Creophilus albertisi, (FAUVEL)
|
publication ID |
https://doi.org/10.1111/j.1096-3642.2011.00725.x |
|
publication LSID |
lsid:zoobank.org:pub:FBFE9195-BE04-4AFE-9417-6E38BCE6AB84 |
|
persistent identifier |
https://treatment.plazi.org/id/039B414F-193C-FFB9-FF16-FD9B4990FEB2 |
|
treatment provided by |
Valdenar (2021-08-31 23:26:14, last updated by Plazi 2023-11-06 13:00:17) |
|
scientific name |
Creophilus albertisi |
| status |
|
9. CREOPHILUS ALBERTISI (FAUVEL) View in CoL
( FIGS 1E, 4K View Figure 4 , 7E View Figure 7 , 28 View Figure 28 , 31 View Figure 31 )
Emus Albertisi Fauvel, 1879: 95 View in CoL , 94 (in key). Type locality: ‘Nouvelle-Guinée, Fly River’; Macleay, 1886: 142.
Creophilus Albertisi View in CoL ; Bernhauer & Schubert, 1914: 398; Scheerpeltz, 1971: 202.
Creophilus albertisi View in CoL ; Gressitt & Hornabrook, 1977: 26, pl. 5h; Herman, 2001b: 3315.
Type material: Emus Albertisi. Lectotype (here designated). ♂, ‘Nuova Guinea | Fly River| L.M.D’Albertis 1876–77/ Co Typus/ Museo Civico| di Genova/ FMNH-INS 0000 016 766/ [red] LECTO- TYPE | Emus | albertisi Fauvel, 1879 | designated by| D.J. Clarke 2008’ (in MCSN). Paralectotypes (15). All with same collecting data as lectotype and with label ‘[yellow] PARALECTOTYPE | Emus | albertisi Fauvel, 1879 | designated by| D.J. Clarke 2008’. Five specimens with label ‘Museo Civico| di Genova’: 2♂, ‘Co Typus/ FMNH-INS 0000 016 767’; ‘Co Typus/ FMNH-INS 0000 016 768’; 3♀, ‘Co Typus/ FMNH-INS 0000 016 769’; ‘Co Typus/ FMNH-INS 0000 016 770’; ‘Typus/ Albertisi| Fauvel./ C. Albertisi | FvL./ FMNH-INS 0000 016 765’ (in MCSN); five specimens with label ‘Ex-Typis’: 3♂, ‘albertisi| FvL./ R.I.Sc.N.B. 17.479| Coll. et det. A. Fauvel/ FMNH- INS 0000 016 760’; ‘Coll. et det. A. Fauvel| Creophilus | albertisi| Fauv.| R.I.Sc.N.B. 17.479/ FMNH-INS 0000 016 761’; ‘Coll. et det. A. Fauvel| Creophilus | Albertisi| Fauv./ R.I.Sc.N.B. 17.479/ FMNH-INS 0000 016 763’; 2♀, ‘Coll. et det. A. Fauvel| Creophilus | albertisi| Fauv.| R.I.Sc.N.B. 17.479/ FMNH-INS 0000 016 762’; ‘Coll. et det. A. Fauvel| Creophilus | Albertisi| Fauv./ R.I.Sc.N.B. 17.479/ FMNH-INS 0000 016 764’ (in IRSNB); ♀, ‘ Emus | albertisi| Fauvel/ coll. L.W.| Schaufauss/[red] SYNTYPUS | Emus | albertisi Fauvel, 1879 | labeled by MNHUB 2004/ FMNH-INS 0000 016 759’ (in ZMHB); 3 specimens on card: 2♂, FMNH-INS 0000 019 622; FMNH- INS 0000 019 623; 1♀, ‘[ Creophilus | albertisi| Fauvel/ FMNH-INS 0000 019 622’ (in MNHN); 1♂,
aedeagus on card, ‘ ♂ / Albertisi| Fauv./[blue] ex coll.| Klima/[blue] ex coll.| Scheerpeltz/ Albertisi| Fauv./ FMNH-INS 0000 012 299’ (in NMW). Fauvel’s description was based on numerous specimens collected by D’Albertis: ‘Nouvelle-Guinée, Fly River ; en nombre (L.M. D’Albertis).’ These were deposited in ‘ Collection de Musee Civique de Genes et la mienne’ ( MCSN and Fauvel’s collection) .
Other material examined: 59 specimens. See supporting information, Appendix S1.
Diagnosis: With characters of the erythrocephalus - group; head orange-red with subtriangular black spot on frons ( Fig. 7E View Figure 7 ), with distinct denticle at hind angles ( Fig. 31A, B View Figure 31 , vl); antennomeres 7–11 variably orange-brown to white; right mandible with two distinct teeth ( Fig. 31C View Figure 31 ); elytra metallic blue-green or violet; abdominal segments VIII –X orange; tergal chaetotaxic formula = 4-6-6-2-4-4.
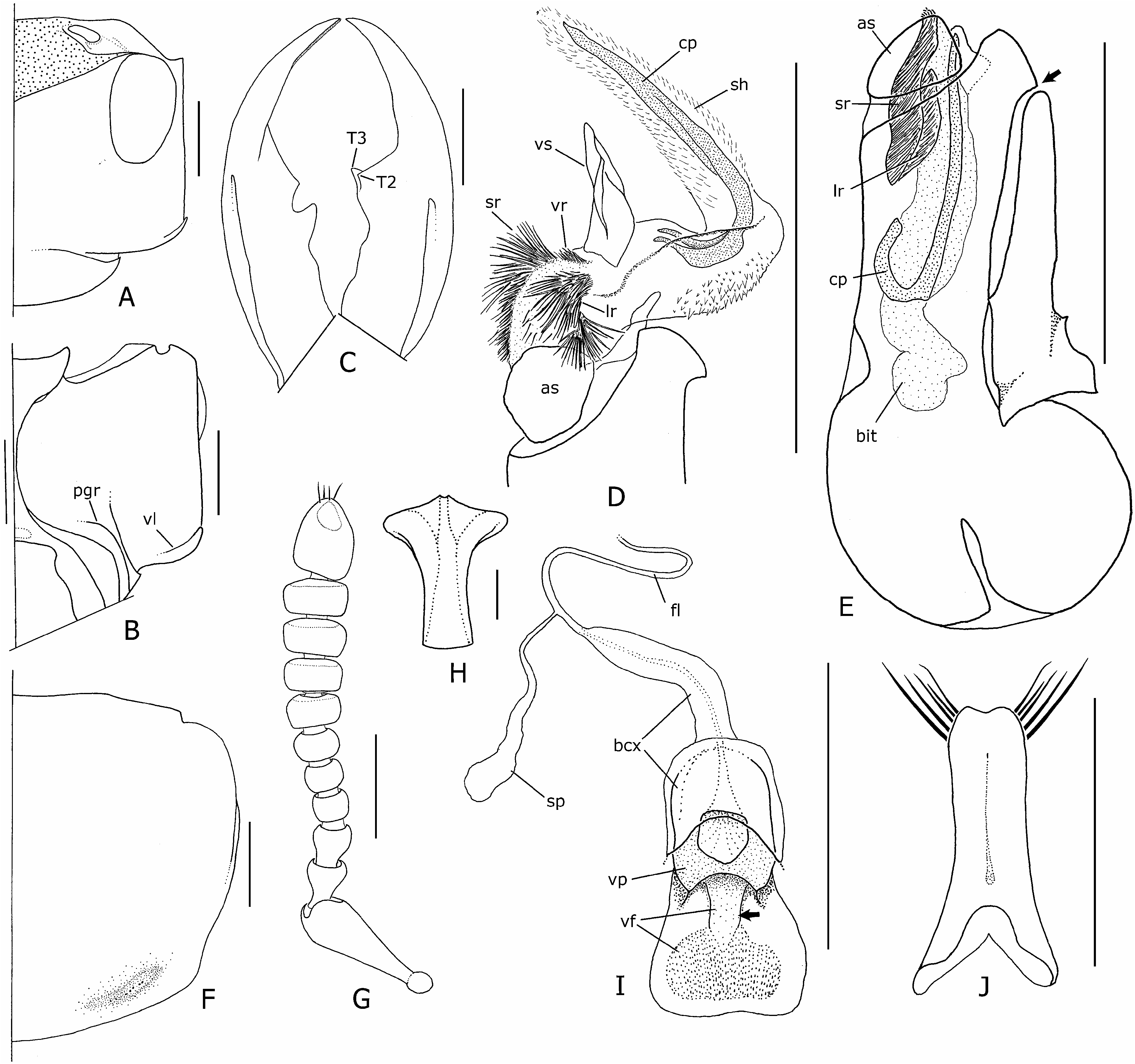
Description: Measurements (N = 10♂, 10♀). Forebody length: ♂ 6.3–9.7 mm, ♀ 6.8–8.4 mm. See supporting Table S5 for comparison of ranges of male and female ratios. Head ( Fig. 27A, B View Figure 27 ) orange-red, narrowly black around mouthparts and antennal fossae, frons with subtriangular black spot usually extending to frontoclypeal margin, and concealing dorsal tentorial pits on vertex or not; very slightly trapezoidal to subquadrate in large males, suborbicular in females and smaller males; HW/HL = 1.14–1.55; hind angles sharply delimited ( Fig. 31A View Figure 31 ); surface dull, with distinctive micropunctate microsculpture and very shallow indistinct and moderately dense impressions (as in Fig. 9A, B); ventrolateral carina present in both sexes ( Fig. 31B View Figure 31 , vl), also visible dorsally ( Fig. 31A View Figure 31 ), developed into distinct anteriorly projecting denticle; postgenal ridge obsolete medially ( Fig. 31B View Figure 31 , pgr); eyes large, protruding ( EYL /HL = 0.47–0.68), lateral to dorsolateral in large males; lateral margins of head usually obscured by eye in dorsal view; HL1/HL2 greater in females than males (♂ = 1.46–2.88, ♀ = 2.29–3.40); antennae as in Figure 31G View Figure 31 , antennomeres 1–6 black, 7–11 yellowish-white to mottled orange-brown ( Fig. 1E), 11 elongate, as long or longer than 9–10 together; mandibles as in Figure 31C View Figure 31 , moderately longer than head in large males, shorter than head in females ( ML /HL ♂ = 0.78–1.27, ♀ = 0.74– 0.92), right mandible with two teeth, T 1 absent, T 3 largest. Thorax and abdomen. Pronotum ( Fig. 31F View Figure 31 ) slightly transverse ( PW / PL = 1.03–1.26); PL 1.09– 1.29 ¥ ESL; with basolateral margins slightly emarginate; hind angles distinct; with sparse peripheral setae, longer sparse vestiture on lower anterolateral declivities, and long medially directed basal setae; small transverse basolateral impressions present, smooth and asetose, with transverse line of tiny punctules; elytra distinctly widened posteriorly, brightly coloured metallic blue-green or violet ( Fig. 1E), sparsely and finely setose, without sculpture; humeri sparsely punctate, narrowly glabrous laterally, not callused; disc shallowly impressed in middle; wings fully developed, clear yellowish-brown, without black spot in medial field between MP 3 and MP 4 veins; abdomen with segments III – VII shining black, VIII –X orange ( Fig. 1E); vestiture fine, moderately dense; microsculpture extremely fine, producing weak iridescent reflection, particularly on ventral side; tergite VII with well-developed palisade fringe. Male genitalia and secondary sexual characters. Sternite IV with densely setose posteromedial emargination of transverse fold (most obvious in larger specimens), and sternites V – VII with sparsely distributed micropores near middle of sternites. Aedeagus as in Figure 31E View Figure 31 ; median lobe distinctly yellowish, not produced apically, with minute projection dorsoapically ( Fig. 31E View Figure 31 , arrow), without lateral recurved teeth, with paired apicolateral sclerites (as) separated from sclerotized median lobe by membranous strip. Paramere nearly as wide as median lobe, with very long apicolateral setae ( Fig. 31J View Figure 31 ). Internal sac inverted as in Figure 31E View Figure 31 , everted as in Figure 31D View Figure 31 ; with ventromedian spiculose strip (vr) reduced, shorter than ventral sclerite (vs), flanked by very large paired basoventral spiculose regions ( Fig. 31D View Figure 31 , sr), with additional lateral spiculose regions ( Fig. 31D View Figure 31 , lr); ventral sclerite expanded distally ( Fig. 31H View Figure 31 ); copulatory piece (cp) completely surrounded by distinctly spiculose membranous sheath (sh). Female internal genitalia. Internal female genitalia as in Figure 31I View Figure 31 ; vaginal plate (vp) small, paired lateral sclerites fused posteriorly to form narrowly sclerotized ring; vaginal fold with medial sclerotized strip proximally ( Fig. 31I View Figure 31 , vf, arrow), and with diffuse patch of minute teeth distally. Chaetotaxy. Basiantennal, parasutural 1, and posterior epipleural macrosetae absent; elytral discal series with three macrosetae; metaventrital macroseta absent or undetected; tergal chaetotaxic formula = 4-6-6-2-4-4, inner lateral macrosetae absent on tergites VI – VIII, medial macrosetae absent on tergites III and VI.
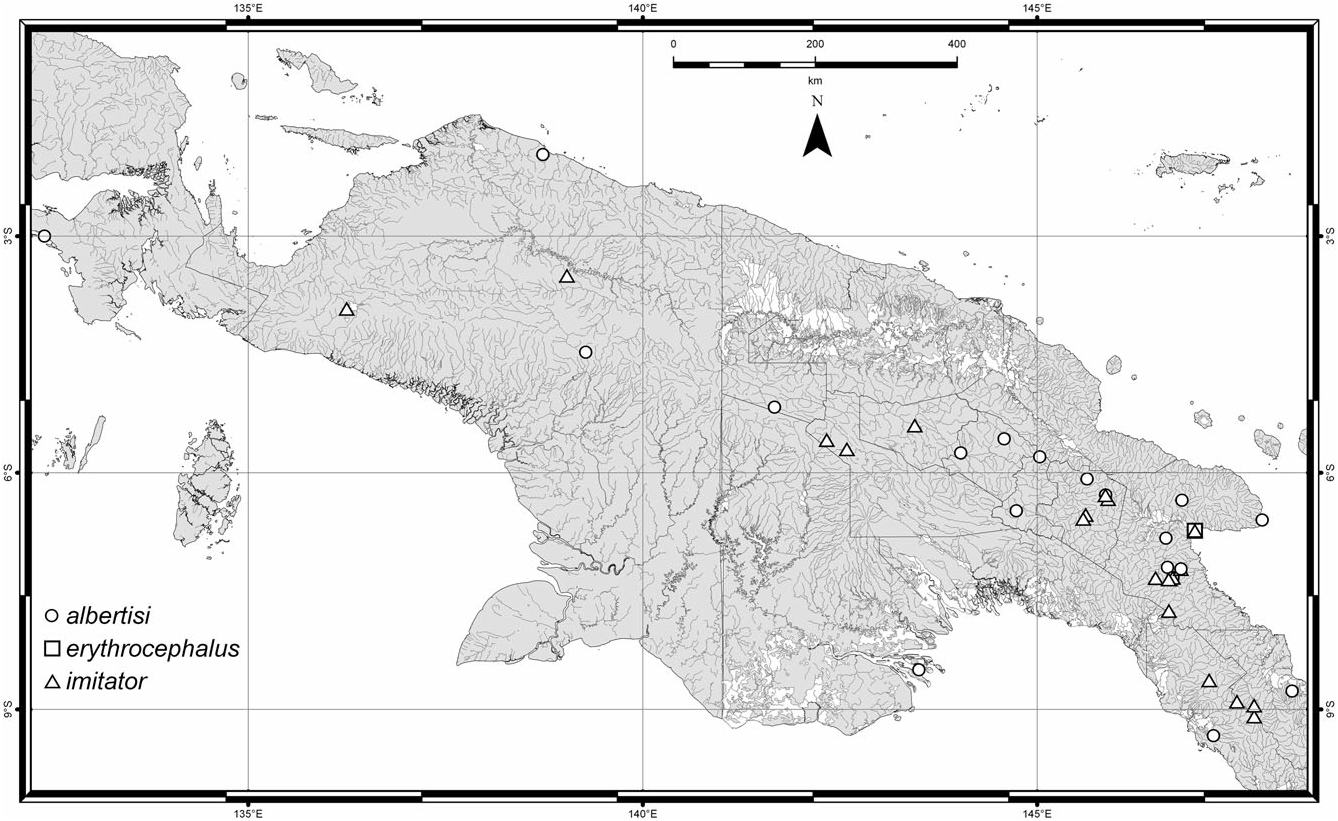
Distribution ( Fig. 28 View Figure 28 , circles): Papua New Guinea, Indonesia: Irian Jaya. Scheerpeltz (1971) notes it is common throughout New Guinea and in (unspecified) neighbouring islands, but I have not seen any specimens from outside mainland New Guinea.
Biology and ecology: Rare in collections; most specimens without habitat or collection data. Fifteen collections are from light traps (mercury vapour, black light), two from carrion. Habitat: unknown, but probably intact forest. Altitude: 60–1700 m. Phenology: throughout the year. Gressitt & Hornabrook (1977: 26) gave the only known published biological observations, noting that C. albertisi ‘occasionally comes to light’ and ‘can discharge a peculiar pungent scent when frightened’. Other biology and life-history characteristics are unknown. Larvae and pupae are unknown.
Remarks: Last (1987) recorded Creophilus from New Guinea, but did not list the species, nor did he provide any records.
Bernhauer M, Schubert K. 1914. Staphylinidae IV. In: Schenkling S, ed. Coleopterorum catalogus, pars 57. Berlin: W. Junk, 289 - 408.
Fauvel A. 1879. Les staphylinides des Moluques et de la Nouvelle Guinee (2. e memoire). Annali del Museo Civico di Storia Naturale di Genova 15: 63 - 121.
Gressitt JL, Hornabrook RW. 1977. Handbook of common New Guinea beetles. Wau, Papua New Guinea,: Wau Ecology Institute.
Herman LH. 2001 b. Catalog of the Staphylinidae (Insecta: Coleoptera). 1758 to the end of the second millennium. VI. Staphylinine Group (Part 3). Staphylininae: Staphylinini: (Quediina + Xanthopygina), Xantholinini. Staphylinidae Incertae Sedis. Fossils, Protactinae †. Bulletin of the American Museum of Natural History 265: 3021 - 3840.
Last HR. 1987. Staphylinidae from Papua New Guinea in the collection of Bernice P. Bishop Museum Honolulu, Hawaii (Insecta, Coleoptera). Entomologische Abhandlungen, Staatliches Museum fur Tierkunde in Dresden 51: 25 - 56.
MacLeay WJ. 1886. The insects of the Fly River, New Guinea, ' Coleoptera. '. Proceedings of the Linnean Society of New South Wales 1: 136 - 157.
Scheerpeltz O. 1971. Staphylinidenausbeuten von Universitatsprofessor Dr. H. Loffler gelegentlich seiner seenkundlichen Studienreisen in tropischen Hochgebirgen 1969 und 1970 (6. Beitrag zur Kenntnis der australisch-polynesischen Staphyliniden, gleichzeitig 11. Beitrag z. K. d. orientalischen St. und 21. Beitrag z. K. d. neotropischen St.). Koleopterologische Rundschau 49: 201 - 207.
Figure 4. Male copulatory pieces of selected species in the Creophilus-complex, dorsal view. In each figure the large paired dark structures are the sclerotized arms of the copulatory piece. A, C. variegatus; B, C. maxillosus maxillosus; C, C. m. villosus; D, C. galapagensis; E, C. flavipennis; F, C. incanus; G, C. oculatus; H, C. erythrocephalus; I, C. imitator; J, C. lanio; K, C. albertisi; L, Hadrotes crassus; M, Thinopinus pictus; N, Hadropinus fossor; O, Liusus hilleri. Abbreviations: iedg, apical inner edge of arms of copulatory piece; por, parallel oblique ridges of copulatory piece (see also Fig. 10); xbar, ‘cross-bar’ of appendix. Additional abbreviations as in Figure 3. Scale bar = 1 mm.
Figure 7. Heads of some species of the Creophilus erythrocephalus species-group (antennal flagella omitted). A, C. erythrocephalus; B, C. lanio; C, C. oculatus; D, C. imitator; E, C. albertisi. A–D, large males; E, small female. Scale bars = 5 mm.
Figure 27. Creophilus imitator, details of morphology (setae of mandibles, antenna, and pronotum omitted). A, mandibles, dorsal (bases omitted); B, right antenna, dorsal (only apical setae shown); C, median lobe apex, ventral; D, aedeagus with details of inverted internal sac, right lateral (parameral setae omitted); E, pronotum (female); F, female internal genitalia, ventral – arrow indicates small sclerotic ridges of vaginal fold; G, paramere, dorsal; H, ventral sclerite of internal sac, ventral (lower) and apical (upper). Abbreviations: T2, T3, mandibular teeth. Additional abbreviations as in Figure 3 (male) and Figure 5 (female). Scale bars: H = 0.1 mm; rest = 1 mm.
Figure 28. Distribution of all georeferenced specimens of C. albertisi (circles), C. erythrocephalus (square) and C. imitator (triangles) in New Guinea.
Figure 31. Creophilus albertisi, details of morphology (setae of head, mandibles, antenna, and pronotum omitted). A, head (large male), dorsal; B, same, ventral; C, mandibles, dorsal (bases omitted); D, apex of median lobe with fully everted internal sac, right lateral; E, aedeagus with details of inverted internal sac, right lateral (parameral setae omitted) – arrow indicates dorsoapical projection of median lobe; F, pronotum (large male); G, right antenna, dorsal (only apical setae shown); H, ventral sclerite of internal sac, ventral; I, female internal genitalia, ventral – arrow indicates thin medial sclerite of vaginal fold; J, paramere, dorsal. Abbreviations: T2, T3, mandibular teeth; lr, lateral spiculose region; pgr, postgenal ridge; vl, ventrolateral carina. Additional abbreviations as in Figure 3 (male) and Figure 5 (female). Scale bars: H = 0.1 mm; rest = 1 mm.
| MCSN |
Museo Civico di Storia Naturale, Verona |
| R |
Departamento de Geologia, Universidad de Chile |
| IRSNB |
Institut Royal des Sciences Naturelles de Belgique |
| MNHN |
Museum National d'Histoire Naturelle |
| NMW |
Naturhistorisches Museum, Wien |
| ML |
Musee de Lectoure |
| T |
Tavera, Department of Geology and Geophysics |
| PW |
Paleontological Collections |
| PL |
Západoceské muzeum v Plzni |
| MP |
Mohonk Preserve, Inc. |
| V |
Royal British Columbia Museum - Herbarium |
| VI |
Mykotektet, National Veterinary Institute |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Creophilus albertisi
| Clarke, Dave J. 2011 |
Creophilus albertisi
| Herman LH 2001: 3315 |
| Gressitt JL & Hornabrook RW 1977: 26 |
Creophilus Albertisi
| Scheerpeltz O 1971: 202 |
| Bernhauer M & Schubert K 1914: 398 |
Emus Albertisi
| MacLeay WJ 1886: 142 |
| Fauvel A 1879: 95 |