Creophilus lanio, (ERICHSON)
|
publication ID |
https://doi.org/10.1111/j.1096-3642.2011.00725.x |
|
publication LSID |
lsid:zoobank.org:pub:FBFE9195-BE04-4AFE-9417-6E38BCE6AB84 |
|
persistent identifier |
https://treatment.plazi.org/id/039B414F-1941-FFBB-FC41-F9894B5BFD87 |
|
treatment provided by |
Valdenar (2021-08-31 23:26:14, last updated by Plazi 2023-11-06 13:00:17) |
|
scientific name |
Creophilus lanio |
| status |
|
8. CREOPHILUS LANIO (ERICHSON) View in CoL
( FIGS 2H–J, 3Q, 4J View Figure 4 , 7B View Figure 7 , 29 View Figure 29 , 30 View Figure 30 )
Staphylinus lanio Erichson, 1839: 352 View in CoL . Type locality: ‘Terra Van-Diemenii’; Gravenhorst, 1806: 126.
Staphylinus oculatus View in CoL var. Gravenhorst; Dejean, 1821: 21; Erichson, 1839: 352 (synonym of C. lanio View in CoL ).
Creophilus lanio View in CoL ; Fauvel, 1875: 56; Bernhauer & Schubert, 1914: 398; Lea, 1925: 229 (synonym of erythrocephalus View in CoL ); Steel, 1949: 58, figs 2, 5, 7 and 8; Radford, 1981: 174 (as C. lania , error for lanio View in CoL ); Herman, 2001b: 3317.
Emus lanio ; Fauvel, 1877: 250; Fauvel, 1878a: 248; Fauvel, 1878b: 541.
Creophilus erythrocephalus var. lanio View in CoL ; Olliff, 1887: 492–493.
Type material: Staphylinus lanio Erichson. Lectotype (here designated). ♀, ‘[orange] Type/ 5886/ [grey] lanio| Er.| terra van Diem [Tasmania], Sch/ [lilac] Hist.-Coll. ( Coleoptera )| Nr. 5886| Staphylinus lanio Erichs. | Terra v. Diem., Schayer| Zool. Mus. Berlin / [red] SYNTYPUS | Staphylinus | lanio Erichson, 1839 | labeled by MNHUB 2004/ FMNH-INS 0000 016 774/ [red] LECTOTYPE | Staphylinus | lanio Erichson, 1839 | designated by| D. J. Clarke 2008’ (in ZMHB). Specimen missing left mesotarsus. Paralectotypes (3). All with labels ‘[orange] Type/ [lilac] Hist.-Coll. ( Coleoptera )| Nr. 5886| Staphylinus lanio Erichs. | Terra v. Diem., Schayer| Zool. Mus. Berlin / [red] SYNTYPUS | Staphylinus | lanio Erichson, 1839 | labeled by MNHUB 2004/ [yellow] PARA- LECTOTYPE | Staphylinus | lanio Erichson, 1839 | designated by| D. J. Clarke 2008’: 2♂, ‘ FMNH-INS 0000 016 775’; ‘ FMNH-INS 0000 016 777’; 1♀, ‘ FMNH-INS 0000 016 776’ (in ZMHB).
Other material examined: 2743 specimens. See supporting information, Appendix S 1.
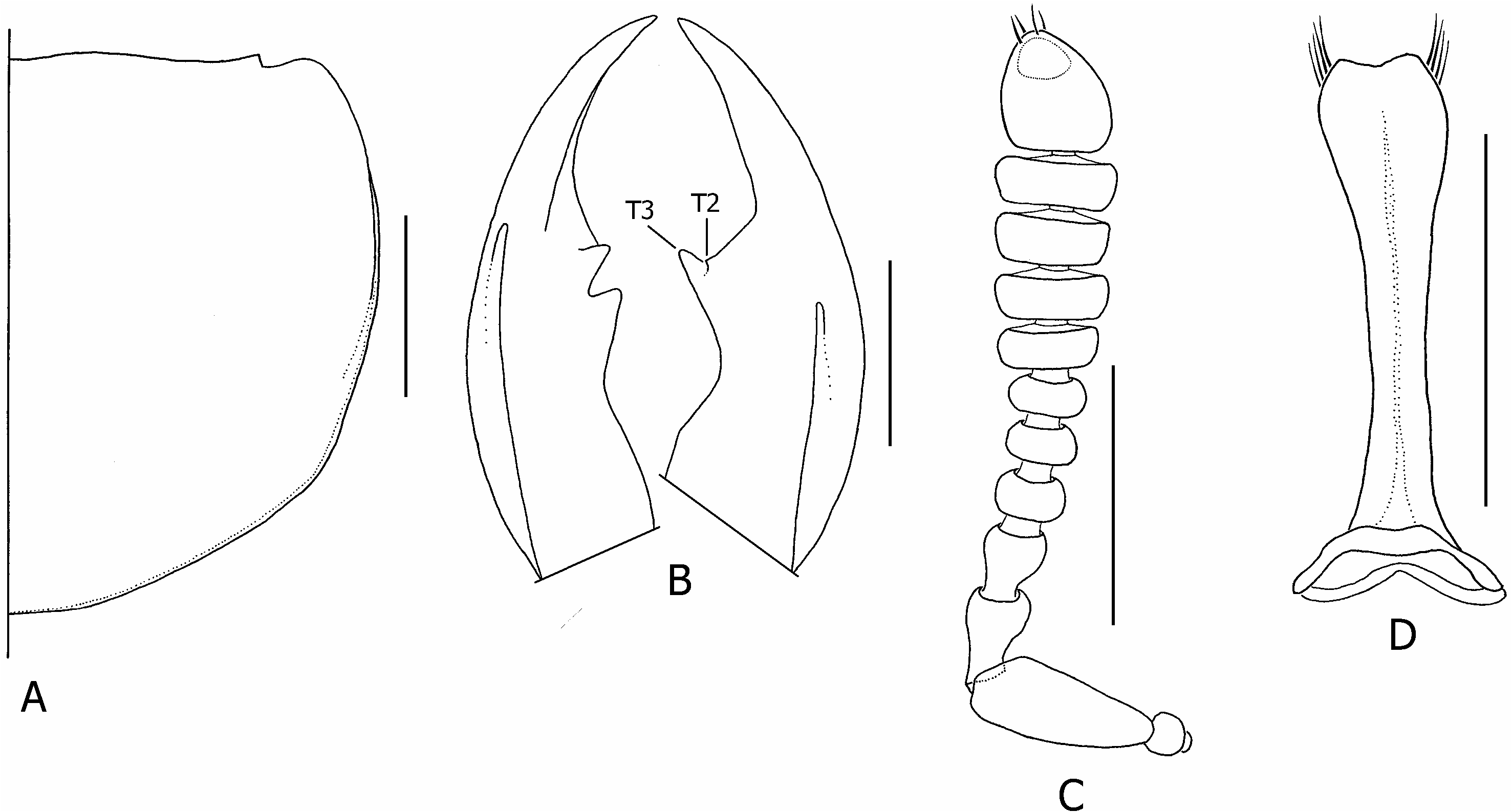
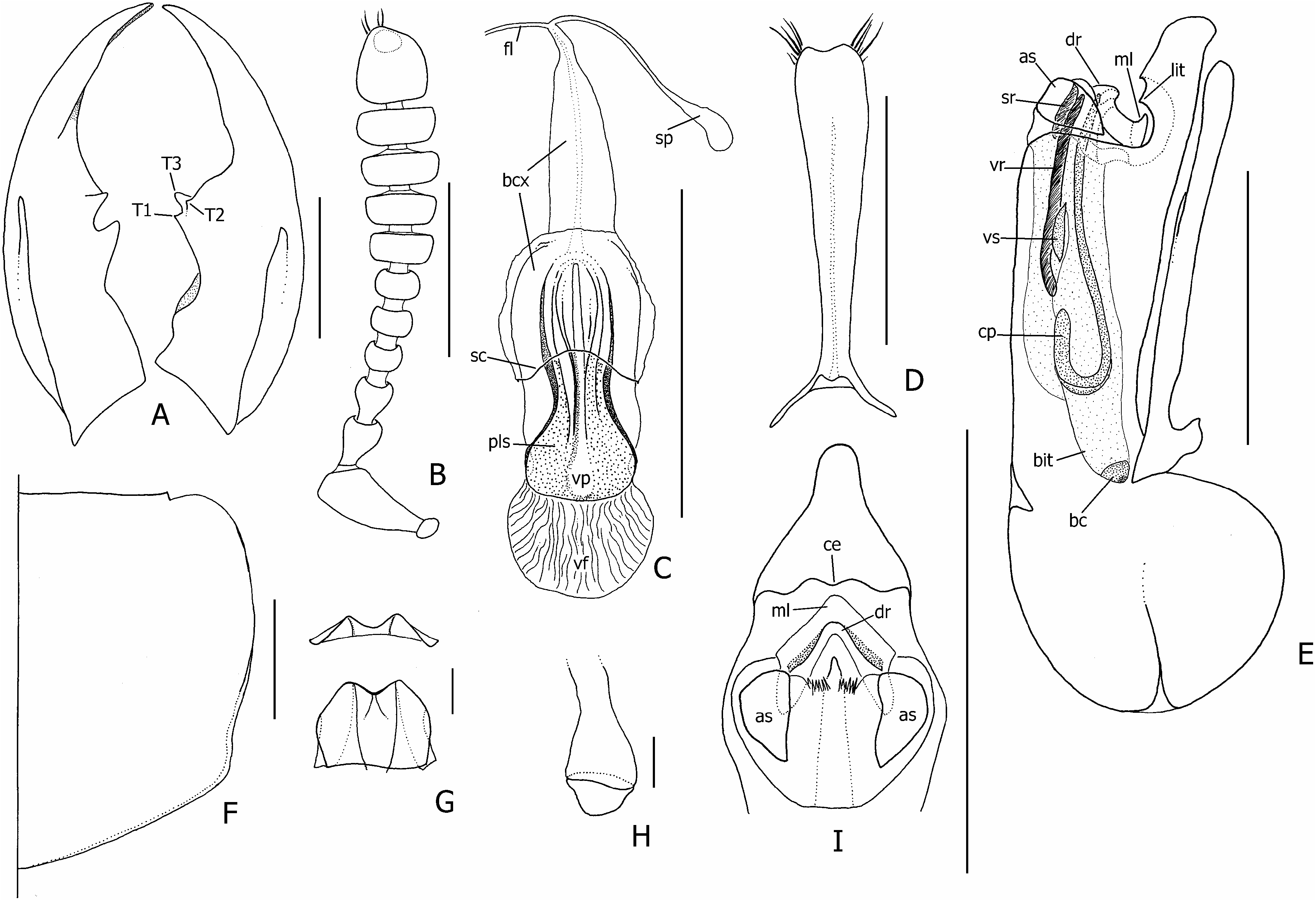
Diagnosis: With characters of the erythrocephalus - group; head orange-red, with large diffuse subquadrate or cordiform black spot ( Fig. 7B View Figure 7 ); right mandible with three teeth ( Fig. 29A View Figure 29 ); elytra black, humeri glabrous, conspicuously sculptured; abdominal segment IX orangeish-brown; parasutural 1 and humeral macrosetae absent, tergal chaetotaxic formula = 4-6-6–6-4-6.
Description: Measurements ( N = 10♂, 10♀). Forebody length: ♂ 5.5–9.8 mm, ♀ 5.3–7.8 mm. See supporting Table S 5 for comparison of ranges of male and female ratios. Head. Head orange-red, narrowly black around mouthparts and antennal fossae, with large diffuse irregularly subquadrate or cordiform black spot ( Fig. 7B View Figure 7 ), usually concealing dorsal tentorial pits, frequently with midlongitudinal line of black pigment continuing to frontoclypeal margin and to neck; strongly trapezoidal, much wider posteriorly; HW/ HL = 1.35–1.59; shining, without distinct microsculpture; eyes small to moderately large ( EYL / HL = 0.38–0.56), dorsolateral, lateral margins of head visible in dorsal view (not obscured by eye); HL 1/ HL 2 greater in females than males (♂ = 1.21–1.83, ♀ = 1.63–2.00); antennae as in Figure 29B View Figure 29 , antennomeres 1–6 black, 7–11 greyish-black, 11 as long or very slightly longer than 9–10 together; mandibles as in Figure 29A View Figure 29 , moderately longer than head in large males, subequal to head in females ( ML / HL ♂ = 0.81– 1.32, ♀ = 0.82–1.05), right mandible with three teeth, T 3 largest. Thorax and abdomen. Pronotum ( Fig. 29F View Figure 29 ) slightly transverse ( PW / PL = 1.09–1.25); PL 1.22–1.45 ¥ ESL; with basolateral margins distinctly emarginate, hind angles distinct; with sparse peripheral setae and short, sparse vestiture on lower anterolateral declivities; elytra uniformly black, humeral regions shining, distinctly callused, disc densely setose, sparsely rugosely sculptured, especially on humeri; wings fully developed, black with distinct black spot in medial field between MP 3 and MP 4 veins; abdomen shining black, except for lightly pigmented orangeish-brown ninth segment; tergite VII with well-developed palisade fringe; styli bicoloured, orangeish-brown basally and black distally. Male genitalia and secondary sexual characters. Ventrolateral carina of large males indistinct, partially obliterated by punctures and secondary anastomosing ridges. Aedeagus as in Figure 29E View Figure 29 ; median lobe apex extended into long blunt point ( Fig. 29I View Figure 29 ), produced slightly dorsally at tip, with paired apicolateral sclerites (as) separated from sclerotized median lobe by distinct membranous strip. Paramere as in Figure 29D View Figure 29 . Internal sac inverted as in Figure 29E View Figure 29 ; ventral sclerite (vs) small, wider than long, distinctly notched apically ( Fig. 25G View Figure 25 ). Female internal genitalia. Internal female genitalia as in Figure 29C View Figure 29 ; vaginal plate with paired lateral sclerites (pls), posterolateral areas membranous; vaginal fold forming large finely rugose sclerite ( Fig. 29C View Figure 29 , vf). Chaetotaxy. Elytra without parasutural 1 and humeral macrosetae; elytral discal series with 3–4 macrosetae; metaventrital macroseta absent or undetected; tergal chaetotaxic formula = 4-6-6-6-4-6, medial macrosetae absent on tergite III, inner lateral macrosetae absent on tergite VII; second gonocoxal macroseta absent.
Variation: The dorsomedial spot in C. lanio is very variable. Occasionally, usually in smaller specimens, it is expanded to cover most of the area between the eyes, although always surrounded by the usual orange coloration of the head.
Comparison: Creophilus lanio may be immediately distinguished from C. erythrocephalus and C. imitator by the diffuse, never circular cranial spot and by the lightly pigmented ninth abdominal segment. Contrary to Steel (1949: 58), all specimens I examined had uniformly shining black elytra, none with a ‘purplish reflection’. Although Cameron (1952: 255) described the elytral sculpturing of C. imitator as ‘less rugose than in lanio ’ it is more distinctive in C. imitator .
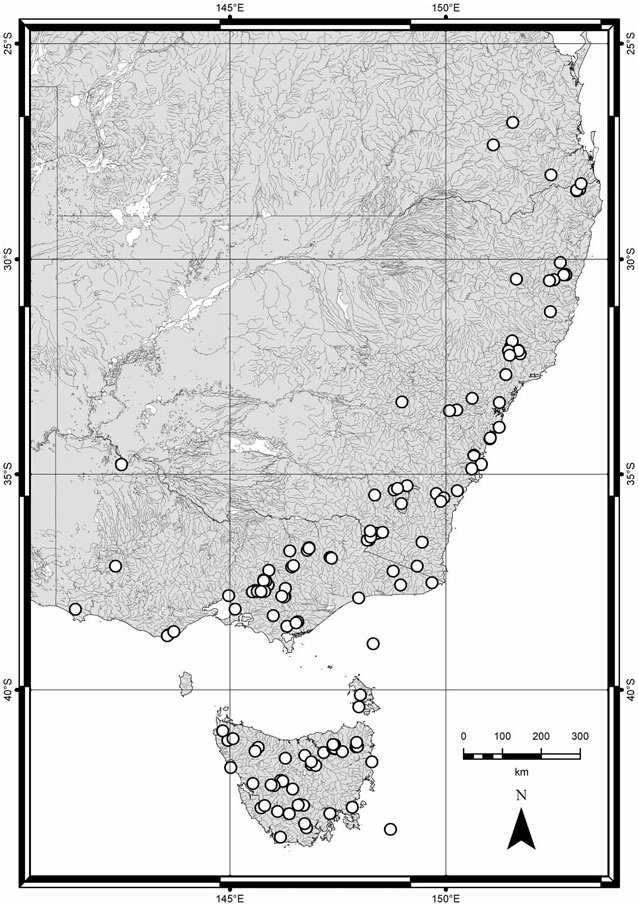
Distribution ( Fig. 30 View Figure 30 ): Australia: New South Wales, Tasmania, Victoria, southern Queensland. One record from Western Australia (no locality given, possibly mislabelled).
Biology and ecology: Creophilus lanio is known mostly from forested areas, with few records from pasture and coastal habitats. Most collections are from carrion-baited pitfall traps. Specimens have also been taken at light, in yellow pan traps, in Malaise and window traps, and in dung of various sorts. Habitat: drier woodlands to temperate rainforests of many kinds (e.g. Acacia , Banksia , Eucalyptus , Nothofagus , and Pomaderris ). Altitude: sea level to 1650 m. Phenology: throughout the year. Other biology and lifehistory characteristics are unknown. Larvae and pupae are unknown.
Bernhauer M, Schubert K. 1914. Staphylinidae IV. In: Schenkling S, ed. Coleopterorum catalogus, pars 57. Berlin: W. Junk, 289 - 408.
Cameron M. 1952. Results of the Archbold expeditions. New species of Staphylinidae (Col.) from New Guinea and Misool. Treubia 21: 241 - 256.
Dejean PFMA. 1821. Catalogue de la collection de Coleopteres de M. Le Baron Dejean, lieutenant-general des armees du roi, commandeur de l'ordre royal de la legion d'honneur, chevalier de l'ordre royal et militaire de Saint- Louis. Paris: Crevot.
Erichson WF. 1839. Genera et species staphylinorum insectorum coleopterorum familiae. Berlin: F. H. Morin.
Fauvel A. 1875. Synopsis des Creophilus. Tijdschrift voor Entomologie 18: 53 - 60.
Fauvel A. 1877. Les staphylinides de l'Australie et de la Polynesie. Annali del Museo Civico di Storia Naturale di Genova 10: 168 - 298.
Fauvel A. 1878 a. Les staphylinides des Moluques et de la Nouvelle Guinee. Annali del Museo Civico di Storia Naturale di Genova 12: 171 - 315 pls 1 - 2.
Fauvel A. 1878 b. Les staphylinides de l'Australie et de la Polynesie (2. e memoire). Annali del Museo Civico di Storia Naturale di Genova 13: 465 - 598.
Gravenhorst JLC. 1806. Monographia coleopterorum micropterorum. Gottingae [Gottingen]: H. Dieterich.
Herman LH. 2001 b. Catalog of the Staphylinidae (Insecta: Coleoptera). 1758 to the end of the second millennium. VI. Staphylinine Group (Part 3). Staphylininae: Staphylinini: (Quediina + Xanthopygina), Xantholinini. Staphylinidae Incertae Sedis. Fossils, Protactinae †. Bulletin of the American Museum of Natural History 265: 3021 - 3840.
Lea AM. 1925. On Australian Staphylinidae (Coleoptera). Part II. Transactions of the Royal Society of South Australia 49: 213 - 253.
Olliff AS. 1887. A revision of the Staphylinidae of Australia. Part III. Proceedings of the Linnean Society of New South Wales 2: 471 - 512.
Radford WPK. 1981. The Fabrician types of the Australian and New Zealand Coleoptera in the Banks collection at the British Museum (Natural History). Records of the South Australian Museum 18: 155 - 197.
Steel WO. 1949. On the Australian species of Creophilus (Coleoptera: Staphylinidae). Proceedings of the Linnean Society of New South Wales 74: 57 - 61.
Figure 4. Male copulatory pieces of selected species in the Creophilus-complex, dorsal view. In each figure the large paired dark structures are the sclerotized arms of the copulatory piece. A, C. variegatus; B, C. maxillosus maxillosus; C, C. m. villosus; D, C. galapagensis; E, C. flavipennis; F, C. incanus; G, C. oculatus; H, C. erythrocephalus; I, C. imitator; J, C. lanio; K, C. albertisi; L, Hadrotes crassus; M, Thinopinus pictus; N, Hadropinus fossor; O, Liusus hilleri. Abbreviations: iedg, apical inner edge of arms of copulatory piece; por, parallel oblique ridges of copulatory piece (see also Fig. 10); xbar, ‘cross-bar’ of appendix. Additional abbreviations as in Figure 3. Scale bar = 1 mm.
Figure 7. Heads of some species of the Creophilus erythrocephalus species-group (antennal flagella omitted). A, C. erythrocephalus; B, C. lanio; C, C. oculatus; D, C. imitator; E, C. albertisi. A–D, large males; E, small female. Scale bars = 5 mm.
Figure 25. Creophilus erythrocephalus, details of morphology (setae of mandibles, antenna, and pronotum omitted). A, pronotum (large male); B, mandibles, dorsal (bases omitted); C, right antenna, dorsal (only apical setae shown); D, paramere, dorsal. Abbreviations: T2, T3, mandibular teeth. Scale bars = 1 mm.
Figure 29. Creophilus lanio, details of morphology (setae of mandibles, antenna, and pronotum omitted). A, mandibles, dorsal (bases omitted); B, right antenna, dorsal (only apical setae shown); C, female internal genitalia, ventral; D, paramere, dorsal; E, aedeagus with details of inverted internal sac, right lateral (parameral setae omitted); F, pronotum (female); G, ventral sclerite of internal sac, ventral (lower) and apical (upper); H, basal cap of internal sac; I, median lobe apex, ventral. Abbreviations: T1, T2, T3, mandibular teeth. Additional abbreviations as in Figure 3 (male) and Figure 5 (female). Scale bars: G and H = 0.1 mm; rest = 1 mm.
| J |
University of the Witwatersrand |
| S |
Department of Botany, Swedish Museum of Natural History |
| N |
Nanjing University |
| HL |
Houghton Lake Wildlife Research Station |
| ML |
Musee de Lectoure |
| T |
Tavera, Department of Geology and Geophysics |
| PW |
Paleontological Collections |
| PL |
Západoceské muzeum v Plzni |
| MP |
Mohonk Preserve, Inc. |
| I |
"Alexandru Ioan Cuza" University |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Creophilus lanio
| Clarke, Dave J. 2011 |
Creophilus erythrocephalus var. lanio
| Olliff AS 1887: 492 |
Emus lanio
| Fauvel A 1878: 248 |
| Fauvel A 1878: 541 |
| Fauvel A 1877: 250 |
Creophilus lanio
| Herman LH 2001: 3317 |
| Radford WPK 1981: 174 |
| Steel WO 1949: 58 |
| Lea AM 1925: 229 |
| Bernhauer M & Schubert K 1914: 398 |
| Fauvel A 1875: 56 |
Staphylinus lanio
| Erichson WF 1839: 352 |
| Gravenhorst JLC 1806: 126 |
Staphylinus oculatus
| Erichson WF 1839: 352 |
| Dejean PFMA 1821: 21 |