Creophilus maxillosus, (LINNAEUS)
|
publication ID |
https://doi.org/10.1111/j.1096-3642.2011.00725.x |
|
publication LSID |
lsid:zoobank.org:pub:FBFE9195-BE04-4AFE-9417-6E38BCE6AB84 |
|
persistent identifier |
https://treatment.plazi.org/id/039B414F-1959-FFDC-FF0B-FD00487AFC11 |
|
treatment provided by |
Valdenar |
|
scientific name |
Creophilus maxillosus |
| status |
|
1. CREOPHILUS MAXILLOSUS (LINNAEUS) View in CoL
( FIGS 1F, 2E–G, 3C, D, I, P, 4B, C View Figure 4 , 5A View Figure 5 , 9C–F, L, M, 10B View Figure 10 , 13 View Figure 13 , 14 View Figure 14 , 15,19B, 24)
Staphylinus maxillosus Linnaeus, 1758: 421 View in CoL . Type locality: ‘Europa’; Herman, 2001b: 3317 (as C. maxillosus View in CoL ); Navarrete-Heredia et al., 2002: 322 (as C. maxillosus View in CoL ). Type specimens: see C. maxillosus maxillosus View in CoL .
Staphylinus anonymus Sulzer, 1761: 17 View in CoL , fig. 49. Type locality: ‘Europa’; Erichson, 1839: 348 (synonym of S. maxillosus View in CoL s.l.); Herman, 2001b: 3321 (synonym of C. maxillosus View in CoL s.l.); Smetana, 2004: 671 (synonym of C. m. maxillosus View in CoL ).
Staphylinus tertius Schaeffer, 1766 View in CoL : plate 20, fig. 1, 1. Type locality: ‘Ratisbon’ (not seen); Erichson, 1839: 348 (synonym of S. maxillosus View in CoL s.l.); Herman, 2001b: 3321 (synonym of C. maxillosus View in CoL s.l.; nomen nudum, Article 11.4).
Staphylinus balteatus DeGeer, 1774: 18 View in CoL . Type locality not cited; Fabricius, 1781: 334 (synonym of S. maxillosus View in CoL s.l.); Herman, 2001b: 3321 (synonym of C. maxillosus View in CoL s.l.); Smetana, 2004: 671 (synonym of C. m. maxillosus View in CoL ).
Staphylinus fasciatus Füessly, 1775: 21 View in CoL . Type locality: Switzerland; Gravenhorst, 1802: 3 (synonym of S. maxillosus View in CoL s.l.); Herman, 2001b: 3322 (synonym of C. maxillosus View in CoL s.l.); Smetana, 2004: 672 (synonym of C. m. maxillosus View in CoL ).
Staphylinus nebulosus Geoffroy, 1785: 165 View in CoL . Type locality: Paris; Olivier, 1795: (42): 10 (synonym of S. maxillosus View in CoL s.l.); Herman, 2001b: 3322 (synonym of C. maxillosus View in CoL s.l.); Smetana, 2004: 672 (synonym of C. m. maxillosus View in CoL ).
Staphylinus bicinctus Mannerheim, 1843: 229 View in CoL . Type locality: ‘insula Sitkha’; Solsky, 1870: 261 (synonym of C. arcticus ); Horn, 1879: 200 (synonym of C. villosus View in CoL ); Ganglbauer, 1895: 415 (synonym of C. maxillosus View in CoL s.l.); Herman, 2001b: 3323 (synonym of C. maxillosus View in CoL s.l.; preoccupied by Staphylinus bicinctus Rossi, 1792 View in CoL ); Navarrete-Heredia et al., 2002: 322 (synonym of C. maxillosus View in CoL s.l.); Smetana, 2004: 671 (synonym of C. m. maxillosus View in CoL ); primary junior homonym of Staphylinus bicinctus Rossi, 1792 View in CoL .
Staphylinus orientalis Motschulsky, 1858b: 67 . Type locality: ‘Iles Kouriles’; Ganglbauer, 1895: 415 (synonym of C. maxillosus View in CoL s.l.); Herman, 2001b: 3324 (synonym of C. maxillosus View in CoL s.l.); Smetana, 2004: 672 (synonym of C. m. maxillosus View in CoL ).
Creophilus fulvago Motschulsky, 1860: 120 View in CoL . Type locality: ‘ Mongolie et Chine boréale’; Ganglbauer, 1895: 415 (synonym of C. maxillosus View in CoL s.l.); Herman, 2001b: 3324 (synonym of C. maxillosus View in CoL s.l.); Smetana, 2004: 672 (synonym of C. m. maxillosus View in CoL ).
Creophilus maxillosus var. pulchellus Meier, 1899: 99 . Type locality: ‘Hamburg’; Blackwelder, 1943: 448 (synonym of C. maxillosus View in CoL s.l.); Herman, 2001b: 3324 (synonym of C. maxillosus View in CoL s.l.); Smetana, 2004: 672 (synonym of C. m. maxillosus View in CoL ).
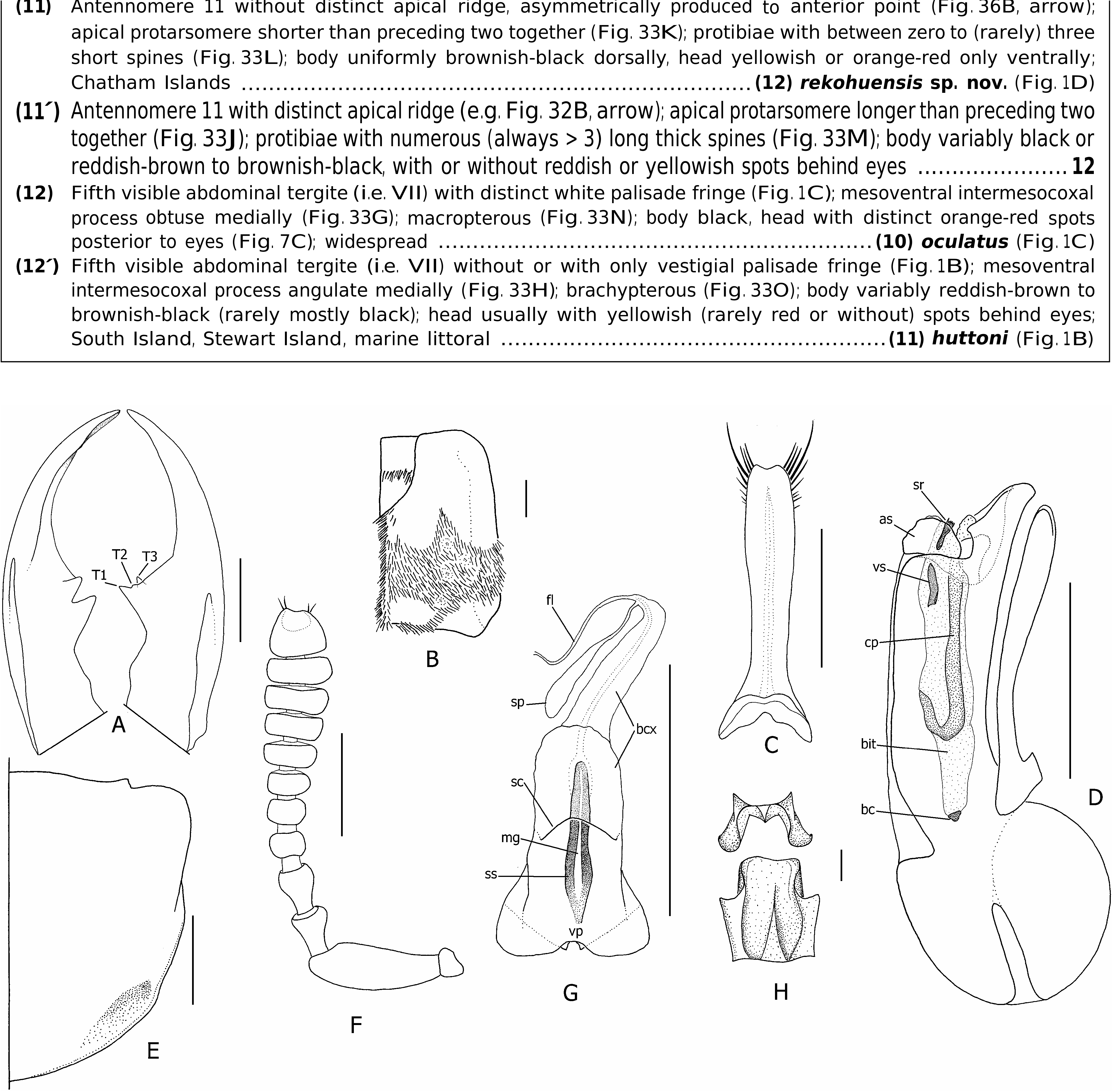
Diagnosis: With characters of the maxillosus -group; integument, including elytra, uniformly black; antennae moderately clavate; apex of antennomere 11 emarginate ( Fig. 13F View Figure 13 ); elytra with white vestiture arranged into well-demarcated (but variable) central transverse fascia ( Figs 1F, 13B View Figure 13 ); tergal chaetotaxic formula 6-6-6-2(4)-4(6)-8(10+).
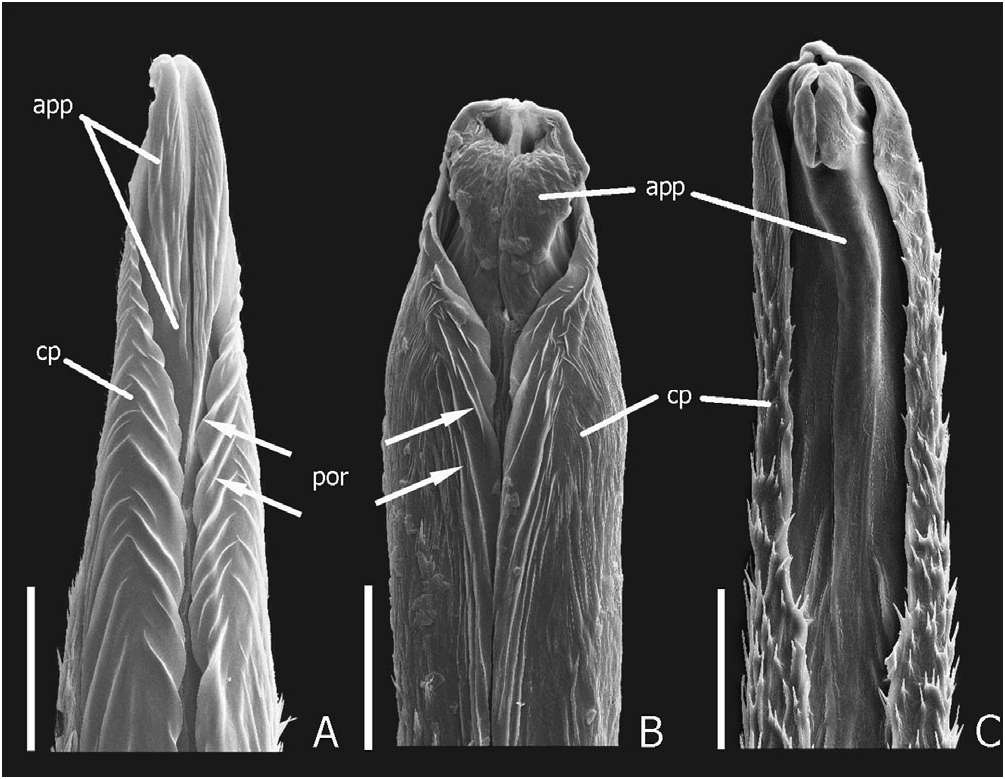
Description: To previous detailed descriptions ( Blackwelder, 1936; Dajoz & Caussanel, 1969) I add measurements and body ratios (listed under each subspecies), chaetotaxic characters, and full description of male and female genital characters. Head. Head slightly wider anteriorly in large males, subtrapezoidal and wider posteriorly to suborbicular in females and smaller males; basal margins densely setose; dorsal punctation moderately dense; eyes large, dorsolateral, lateral margins of head visible in dorsal view (not obscured by eye), HL1/HL2 greater in females than males; antennae as in Figure 13F View Figure 13 , moderately clavate; antennomeres 1–6 brownish-black, 7–11 greyish-black; apex of antennomere 11 slightly to moderately emarginate medially; each pair of apical setae widely separated, one on each side of apex; mandibles as in Figure 13A, T View Figure 13 1 and T 3 subequal in length, in large males T1 much larger than T2 and T3. Thorax and abdomen. Pronotum ( Figs 2G, 13E View Figure 13 ) moderately to distinctly narrowed posteriorly; basolateral margins very shallowly emarginate, hind angles indistinct; with dense and long peripheral setae and coarse long black or whitish-grey vestiture on anterolateral declivities; scutellum with indistinct anterior band of whitish-grey vestiture; elytra uniformly black, densely setose, with characteristic welldelimited fascia of whitish-grey setae at middle; wings fully developed, clear yellowish-brown to black, without black spot in medial field between MP3 and MP4 veins; ventral thoracic vestiture entirely whitish-grey or black ( Fig. 14 View Figure 14 ); anterior and middle legs with black vestiture, hind coxae, trochanters, and femora with white vestiture; abdominal vestiture arranged into characteristic pattern of whitish-grey or golden brown maculation dorsally, and mostly or entirely white on sternites and parasclerites IV–VI, sternite III, and sternites VII–IX; extensive whitishgrey vestiture interrupted dorsally by a large medial and two smaller anterolateral black patches, and ventrally by more or less circular black patches laterally; abdominal tergite VII with well-developed palisade fringe. Male genitalia. Aedeagus as in Figures 3C, 13D View Figure 13 ; paired apicolateral sclerites articulated to internal edge of median orifice. Paramere as in Figure 13C View Figure 13 . Internal sac inverted as in Figure 13D View Figure 13 , everted as in Figure 3C, D; with short ventral process ( vp), ventromedian spiculose strip slightly longer than the box-like ventral sclerite ( Figs 3I, 13H View Figure 13 , vs). Female internal genitalia. Internal genitalia as in Figures 5A View Figure 5 , 13G View Figure 13 ; vaginal plate produced into two broad lobes posteriorly, and with median sclerotized strip ( ss); vaginal fold ( vf) completely membranous; spermatheca ( sp) elongate sacculate, with distinct duct connecting to bursa copulatrix ( bcx). Chaetotaxy. Elytral discal series with 4–8 macrosetae, frequently with additional macroseta medially disposed from series; tergal chaetotaxic formula = 6-6-6-2(4)-4(6)- 8(10+), outer laterals present or absent and inner laterals absent on tergite VI, inner laterals present or absent on tergite VII; tergite VIII often with inconsistent additional macrosetae.
Variation: Coloration and the patterning of body vestiture are the only notable geographically variable characters, and with the exception of North American and Palearctic forms (discussed under each subspecies) appears to be continuous. These forms exhibit more or less identical male and female genitalia, with only subtle differences in the copulatory piece/ appendix that may be significant for reproductive isolation but which require more detailed study. Much of the synonymic diversity is attributable to variations in vestiture coloration and to allometric characters of the head and thorax.
Comparison: Creophilus maxillosus is distinctive in habitus ( Fig. 1F) and most similar to C. incanus . It is reliably distinguished from this species by the concave apex of the apical antennomeres, and from all species by the definite whitish-grey elytral fascia, and dorsal pattern of body vestiture.
Distribution: Widespread throughout the northern hemisphere; apparently absent from austral regions (except as adventive), South East Asia, and the East Indies. See under each subspecies for details.
Biology and ecology: Creophilus maxillosus is probably the best-studied rove beetle species. Herman (2001b) lists many papers on its biology, morphology, and life history. The following papers provide details of: general biology and life history ( Abbott, 1938; Fichter, 1949b; Kramer, 1955; Greene, 1996); morphology and anatomy ( Talbot, 1928; Blackwelder, 1936; Dajoz & Caussanel, 1969); diet ( Fichter, 1948); chemical defence ( Jefson et al., 1983); olfaction ( Abbott, 1936); and immature morphology ( Voris, 1939; Paulian, 1941). Kirkpatrick & Olson (2002) note increases in predatory activity at night. Moon & Kajii (1997) observed fully grown larvae feeding on fly maggots beneath rabbit carcasses, where they are presumably able to evade the cannibalistic tendencies of adults ( Abbott, 1937, 1938) by burrowing into the substrate (as observed by Fichter, 1948).
Dynamics of arrival and departure at carrion have been recorded frequently in the forensic entomology literature (e.g. Early & Goff, 1986; Goff, 1991; Anderson & VanLaerhoven, 1996; Moon & Kajii, 1997; Richards & Goff, 1997; Sánchez Piñero, 1997; Avila & Goff, 1998). Summarizing these papers, adults arrive early at a carcass during the ‘bloat’ and ‘decay’ stages ( Payne, 1965; length of stages varies depending on environmental conditions), remaining there up to 13 days post-mortem. Creophilus maxillosus prefers open disturbed habitats over dense forest, and is typically found in synanthropic situations. Habitat: seashore, grassland, prairie, mixed forest, savannah, woodland, swamp, alpine meadow, etc. Altitude: sea level to 3640 m. Phenology: throughout the year, tending to be most common from late spring through autumn in northern USA.
Remarks: Measurement and ratio data for this species are provided for each subspecies, below. I could not determine details and/or depositions for type material of the following synonyms: Staphylinus anonymus Sulzer , Staphylinus tertius Schaeffer , Staphylinus balteatus De Geer , Staphylinus fasciatus Füessly , Staphylinus nebulosus Geoffroy , Staphylinus bicinctus Mannerheim ( syntypes in ZMHB?), and C. maxillosus var. pulchellus Meier ( holotype by monotypy). Syntypes (?) of S. orientalis and C. fulvago are presumably in ZMUM but according to A. Gussakov (ZMUM) these specimens are in poor condition and their identity cannot be confirmed. I list these nine names here because I could not confirm their status as synonyms of the nominate subspecies, which for most is nevertheless suggested by their type localities and synonymic history. All other synonyms and type material are listed under the subspecies to which they apply. Previous authors (e.g. Horn, 1879; Hatch, 1957) have treated C. bicinctus as a synonym of C. m. villosus or C. arcticus (see Hatch, 1938: 148). Motschulsky (1858b, 1860) notes the similarity of S. orientalis to C. m. maxillosus , noting that it differs only in (trivial) dimensions of the head and thorax. According to the original descriptions, C. fulvago Motschulsky and C. m. var. pulchellus Meir may represent forms similar to the C. ciliaris holotype, characterized by extensive golden or reddish-brown vestiture over most of the body. Most authors prior to 1938 treated C. villosus ( Gravenhorst, 1802) as either a separate species or a variety of C. maxillosus ( Herman, 2001b) . Hatch (1938) was the first to treat C. villosus as a subspecies of C. maxillosus , although Blackwelder (1943) later synonymized it with C. maxillosus , which was followed by Herman (2001b). The subspecific division of C. maxillosus has been readopted in some recent works ( Smetana & Davies, 2000; Smetana, 2004), including this one, because although superficial, the differences between them are more or less discrete, and may be supported by some internal sac differences that require further study.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Creophilus maxillosus
| Clarke, Dave J. 2011 |
Creophilus maxillosus var. pulchellus
| Smetana A 2004: 672 |
| Herman LH 2001: 3324 |
| Blackwelder RE 1943: 448 |
| Meier W 1899: 99 |
Creophilus fulvago
| Smetana A 2004: 672 |
| Herman LH 2001: 3324 |
| Ganglbauer L 1895: 415 |
| Motschulsky V 1860: 120 |
Staphylinus orientalis
| Smetana A 2004: 672 |
| Herman LH 2001: 3324 |
| Ganglbauer L 1895: 415 |
| Motschulsky V 1858: 67 |
Staphylinus bicinctus
| Smetana A 2004: 671 |
| Navarrete-Heredia JL & Newton AF & Thayer MK & Ashe JS & Chandler DS 2002: 322 |
| Herman LH 2001: 3323 |
| Ganglbauer L 1895: 415 |
| Horn GH 1879: 200 |
| Solsky SM 1870: 261 |
| Mannerheim CG 1843: 229 |
Staphylinus tertius
| Herman LH 2001: 3321 |
| Erichson WF 1839: 348 |
Staphylinus nebulosus
| Smetana A 2004: 672 |
| Herman LH 2001: 3322 |
| Geoffroy EL 1785: 165 |
Staphylinus fasciatus Füessly, 1775: 21
| Smetana A 2004: 672 |
| Herman LH 2001: 3322 |
| Gravenhorst JLC 1802: 3 |
| Fuessly JC 1775: 21 |
Staphylinus balteatus DeGeer, 1774: 18
| Smetana A 2004: 671 |
| Herman LH 2001: 3321 |
| Fabricius JC 1781: 334 |
| DeGeer C 1774: 18 |
Staphylinus anonymus
| Smetana A 2004: 671 |
| Herman LH 2001: 3321 |
| Erichson WF 1839: 348 |
| Sulzer JH 1761: 17 |
Staphylinus maxillosus
| Navarrete-Heredia JL & Newton AF & Thayer MK & Ashe JS & Chandler DS 2002: 322 |
| Herman LH 2001: 3317 |
| Linnaeus C 1758: 421 |