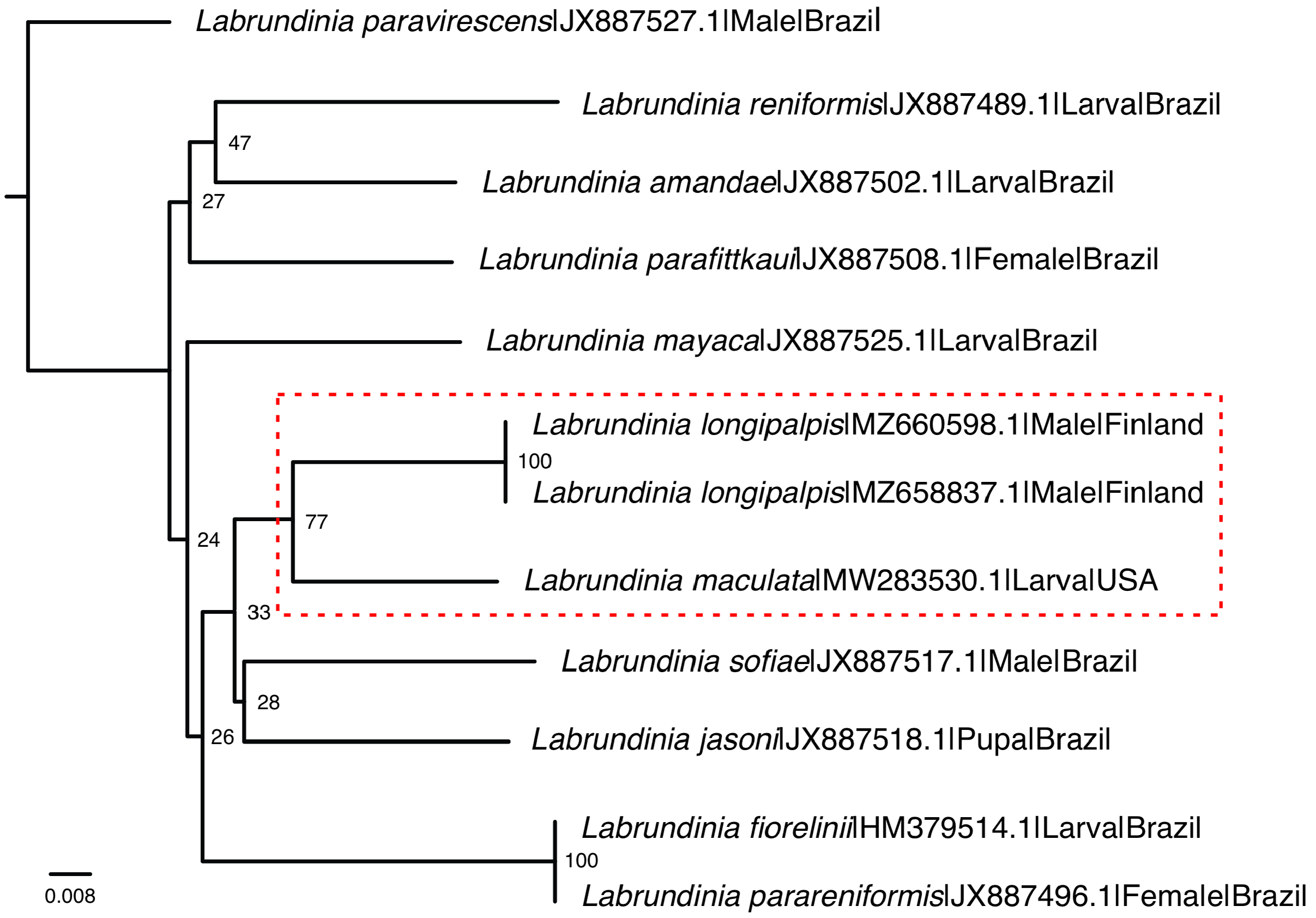
In recent years, significant advancements have been made in the molecular investigation of Labrundinia longipalpis . Notably, Krosch et al. (2017, 2022) conducted studies analyzing genetic material, which included a population of the species found in California, United States, previously identified as L. maculata . Using fragments of the mitochondrial cytochrome c oxidase (COI) gene, they conducted a molecular phylogenetics study on the subfamily Tanypodinae . In a more recent study, Roslin et al. (2022) collected L. longipalpis specimens from Finland and employed DNA barcoding techniques, specifically sequencing the COI gene using universal primers ( Folmer et al. 1994). Herein, through the comparison of these DNA sequences, in association with additional material from the Neotropical region in southeast Brazil ( Table 1 View TABLE 1 ), a clustering pattern between the North American and European populations of L. longipalpis was recovered ( Fig. 3 View FIGURE 3 ), although supported to a limited extent.
Partial COI gene sequences were retrieved from 12 specimens representing 10 species of the Labrundinia genus, resulting in an alignment of 658 base pairs. Among these sequences, there were 184 variable sites (27.9%), with 134 sites (20.3%) potentially informative for parsimony analysis. Notably, a maximum intraspecific divergence of 8.00% between the North American and European populations of Labrundinia was observed. This level of divergence is higher than previously reported findings by Silva et al. (2013), who reported a maximum intraspecific divergence of approximately 5.00% within Labrundinia species. The increased maximum withinspecies observed in our study may be attributed to the geographical isolation of the populations. Importantly, it is worth noting that a higher threshold of 5–8% is not uncommon within the family Chironomidae , as documented for closely related species ( Carew & Hoffmann 2015, Song et al. 2018).
The findings of the study suggest the possibility of gene flow or a shared ancestral lineage between the North American and European populations of Labrundinia . These hints could lend support to the hypothesis proposed by Silva et al. (2011) regarding the synonymization of L. maculata with L. longipalpis , which was further reinforced by the comprehensive phylogenetic study of the genus conducted by Silva et al. (2014b), utilizing a total evidence matrix. It is also worth noting that certain variations in the abdominal coloration pattern were observed within the European populations when comparing the newly sequenced material ( Roslin et al. 2022) with the typematerial analyzed in this study. Overall, the plausibility of considering the Palearctic and Nearctic populations as a single species is grounded in their remarkable morphological similarity, despite the minor discrepancies in their morphology. While these variations could potentially be attributed to the freshness of the specimens and their preservation in alcohol, they also prompt consideration of morphological population variation or the possibility of cryptic species. Consequently, it is pivotal to acknowledge the need for further investigation, which should entail integrating additional molecular markers, conducting meticulous analyses of morphological and ecological characteristics, and expanding the geographical sampling. Such endeavors are essential to provide a more comprehensive understanding of these findings, enhancing their validity and broader significance.
Geographical range
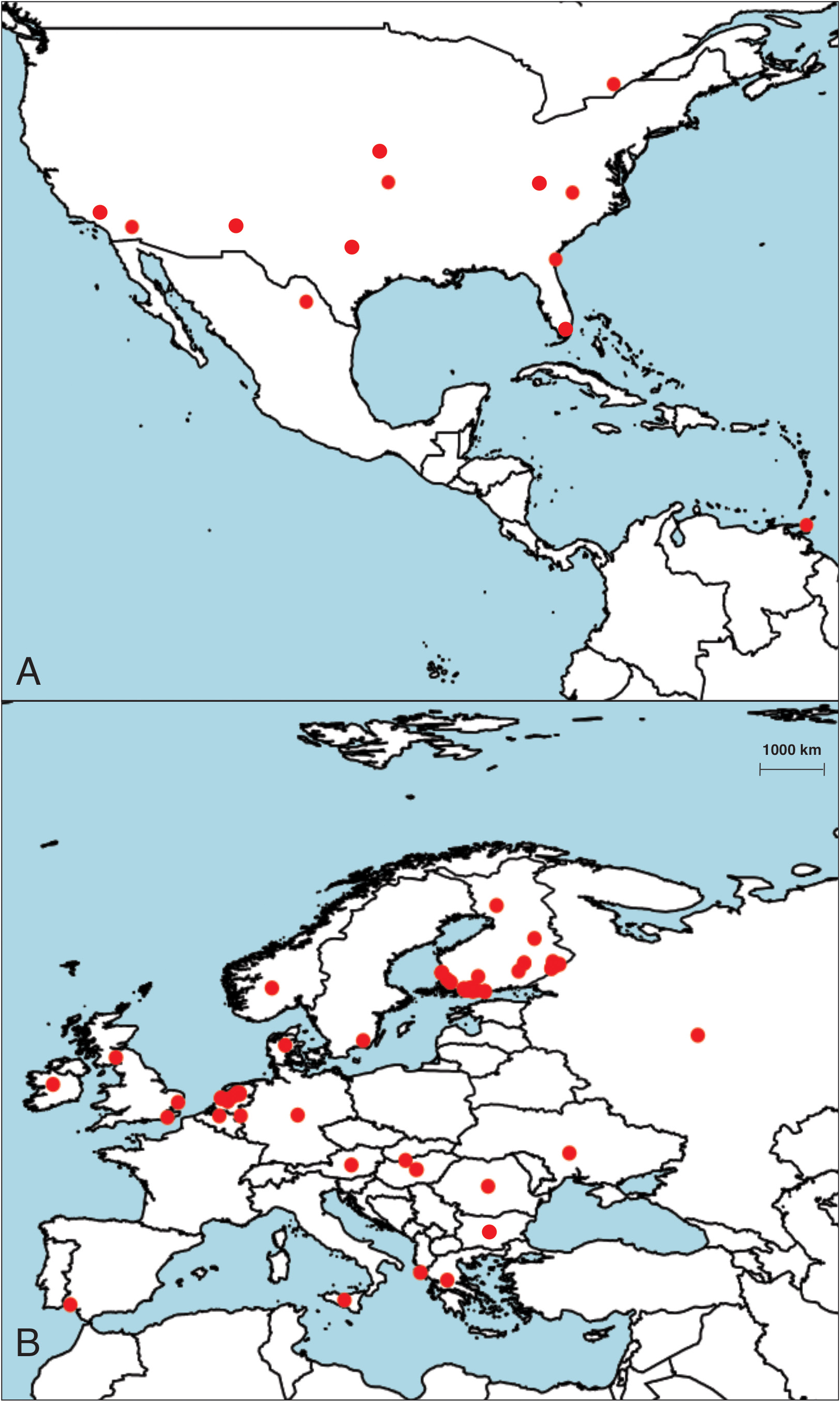
The distribution of Labrundinia longipalpis is of particular interest due to its contrasting pattern with the predominantly tropical distribution observed in other species of the genus. This is aquatic midge that has a wide geographical range ( Ashe & O’Connor 2009), spanning across multiple continents ( Fig. 2 View FIGURES 2 ). In addition to Canada (Ontario), Mexico (Coahuila) and Trinidad and Tobago ( Roback 1987, Silva et al. 2011), L. longipalpis has been found in various European countries such as Austria, Belgium (Flanders), Bulgaria ( Hubenov 2021), Croatia ( Ivković et al. 2020), Danish mainland, East European Russia, Finland ( Roslin et al. 2022), Germany, Greece, Hungary, Ireland, Italy (Sicily), Netherlands ( de Jong 2014, Vallenduuk & Moller-Pillot 2007), Norway (mainland), Romania, Slovakia ( Hamerlík 2002, 2007), Spain (Andalusia), Sweden (Gotland), and Ukraine ( de Jong 2014). It has also been reported in several locations across United States including California (Orange and Riverside Counties), Georgia (Glynn County), Kansas (Cherokee and Jefferson Counties), North Carolina (Forsyth County), and Texas (Blanco County) ( Roback 1987, Silva et al. 2011). The ability of this species to thrive in diverse environments is reflected in its wide distribution across North America and Europe, and raises questions about how it has been able to achieve long-distance dispersal.
The presence of shared species between the Palearctic and Nearctic regions, particularly in northern areas, has been documented in several studies ( Ekrem et al. 2018, Marusik & Koponen 2005, Silva et al. 2023). This phenomenon could be attributed to faunal exchanges that occurred across the Bering land bridge between 135,000 and 70,000 years ago. It should be noted, however, that these exchanges were primarily limited to larger species adapted to cold environments ( Rodríguez et al. 2006), which now dominate the Holarctic realm. Additionally, it has been observed that chironomids can also serve as aerial plankton ( Hardy & Milne 1938, Gressitt et al. 1960, Cotoras & Zumbad 2020) and one cannot disregard the possibility of long-distance dispersal through airborne transportation as a putative explanation for the observed trans-Atlantic distribution patterns ( Ekrem et al. 2018), including those observed in Labrundinia species. This mode of transportation could open up the possibility for chironomids to traverse vast distances and colonize new habitats, expanding their geographical range and establishing populations in distant locations.
Understanding the colonization patterns of Labrundinia longipalpis is essential given its widespread distribution across the Holarctic region. By studying its distributional patterns, insights into how it interacts with other species may be gained and how it may influence ecosystem functioning. That being said, Labrundinia is primarily distributed in the Neotropical and Nearctic regions, with some species found also in the Caribbean islands ( Silva et al. 2014a). Recently, a comprehensive phylogeny of the genus was conducted using molecular and morphological evidence, recovering the monophyletic nature of the group and shedding light on its evolutionary history. The study revealed that Labrundinia initially diversified in the Neotropical region, indicating its origin in that area. Moreover, according to the authors, the current presence of Labrundinia in the Nearctic region and southern South America, however, is believed to be the result of subsequent dispersal events. Additionally, as mentioned earlier, the distribution patterns of the genus in the Palearctic region may be attributed to long-distance transoceanic dispersal events after vicariance ( Silva et al. 2014b). Nevertheless, it is important to note that further investigations are required to comprehensively determine whether the observed differences between the North American and European populations of L. longipalpis represent distinct species within the genus Labrundinia .
Ecological significance
The available data on the ecology of Labrundinia longipalpis is limited but informative. Larvae have been found in the seepage zone between the Pleistocene and Holocene parts in the Netherlands, where water has a pH of 7 to 8 ( Vallenduuk & Moller-Pillot 2007). Recently, larvae of L. longipalpis were collected in the waterfall complex of the Plitvice Lakes in Croatia ( Ivković et al. 2020). These oligotrophic lakes, renowned for their unique karst features, have been found to serve as a suitable habitat for L. longipalpis . The lakes are characterized by a low concentration of organic solutes, supersaturation with calcium salts, a pH level above 8.0, and the presence of algae and mosses that contribute to the formation of tufa barriers ( Srdoč et al. 1985, Stilinović & Božičević 1998). Additionally, this species is also known to be characteristic of mesohumic and polyhumic lakes, as noted by Brundin (1949) and Saether (1979). In Slovakia, L. longipalpis has been documented in potamon-like environments, which refer to riverine or stream habitats characterized by continuous water flow ( Hamerlík 2007). In the Nearctic region, L. longipalpis has been documented in the Florida Everglades, a vast marsh ecosystem created by the flooding of a shallow limestone depression ( Jacobsen 2008).
The adaptability of Labrundinia longipalpis in North America is truly remarkable.The discovery of L. longipalpis larvae in an unconventional water body in Kansas, characterized by high concentrations of heavy metals ( Roback 1987), not only expands the understanding of the species’ adaptability but also highlights its ability to thrive in diverse aquatic environments, even those with atypical conditions. These findings demonstrate the extraordinary resilience of Labrundinia and its capacity to successfully inhabit a range of ecological niches. In terms of feeding behavior, L. longipalpis larvae are believed to be predators, primarily preying on chironomids or other prey, as very few specimens have been found without this type of prey ( Silva et al. 2011). This specialized feeding behavior further supports the species’ ability to adapt to different aquatic environments and underscores its role as a key predator within these ecosystems. Although adult L. longipalpis have been observed in Sweden during June and July ( Brundin 1949), and larvae have been found in the Netherlands from April to the end of August, along with prepupae, pupae, and exuviae in May, June, and August, conclusive information about their life cycle remains elusive ( Vallenduuk & Moller-Pillot 2007). Further research is needed to unravel the intricacies of their life cycle and fully comprehend their ecological dynamics across various environments.
| R |
Departamento de Geologia, Universidad de Chile |
| IRSNB |
Institut Royal des Sciences Naturelles de Belgique |
| VI |
Mykotektet, National Veterinary Institute |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Labrundinia longipalpis ( Goetghebuer, 1921 )
| Silva, Fabio Laurindo Da 2023 |
Labrundinia longipalpis (Goetghebuer)
| Silva, F. L. & Fonseca-Gessner, A. A. & Ekrem, T. 2011: 234 |
Labrundinia maculata
| Silva, F. L. & Fonseca-Gessner, A. A. & Ekrem, T. 2011: 234 |
| Roback, S. S. 1987: 192 |
| Roback, S. S. 1971: 271 |
Labrundinia longipalpis (Goetghebuer)
| Fittkau, E. J. 1962: 376 |
Ablabesmyia longipalpis (Goetghebuer)
| Goetghebuer, M. 1936: 43 |
Pentaneura longipalpis (Goetghebuer)
| Edwards, F. W. 1929: 294 |
Tanypus longipalpis
| Goetghebuer, M. 1921: 18 |