Chaetophractus villosus ( Desmarest, 1804 )
|
publication ID |
https://doi.org/10.1093/mspecies/seab017 |
|
persistent identifier |
https://treatment.plazi.org/id/039C171F-3956-7E54-FF71-439AA2E9F84A |
|
treatment provided by |
Felipe |
|
scientific name |
Chaetophractus villosus ( Desmarest, 1804 ) |
| status |
|
Chaetophractus villosus ( Desmarest, 1804) View in CoL
Large Hairy Armadillo
Dasypus octocinctus G. I. Molina, 1782:30 . Type locality “Nel Cujo,” Chile (=provincia Mendoza, Argentina, see Tamayo, 1968:6); preoccupied by Dasypus octocinctus Schreber, 1774 , a junior synonym of Dasypus novemcinctus Linnaeus, 1758 View in CoL .
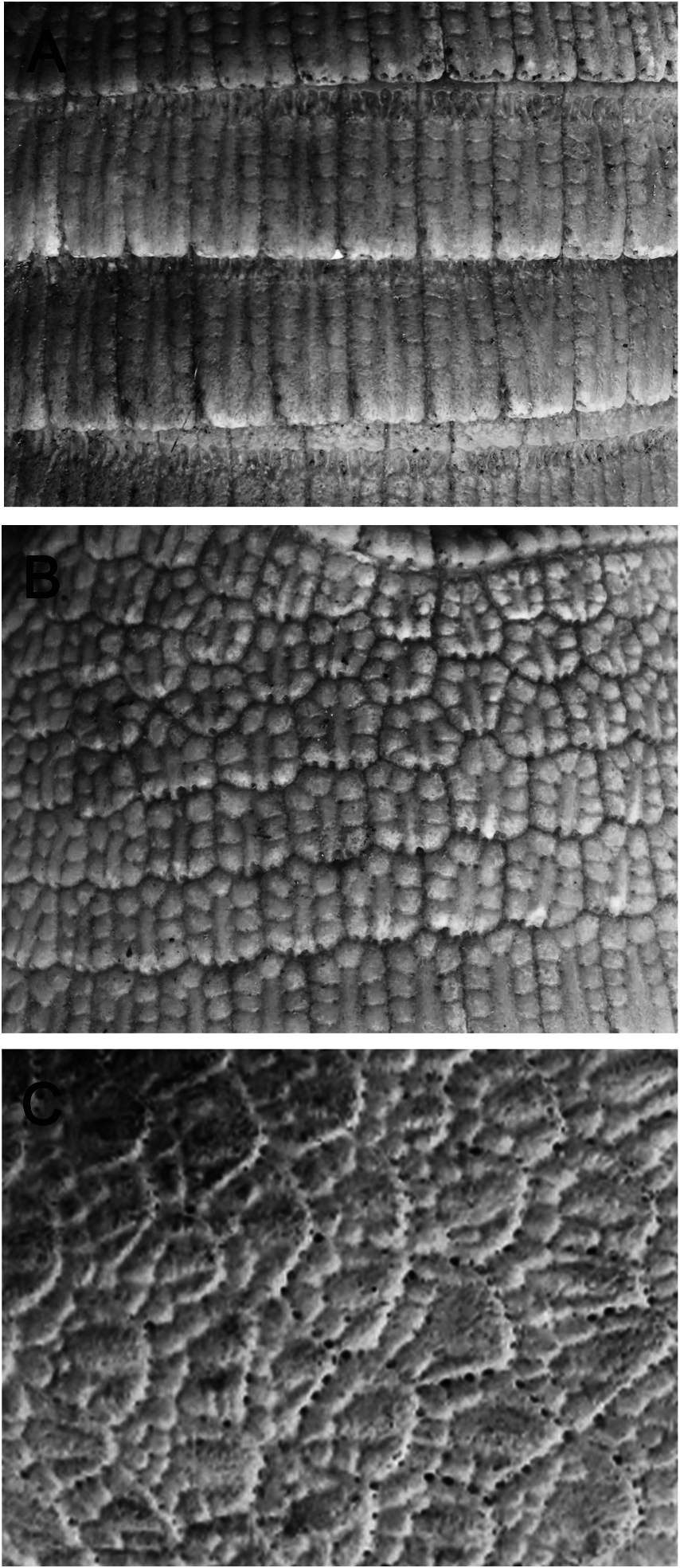
lor [ icatus]. villosus Desmarest, 1804:28 View in CoL . Based on “Le tatou velu de d’Azara” therefore, the type locality is “Les Pampas” [of Buenos-Aires, Argentina], south of Río la Plata, between latitudes 35ºS and 36ºS ( Azara, 1810:164). Fig. 1. —A Chaetophractus villosus View in CoL from the Patagonian steppe,
T [ atus]. villosus : Olfers, 1818:220. Name combination.
Tatusia villosa : Lesson, 1827:312. Name combination.
Dasypus ( Tatusia) villosus : Rapp, 1852:10. Name combination.
Dasypus View in CoL [( Euphractus View in CoL )] villosus : Burmeister, 1861:427. Name combination.
Euphractus villosus : Gray, 1865:376. Name combination.
Chaetophractus villosus View in CoL : Fitzinger, 1871:268. First use of current name combination.
[ Dasypus ( Choetophractus) ] villosus View in CoL : Trouessart, 1898:1146. Name combination.
D [ asypus]. pilosus Larrañaga, 1923: 343 View in CoL Type locality “campis bonaerensibus;” preoccupied by Dasypus pilosus ( Fitzinger, 1856) View in CoL .
Euphractus ( Chaetophractus) villosus : Moeller, 1968:514. Name combination.
Choetophractus villosus : Seelwische, 1980. Name combination
Chaethophractus villosus : Ramírez Pinto and Velázquez, 2010:24. Incorrect subsequent spelling of Chaetophractus villosus ( Desmarest, 1804) View in CoL .
CONTEXT AND CONTENT. Order Cingulata View in CoL , family Chlamyphoridae , subfamily Euphractinae , tribe Euphractini ( Wetzel et al. 2008; Gibb et al. 2016). The most recent study on the phylogenetic systematics of hairy armadillos ( Abba et al. 2015a) recognizes two species: Chaetophractus villosus View in CoL and C. vellerosus View in CoL . Chaetophractus villosus View in CoL is monotypic ( Superina and Abba 2018). Synonymy is modified from Wetzel et al. (2008).
NOMENCLATURAL NOTES. The generic name, Chaetophractus , means protected by hair [chaet from chaeta, Neo-Latin, meaning hair or bristle + phract from phraktos, Greek, meaning protected; see Braun and Mares (1995)]. The species name refers to its long hair ( villosus, Latin , means hairy— Braun and Mares 1995). Common names in Spanish are peludo, peludo grande, quirquincho, quirquincho peludo, and tatú pecho amarillo. It is sometimes called big, greater or large hairy armadillo in English.
DIAGNOSIS
Chaetophractus villosus (Fig. 1) is second in size only to Euphractus sexcinctus (six-banded armadillo) among the euphractines, with a total head–body length of about 335 mm (range: 260–400 mm) and a mean body mass of 2.9 kg (range: 2–5kg — Superina and Abba 2018) versus total head–body length of up to 480 mm and mean body mass of 4.6 kg in the six-banded armadillo ( Wetzel 1985). The ornamentation of the head-shield osteoderms is conspicuous in C. villosus , whereas it is subtle in the six-banded armadillo ( Krmpotic et al. 2008). The ventral and lateral sides of the body are covered with bristles that are longer and thinner than in the six-banded armadillo ( Fitzinger 1871). Chaetophractus villosus has one complete movable band at the anterior edge of the scapular shield, which is not movable in the six-banded armadillo ( Wetzel 1985).
Compared to C. vellerosus (screaming hairy armadillo), C. villosus is larger ( 2–5 kg versus 0.6–1.2 kg) and has a darker carapace and proportionally shorter ears (< 30 mm length versus 29–32 mm for C. vellerosus — Superina and Abba 2018). Dorsal hair is prominent in both species and varies in color from tan to buffy in C. vellerosus , but it is black and brown in C. villosus ( Wetzel et al. 2008) . Also, the head shield of C. villosus is broad (width:length ratio averaging 0.95) and has conspicuously ornamented osteoderms, whereas it is narrower (width:length ratio averaging 0.90) and with flat and smooth osteoderms in C. vellerosus ( Wetzel et al. 2008; Carlini et al. 2016). The posterior margin of the head shield is not straight, but conforms to the outline of individual marginal osteoderms ( Wetzel et al. 2008). The scapular shield consists of five or six rows of osteoderms and is larger than that formed by the four rows in C. vellerosus ( Carlini et al. 2016) . The marginal osteoderms of the dorsal carapace have pointed apices that are sharper than in C. vellerosus ( Carlini et al. 2016) , and those of the pelvic shield are subtriangular both in C. villosus and C. vellerosus ( Krmpotic et al. 2008) . The pelvic shield frequently bears one to four pores in the dorsal medial line (one or two in C. vellerosus — Carlini et al. 2016), which are the openings of pelvic glands ( Fernández 1922; Estecondo and Casanave 1999; Estecondo et al. 2000).
GENERAL CHARACTERS
The dorsum of Chaetophractus villosus is covered by a bony carapace consisting of a cephalic or head shield, one movable nuchal band, a scapular shield, six to nine (most commonly seven or eight) movable bands, a pelvic shield, and a caudal sheath that completely protects the tail (Figs. 1 and 2; Fernández 1931; Krmpotic et al. 2008; Wetzel et al. 2008; Superina and Abba 2018). One to four glandular pores are often visible in the middorsal line of the pelvic shield ( Fernández 1922; Estecondo and Casanave 1999).
Two main types of osteoderms can be distinguished in the dorsal part of the carapace: fixed osteoderms, forming the scapular and pelvic shields, and movable osteoderms, arranged in rows, forming the bands ( Krmpotic et al. 2008). The external surface of the fixed osteoderms of the scapular and pelvic shields is ornamented with an elongated central figure and several smaller peripheral figures, all of them convex and delimited by well-defined sulci ( Fig. 2A View Fig ). The central figure occupies the posterior two-thirds of the osteoderm, whereas the peripheral figures are located anterior and lateral to the central one ( Fig. 2A View Fig ). There are piliferous foramina on the posterior margin of each osteoderm ( Krmpotic et al. 2008). The osteoderms of the movable bands have two distinct regions: a cranial portion that is elevated, not exposed (and, hence, not visible in living animals), without ornamentation, and which occupies about the proximal one-third of the osteoderm. The other distinct region overlaps the cranial portion of the caudally adjacent osteoderm; its external surface is ornamented with an elongated central figure and smaller peripheral figures ( Fig. 2B View Fig ). In contrast to the fixed osteoderms, usually only lateral peripheral figures are present on these movable osteoderms ( Fig. 2B View Fig ; Krmpotic et al. 2008). Osteoderms of the cephalic shield are not arranged in rows. The osteoderms of the middle and posterior regions are the largest, with a similar ornamentation to that of the scapular and pelvic shields ( Fig. 2C View Fig ; Krmpotic et al. 2008). The anterior marginal osteoderms are smaller, lack ornamentation, and although they vary in shape, they are generally polygonal ( Krmpotic et al. 2008). The shape, size, and arrangement of the osteoderms of the cephalic shield have been used by one of us (MS) to identify individuals in a population.
In general, osteoderms are composed of compact bone. There are three zones arranged in dorsoventral succession, with the outer and inner zones being comparatively thin and formed by regular compact bone with primary and secondary osteons ( Krmpotic et al. 2008). The middle zone is thicker, representing about 50% of osteoderm thickness. It consists of osseous lamellae that lay concentrically around large cavities mainly filled with adipose tissue, hair follicles, and sweat and sebaceous glands ( Krmpotic et al. 2008, 2015).
Mean external measurements (mm ± SD, with parenthetical n) given by Wetzel (1985) for adult specimens (mixed sexes) from Argentina ( n = 9) and Paraguay ( n = 9) were: total body length (head + body), 330.1 ± 31.3 (18); tail length, 149.3 ± 12.1 (16); length of hind foot, 67.1 ± 5.8 (14); ear length 28.5 ± 2.2 (12). Mean external measurements (mm ± SD, with parenthetical n for adult specimens [mixed sexes] from several localities in Argentina, including the introduced population of Tierra del Fuego [obtained by AMA and JAG] and individuals from Mendoza province [obtained by MS]) were: total body length, 339.4 ± 51.4 ( n = 53); tail length, 135.4 ± 9.5 ( n = 49); ear length, 26 ± 5.5 ( n = 49). Mean body mass of seven adults from Argentina and Paraguay given by Wetzel (1985) was 2.02 kg ± 0.59 SD; a range of 2–5 kg for body mass was reported by Superina and Abba (2018).
Mean cranial measurements (mm ± SD) of adult specimens ( 10 females and nine males) from Buenos Aires province, Argentina given by Squarcia et al. (2006) were: nasal length, 34.3 ± 1.4; nasal width, 6.5 ± 0.4; frontal length, 40.7 ± 2.4; frontal width, 22.2 ± 1.0; parietal length, 26.6 ± 1.0; parietal width, 19.0 ± 0.7; premaxillary length, 17.9 ± 1.2; premaxillary width, 12.0 ± 0.8; lacrimal length, 11.3 ± 1.2; lacrimal height, 6.7 ± 0.6; jugal length, 24.7 ± 1.0; jugal height, 8.2 ± 0.7; palatine length, 21.6 ± 1.2; palatine width, 7.6 ± 0.4; pterygoid length, 9.2 ± 0.4; pterygoid height, 5.6 ± 0.4; temporal length, 25.3 ± 0.7; vomer length, 36.6 ± 2.0; vomer width, 6.7 ± 0.6; occipital length, 16.9 ± 0.5; occipital width, 37.3 ± 1.3; dentary length, 71.5 ± 1.9; dentary height, 39.2 ± 1.9. Morphometric differences between bones lying in the rostrocaudal plane (total nasal length males 34.8 ± 0.9, females 33.7 ± 0.9; and frontal length males 40.7 ± 2.4, females 38.8 ± 2.2; n = 9 and 10, respectively) and in mandible dimensions (total mandibular length males 70.5 ± 0.2, females 73.2 ± 0.3; n = 37 and 34, respectively) suggest a sexual dimorphism; for details, see Squarcia et al. (2006, 2009). Sexual dimorphism has also been observed in the humerus and ulna, with females having a more robust humerus and an ulna with a more pronounced curvature along its main axis, a caudal displacement of the lateral fossa, a longer olecranon, and a deeper trochlear notch ( Acuña et al. 2017). Nevertheless, the length of these bones is similar in both sexes ( Acuña et al. 2017); humerus length is 66.2 mm ( Fariña and Vizcaíno 1997) and ulnar length is 63.4 ± 2.8 mm ( Vizcaíno et al. 1999).
The mean number of movable bands of 30 individuals from several localities in Argentina was 7.3 ± 0.5 (obtained by AMA and JAG). Thirty-four individuals analyzed by Langmann (1931) had seven, 159 had eight, and eight had nine movable bands (mean ± SD: 7.81 ± 0.4, n = 201). The number of osteoderms of the movable bands was 36–44 per band ( n = 201). Carapace abnormalities, such as interspersed osteoderms, incomplete bands, double osteoderms, or joined osteoderms most frequently occurred in the first movable band and in the pelvic shield ( Langmann 1931).
DISTRIBUTION
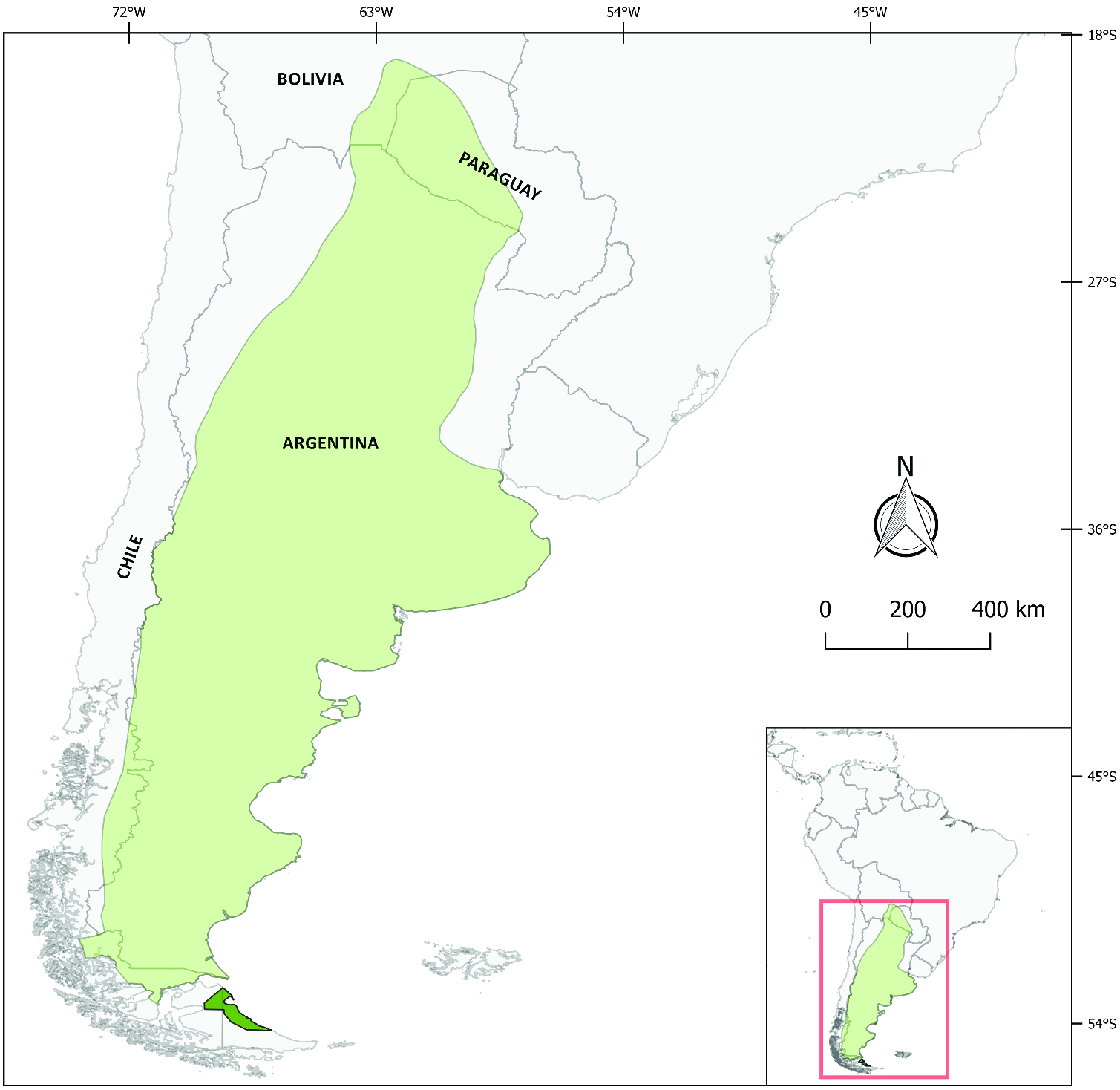
Chaetophractus villosus is found in Argentina, the “Gran Chaco ” of Bolivia and Paraguay, and southern Chile ( Fig. 3 View Fig ; Abba et al. 2014b; Superina and Abba 2018; Gallo et al. 2019b). In Argentina it is widely distributed except it is absent in the northwest ( Jujuy province and western parts of Salta, Catamarca, La Rioja, and San Juan) and in the northeast (provinces of Entre Rios, Corrientes, and Misiones). Records in northeastern Argentina are probably misidentified six-banded armadillos ( Abba and Vizcaíno 2008; Pautasso 2009). Its presence in the south-central part of continental Patagonia was first reported about 100 years ago (Abba et al. 2014a). Chaetophractus villosus was introduced in the Argentinean portion of Isla Grande of Tierra del Fuego ( Fig. 3 View Fig ) 35 years ago, when eight individuals were released north of Rio Grande City for aesthetic reasons ( Poljak et al. 2007; Gallo et al. 2020). The current area occupied on the island is 5,217.92 km 2, with a linear expansion rate of 10.9 km /year ( Gallo et al. 2020). It is now also present in the Chilean portion of the island ( Cabello et al. 2017). It has also been introduced in Isla Leones, Chubut ( Gallo et al. 2019b). In Bolivia it is present in Arenales del Chaco, Bosque Tucumano- Boliviano, Gran Chaco Norte, and Gran Chaco Sur ( Tarifa and Romero-Muñoz 2009; Noss et al. 2010). In Paraguay C. villosus is most common in the Dry Chaco, with marginal occurrence in the Humid Chaco and possibly the Cerrados del Chaco ecoregions ( Smith and Rios 2018). In Chile it was originally found from the region of BioBío south to Magallanes ( Fig. 3 View Fig ; Abba et al. 2014b), but recent records are restricted to Aysén and Magallanes ( Pasutti Morales 2017). The absence of C. villosus in Uruguay may be related to the water barrier presented by the La Plata River ( Poljak et al. 2010).
FOSSIL RECORD
Earliest fossils of Chaetophractus are from the Pliocene to Holocene of South America ( Carlini and Scillato-Yané 1996; McKenna and Bell 1997). The first paleontological record of C. villosus came from fossilized osteoderms found in the locality of Chapadmalal ( Buenos Aires province, Argentina) in a stratigraphic section over the Atlantic coast of the Pampean Region that corresponds to the Chapadmalalan mammal age (4–3.2 Ma, late Pliocene—Cione and Tonni 1995; Carlini and Scillato-Yané 1996). Molecular clock analyses suggest that C. villosus split from other related species about 9.1 ± 2.4 My BP ( Gibb et al. 2016). The presence of C. villosus in archaeological sites of the Pampean Region is very frequent and dates back to 4,600 to 7,700 years BP (see Abba and Vizcaíno 2011).
FORM AND FUNCTION
Form. ––Skull length of an adult Chaetophractus villosus is between 86 and 96.5 mm ( Fig. 4 View Fig ). Skull components are morphologically similar in both sexes ( Squarcia et al. 2006), but females have larger skulls and mandibles than males (see “General Characters” and Squarcia et al. 1994, 1999, 2006, 2009).
Finite element analyses have shown that C. villosus has a stronger jaw, with a masseter force of 0.66 N m−2 and temporalis force of 0.34 N m−2 ( Serrano-Fochs et al. 2015). These values are higher than those found in insectivorous armadillo species such as the southern three-banded armadillo ( Tolypeutes matacus ) with a masseter force of 0.35 N m−2 and temporalis force of 0.14 N m−2, and the pink fairy armadillo ( Chlamyphorus truncatus ), with a masseter force of 0.04 N m−2 and temporalis force of 0.09 N m−2 ( Serrano-Fochs et al. 2015). The 9 maxillary and 10 mandibular teeth are simple, oval, and without enamel. Teeth have open roots and are continuously growing ( Squarcia et al. 2006; Vizcaíno 2009).
The tympanic bulla is hypertrophied, that is, well expanded and swollen ( Roig 1971; Squarcia et al. 2007; Sidorkewicj and Casanave 2012). It is completely ossified in adults and lacks defined limits between the tympanic and the endotympanic bones ( Roig 1971; Squarcia et al. 2007; Basso et al. 2017).
Like all xenarthrans, C. villosus has a small, J-shaped, intramembranous paired bone of 3 mm width in the anterior nasal cavity. It was initially called “nasal floor bone” ( Broom 1897), then “ os nariale ” ( Wegner 1922), and is often absent in museum specimens. Although first thought to be a neomorphic structure and not homologous to the septomaxilla of monotremes or lepidosaurs ( Wible et al. 1990), it was later defined as a true septomaxilla based on studies of other Xenarthra ( Zeller et al. 1993). In armadillos, the presence of these small bones may be linked to their fossorial habits, contributing to the protection of the nares while digging ( Squarcia et al. 2006).
Hyoid morphology differs markedly from the generalized pattern of Mammalia. The thyroid cartilage is partially ossified. The thyrohyal and basihyal bones are fused, forming a V-shaped element (sometimes called V-bone) that is diagnostic for Xenarthra ( Pérez et al. 2010). In living armadillos, including C. villosus , the V-bone is dorsoventrally flattened, producing an elliptical section ( Pérez et al. 2010). In combination with the long and anteroposteriorly directed geniohyoid, it allows protrusion of the tongue considerably beyond the oral cavity, which is important for food acquisition and intake ( Pérez et al. 2010).
The tongue is triangular, elongated in anteroposterior direction, with a sharp apex and filiform, fungiform, and vallate, but no foliate, papillae ( Estecondo et al. 2004). Filiform papillae are the most numerous, covering the apex and body of the tongue ( Estecondo et al. 2004).
The brain weighs about 20 g in an adult of 3 kg body mass ( Jakob and Onelli 1913). It is lissencephalous, macroptic, and with highly developed cerebellum, pyramids, and pons ( Jakob and Onelli 1913). The acoustic nerve is very well developed ( Hübschmann 1903). The well-developed olfactory mucosa and the functional vomeronasal organ strongly suggest an acute sense of smell (Ferrari et al. 1998; Carmanchahi et al. 1999). The glycoconjugate sugar residues in the vomeronasal organ of C. villosus have been characterized by Carmanchahi et al. (2000). The Harderian gland is, in general terms, similar to that of other mammals, but it has unique membranous bodies in its supranuclear region that are organized in a geometrical pattern similar to a “Star of David”; they are probably involved in the formation of the lipid-like secretion ( Aldana Marcos and Affanni 2005). The lacrimal and nictitans glands have the general pattern found in other mammals, but intercellular canaliculi are present between the seromucous cells, suggesting that seromucous components of the lacrimal gland could be involved in bulk fluid and ion transport ( Aldana Marcos et al. 2002).
Females have two pectoral mammary glands, a large vulva of 2–3 cm length, and a urogenital sinus instead of a true vagina ( Cetica et al. 2005). The uterus is pyramid-shaped, with a well-developed body and two small lateral horns ( Fernández 1915; Cetica et al. 2005). The placenta is of the hemochorial type ( Adamoli et al. 2001; Rezende et al. 2012). The ovary is ovoid, elongated, 0.6–1.0 cm long, and with a longitudinal polarization of the cortex and medulla. The cortex faces toward the infundibulum and consists of a stroma containing groups of oocytes surrounded by a simple squamous or cubic follicular cell layer, and typical follicles with a single oocyte ( Cetica et al. 2005). The structure of ovarian follicles is similar to that in other mammalian species ( Codón et al. 2001). The oviduct has a general structure similar to other mammals ( Codón and Casanave 2009), but an unusual cellular distribution, with an increase in the number of ciliated cells from the fimbriae to the uterus, an isthmus that is almost completely lined with ciliated cells, and a higher abundance of secretory cells in the apical zone of the ampulla and fimbriae than in the isthmus ( Codón and Casanave 2009). This unusual cellular distribution may regulate the progression of spermatozoa ( Codón and Casanave 2009).
Males have a long penis (around 55% of body length— Affanni et al. 2001), intra-abdominal testes ( Grassé 1955), and a large, thick, subquadrangular prostate gland ( Fauvel 1894). Sperm heads are very large, flat and spoon-shaped, their length and width being among the largest of any mammalian sperm ( Cetica et al. 1993). Spermatogenesis seems to be seasonal with a temporal interruption between mid and end of autumn ( Luaces et al. 2013).
The digestive system is monogastric ( Estecondo et al. 1995). Stomach size is variable depending on the amount of its content; the length of its greater curvature varies from 6.4 to 38.5 cm (mean 16.3 cm, n = 48—M. C. Ezquiaga et al., in litt., Centro de Estudios Parasitológicos y de Vectores, Buenos Aires, Argentina, 1 February 2020). The small intestine is 121–255 cm (mean 181.08 cm, n = 52) and the large intestine 15–39.3 cm (mean 27.3 cm, n = 49) long (Gallo et al. in press). Cecum length varies between 3.3 and 15 cm (mean 7.06 cm, n = 50––Gallo et al. in press). Olocco Diz et al. (2006) registered the intestine length from one individual: 242 cm for the small intestine, 38.5 cm for the large intestine, and 8.5 cm for the cecum. There is a sphincter that clearly separates the cecum from the small intestine, thus retarding intestinal flow ( Estecondo et al. 1995). Fecal bile acids have 15 compounds: seven known bile acids, cholesterol, and seven unidentified compounds ( Araujo et al. 2010). Deoxycholic acid was only found in C. villosus , but not in six other Xenarthra species ( Araujo et al. 2010).
The spleen has primitive characteristics, such as an intermediate zone between the red and white pulps and persistent hemato-poiesis in adult individuals. It is less differentiated and myeloid in neonates ( Galíndez et al. 1997). The spleen is trilobated and of the nonsinusoidal type ( Galíndez et al. 1997). Its size is variable and represents 0.1–1.0% of body mass in adult individuals ( Galíndez et al. 1997). Its proportionally large size compared to other taxa, such as primates and nonmurid rodents, could be related to fossorial habits and the associated hypoxia ( Galíndez et al. 1997).
One to four pelvic glands are located in concavities of osseous protuberances on the internal face of the pelvic shield. They consist of sebaceous and apocrine sudoriparous acini that release an oily substance in stress situations ( Fernández 1922; Estecondo et al. 2000).
The forefoot has five digits, with a robust third and a reduced first digit ( Galliari et al. 2010). A number of characteristics in the carpus, scaphoids, trapezium-trapezoids, and the forelimb myology, are typical of scratch-diggers ( Galliari et al. 2010; Marshall et al. 2021). Vizcaíno and Milne (2002) and Milne et al. (2009) classified the Euphractini as generalized diggers, with only Tolypeutes and the Dasypodini being less fossorial. The vertebral formula of C. villosus is 7 cervical, 11–12 thoracic, 3–4 lumbar, and 14–15 thoracolumbar vertebrae ( Galliari et al. 2010). The synsacrum of C. villosus and all other Euphractinae has a mode of eight vertebrae (range: 7–9— Galliari and Carlini 2019). The first three synsacral vertebrae are fused to the iliac bones, the next two form the dorsal border of the sacroischial fenestra, and the last three are fused to the ischial bones through the tip of their transverse processes ( Galliari and Carlini 2019). The evolution of the axial skeleton is described in Galliari et al. (2010) and the morphology and evolution of the hand skeleton in Galliari (2014).
Hair is coarse, nearly oval in cross-section, and ranges in color from buffy white or tan to black and in density from sparse to relatively dense ( Ridewood 1901; Wetzel 1985). It has a distinct slit-like medullary cavity and a rather thick cuticle. The granules in the cortex are most thickly set at a slight distance from the margin, thus leaving a central part and a peripheral part of the cortex relatively clear ( Ridewood 1901). The cuticular scales form a mosaic pattern ( Chehébar and Martín 1989).
Function. ––Like all Xenarthra, Chaetophractus villosus has poor thermoregulatory abilities. The rectal temperature of wild individuals varies from 33.3 to 37.0°C, with a mean of 36.0°C (Abba 2008). Body temperature remains stable, at 35.1°C, at ambient temperatures of 10–30°C ( McNab 1980). Rectal temperature can vary from 24 to 36°C over the course of the day ( Roig and Henriquez 1984).
Basal rate of metabolism in a captive individual was 0.178 cm 3 O 2 g−1 · h−1 ± 0.013 SE and minimal conductance 0.031 cm 3 O 2 g−1 · h−1 °C− 1 ± 0.0014 SE ( McNab 1980). Normal heart rate is around 116 beats per minute. The respiratory rate in relaxed individuals is 30–40 breaths per minute; it can rise to 160 breaths per minute in stress conditions ( Aguilar and Superina 2015). The electrocardiogram of C. villosus is similar to that of other mammals ( Maldonado and Casanave 1996). The P-wave is usually very low, Q- and S-waves are almost absent, and the height of the T-wave is variable ( Maldonado and Casanave 1996).
Like other burrowing animals, armadillos are exposed to hypoxic and hypercapnic conditions when inside their burrows ( Maclean 1981; Spencer 2011). Chaetophractus villosus covered with soil is able to use the oxygen trapped between soil particles. In parallel, it reduces body temperature and heart rate in a manner reminiscent of that exhibited by diving mammals ( Casanave and Affanni 1995; Casanave et al. 1995).
In a laboratory environment, C. villosus mostly sleeps in dorsal or lateral decubitus ( Affanni et al. 2001). Periods of uninterrupted sleep of more than 6 h are frequent, and the general characteristics of the brain waves during wakefulness, slow-wave sleep, and paradoxical sleep are similar to those of other mammals ( Affanni 1972). Penile erections and movements are absent during paradoxical sleep, and an intense tremor of the four limbs can be observed after the initiation of slow-wave sleep ( Affanni et al. 2001).
With respect to humoral immunity, C. villosus shows high levels of IgM (Sasiain etal. 1977). Its lymphocytes have receptors for sheep red blood cells (SRBC), the C3 complement fraction, and surface immunoglobulins (Ig-s), but lack receptors for the Fc-segments of immunoglobulins ( Sasiain et al. 1977). Plasma protein polymorphism differs from that in the nine-banded armadillo ( Dasypus novemcinctus ), with a 90% difference in loci encoding for allelic proteins present in blood of these two species, which may explain differences in their susceptibility to disease ( Ramsey and Grigsby 1985).
Blood can be collected from the coccygeal vein ( Superina 2000; Luaces et al. 2011b). Hematological and biochemical parameters are within the range of other xenarthrans ( Maldonado and Casanave 1993; Casanave and Polini 1999; Polini and Casanave 1999; Polini et al. 1999). Hematological parameters may vary among populations and, possibly, age classes ( Casanave and Polini 1999). The basic coagulation system and hemostatic process are similar to those in other mammals, but prothrombin and thrombin times are longer (Casanave et al. 2005, 2006). Blood platelets of C. villosus are circular-shaped at rest and produce pseudopodia when activated, as in other mammals. However, their platelet count range (80–941· 109 /liter) is wider than that of other mammals ( Bermúdez et al. 2004).
ONTOGENY AND REPRODUCTION
Skeletal and intertegument maturity at birth suggests that neonates are altricial ( Galliari 2013). Offspring are born with closed eyes and ears folded, but with well-developed forelegs and claws ( Roberts et al. 1982). They open their eyes about 16–30 days after birth ( Roberts et al. 1982). A Chaetophractus villosus born at Krefeld Zoo, Germany, weighed 145 g at birth and measured 18 cm in total length (from the tip of the snout to the tip of the tail— Encke 1965). Body weight and total length, respectively, were 280 g and 23 cm on day 6; 410 g and 25 cm on day 11; 560 g and 28 cm on day 16; 700 g and 31 cm on day 21; 870 g and 34 cm on day 26; and 1,050 g and 36 cm on day 31. Hence, its daily weight gain during lactation was 26–36 g. At 12 days of age, the offspring started walking around independently, its skin flaked off, and the bristles on the carapace border became visible. It opened its eyes and ears on day 32 ( Encke 1965). Individuals born at Frankfurt Zoo, Germany, weighed 142–145 g on day 2, doubled their weight 9 days later, and opened their eyes at 23–25 days of age ( Kühn 1953). Birth weight of two males at Temaikèn Zoo, Argentina, was 118.53 and 108.33 g ( Olocco Diz and Duggan 2004). The young usually do not leave the burrow until they are weaned, which occurs after about 55 days ( Roberts et al. 1982). Sexual maturity is attained at 1 year of age ( Superina and Abba 2018).
The mating period is probably from the end of winter throughout spring ( Superina and Abba 2018). Spermatogenesis is seasonal, with temporary activation during the mating period ( Luaces et al. 2013). Seasonal changes in different portions of the oviduct, and in fecal steroid hormone concentrations in females, occur from the end of winter to the middle of spring ( Codón and Casanave 2009; Luaces et al. 2011a). A preovulatory genital hemorrhage is sometimes observed ( Aguilar and Superina 2015).
Gestation length varies between 60 and 75 days ( Wetzel 2008). The female constructs a nest prior to giving birth to one or two offspring. Of 34 pregnant uteri examined by Fernández (1915), only five had a single embryo, whereas 29 had two embryos of the same or of different sex.
In the Bolivian Chaco, C. villosus appears to reproduce from November to May, with the majority of lactating females being found in December and April ( Cuéllar and Noss 2003). The milk contains 7.83 g /dl proteins and 8.23 g /dl fat ( Hernandez et al. 1999).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Chaetophractus villosus ( Desmarest, 1804 )
| Gallo, Jorge Alberto, Superina, Mariella, Abba, Agustín Manuel, Rose, Robert K & Hamilton, Meredith J 2022 |
Chaethophractus villosus
| Ramirez Pinto F. & Velazquez M. C. 2010: 24 |
Euphractus ( Chaetophractus ) villosus
| Moeller W. 1968: 514 |
Dasypus ( Choetophractus )
| Trouessart E. L. 1898: 1146 |
Chaetophractus villosus
| Fitzinger L. J. 1871: 268 |
Euphractus villosus
| Gray D. E. 1865: 376 |
Dasypus
| Burmeister H. 1861: 427 |
Dasypus ( Tatusia ) villosus
| Rapp W. 1852: 10 |
Tatusia villosa
| Lesson R. P. 1827: 312 |
Dasypus octocinctus G. I. Molina, 1782:30
| Tamayo H. M. 1968: 6 |
| Molina G. I. 1782: 30 |