Omalus sculpticollis Abeille, 1878
|
publication ID |
https://doi.org/ 10.2478/vzoo-2014-0002 |
|
DOI |
https://doi.org/10.5281/zenodo.6406068 |
|
persistent identifier |
https://treatment.plazi.org/id/03998780-FFE5-7621-30E1-FF35B9B75752 |
|
treatment provided by |
Felipe |
|
scientific name |
Omalus sculpticollis Abeille, 1878 |
| status |
|
Omalus sculpticollis Abeille, 1878
T a x o n o m i c a l a n d m o r p h o l o g i c a l n o t e s
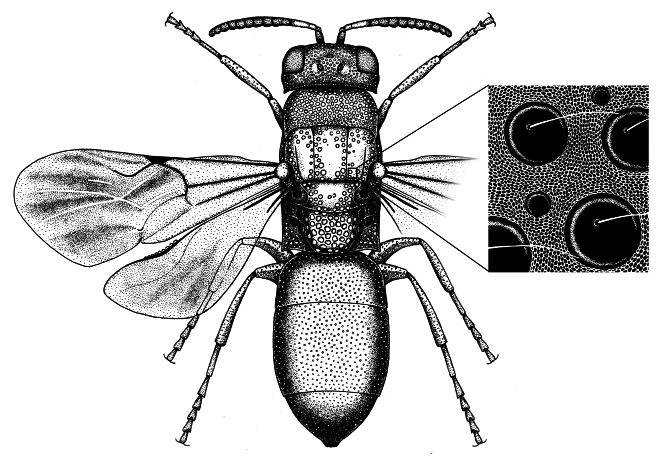
The chrysidid wasp found in the Psenulus nests was identified as Omalus (Chrysellampus) sculpticollis Abeille, 1878 ( Linsenmaier 1959 a, b, 1968, 1987, 1997). This species is easily identifiable for the unique punctuation of the mesosoma and the characteristic shape of the third tergite ( fig. 3 View Fig ). Omalus sculpticollis was transferred into the genus Philoctetes Abeille, 1879 by Kimsey and Bohart (1991). Rosa and Soon (2013) also consider it belonging to the genus Philoctetes .
It should be noted that Linsenmaier divided the genus Omalus Panzer, 1801 into five subgenera: Omalus s. str., Holophris Mocsáry, 1890 , Philoctetes Abeille, 1879 , Elampus Spinola, 1806 (= Notozus Förster, 1853 ) and Chrysellampus Semenov-Tian-Shanskij, 1932, and placed sculpticollis in Chrysellampus. The Swiss author considered the original external morphological characters given by Abeille de Perrin to define the subgenus Philoctetes , as other authors did ( Mocsáry, 1889; Radoszkowski, 1889; du Buysson, 1891; etc.). Kimsey and Bohart (1991), reviewing the tribe Elampini , considered different characters to identify the genera and elevated many subgenera to generic rank. The final result was that part of the former subgenera were elevated to genera, but with different diagnoses and species included, if compared to Linsenmaier’s systematics. Therefore the genus Philoctetes sensu Linsenmaier is not comparable to Philoctetes sensu Kimsey, Bohart 1991 . Some recent taxonomical publications demonstrated that Kimsey and Bohart’s (1991) genera system needs corrections. Many taxa were recently moved from one genus sensu Kimsey, Bohart (1991) to other genera ( Niehuis, 2001; Rosa, 2005 b, 2006, 2009; Strumia, 1995). However, Kimsey and Bohart’s generic system is not accepted by many European and Russian authors ( Kunz, 1994; Mingo, 1994; Vinokurov, 2008). A new revisional work on the Elampini tribe is needed. In the present paper we follow Linsenmaier’s system ( Linsenmaier, 1959 a, b, 1968, 1987, 1997).
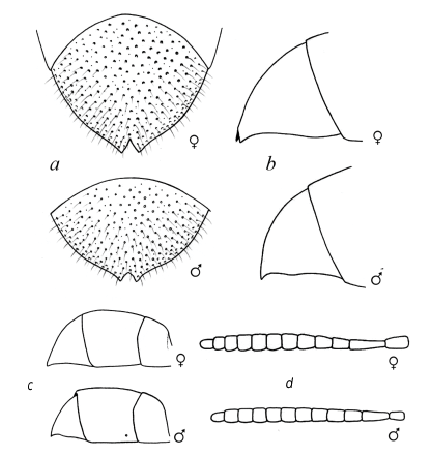
Du Buysson (1891) was the first author to observe the remarkable sexual dimorphism in O. sculpticollis : females have a more elongated metasoma and the third tergite (T–III) ( fig. 4 View Fig , b, c), the T–III is somehow protuberant ( fig. 4 View Fig , a, b), flagellomeres are swollen ( fig. 4 View Fig , d); seen in dorsal view, the males’ head, main part of pronotum, mesonotum, propodeum, and upper part of metasoma are darker (matt black) ( fig. 3 View Fig ). The matt effect on mesosoma in both sexes is originated from a special complex microsculpture, which could be named “coriaceous and punctate, with hairs” according to the terminology given by Eady (1968) ( fig. 3 View Fig ). It should be noted that the coriaceous microsculpture preserves the structural coloration (except for black zones), but eliminates the iridescence effect in both sexes.
S u m m a r y o f n e s t d a t a
Only 90 specimens of O. sculpticollis emerged from the nests overall, but being the larvae they destroyed 435 cells of P. fuscipennis (see the discussion below). Therefore the common correlation “one parasitoid individual per one host cell”is not valid.At the moment of laboratory examination chrysidids were at prepupal or pupal stage, enclosed in cocoons, similarly to their hosts. As the examination took place early in spring (after winter stay) we can conclude that also O. sculpticollis overwinters in prepupal stage in the Crimea. The emergence of imagoes had been taking place from the second decade of May to the first days of June. But two of these 90 specimens emerged much earlier, during summertime: we observed one abandoned cocoon and one with dead imago. Therefore these two should be referred to the so called “facultative generation”, and the studied population of O. sculpticollis could be considered as monovoltine.
Moreover, we can state the following: in the present study the 698 individuals of P. fuscipennis were monovoltine and emerged after winter stay, while other 87 (11.1 %) are referred to facultative generation; correspondingly, 88 individuals of O. sculpticollis were monovoltine, while two (2.2 %) emerged without winter stay (gave the facultative generation). These data prove a clear evidence of connection between the host’s life cycle and its chrysidid enemy. In this sense the long-term evolution process becomes obvious, as the development of chrysidid wasp is synchronized with that of host (the dates of emergence are close and the facultative generations are shown in both host and chrysidid).
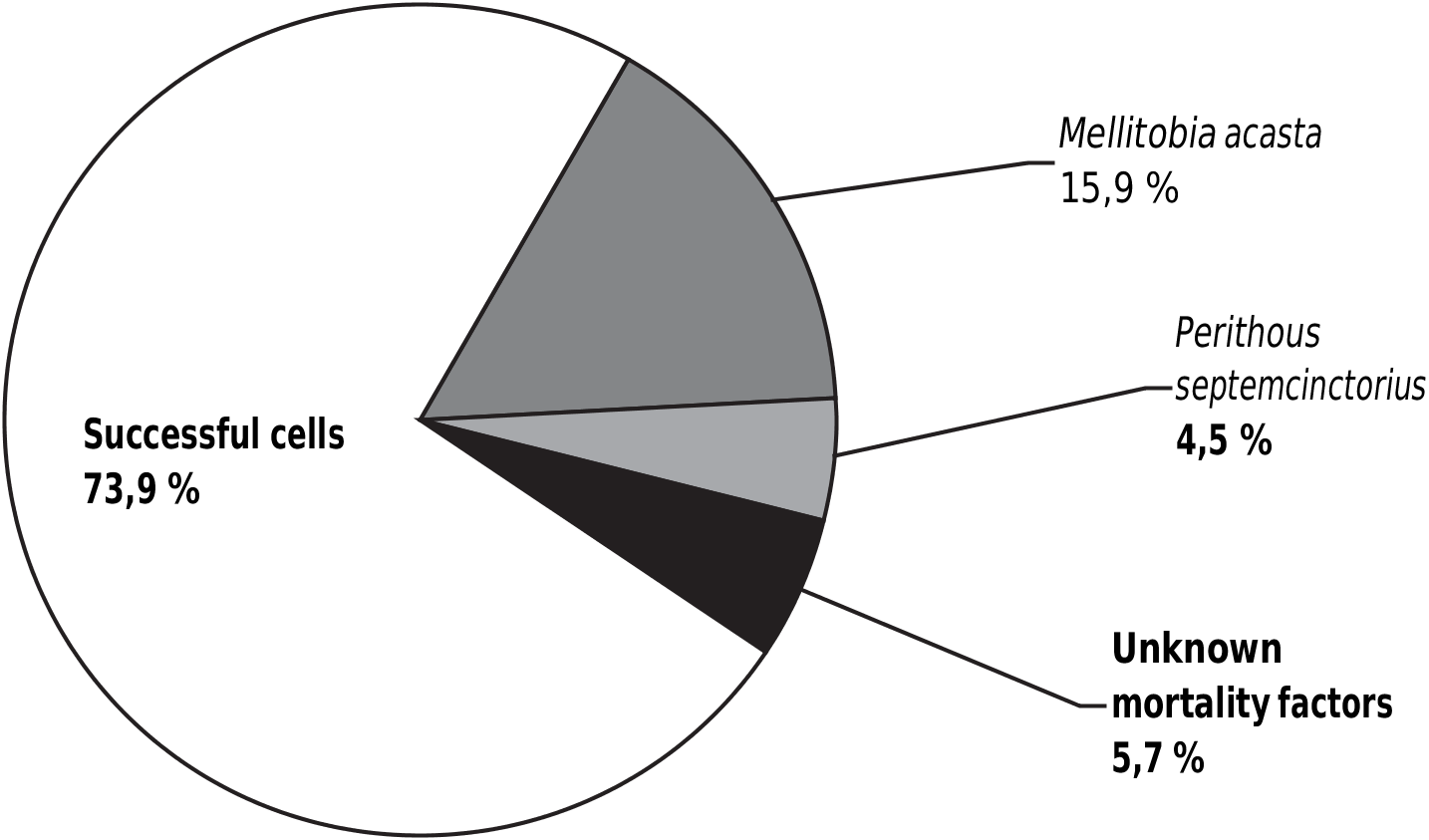
Not all O. sculpticollis successfully developed into imagoes. As shown in figure 5 View Fig , the death of prepupae/pupae in cocoon was registered in five cases, 14 chrysidid cocoons were occupied by Melittobia acasta , four cocoons by the ichneumonoid Perithous septemcinctorius . In total, only the 73.9 % of O. sculpticollis emerged in about the same period, as the host. The sex ratio obtained for O. sculpticollis was almost 1 ♂: 1 ♀.
Omalus sculpticollis was found in 54 (56.8 %) of 95 nests constructed by P. fuscipennis . In each one of these 54 nests we observed that the series of intercellar partitions between the cells were damaged ( fig. 6 View Fig ). The holes in the partitions were rather large, and the spaces of cells in such series appeared to be united. These united cells were always full of aphids and there was only one chrysidid cocoon always present per one series of cells ( fig. 5 View Fig ). The series included from 2 to 14 cells (average — 5.0). In more than the 50 % of the cases, the nests contained two or three series of cells (respectively two or three O. sculpticollis cocoons per nest were obtained). The location pattern of these series in the nests of host was accidental — no regularity could be shown.
There are no doubts that this damage is caused by chrysidids. We did not find any other enemy inside the cell series as there were no extraneous holes found (which could explain the presence of other host enemies). These series were often situated in the “middle of the nest” being surrounded from both sides by cells with normally developing P. fuscipennis .
Though, one chrysidid wasp per one host cell was observed in seven cases on 90. Each such cell contained the cocoon of O. sculpticollis with prepupa/pupa inside and the remainder of an empty P. fuscipennis cocoon with a big hole (there were no aphids). All these facts indicate that chrysidid wasp devoured the mature larva of P. fuscipennis , which had already finished the feeding on aphids and spun its own cocoon. The hole in the host cocoon was made by the chrysidid larva.
D e s c r i p t i o n o f c o c o o n
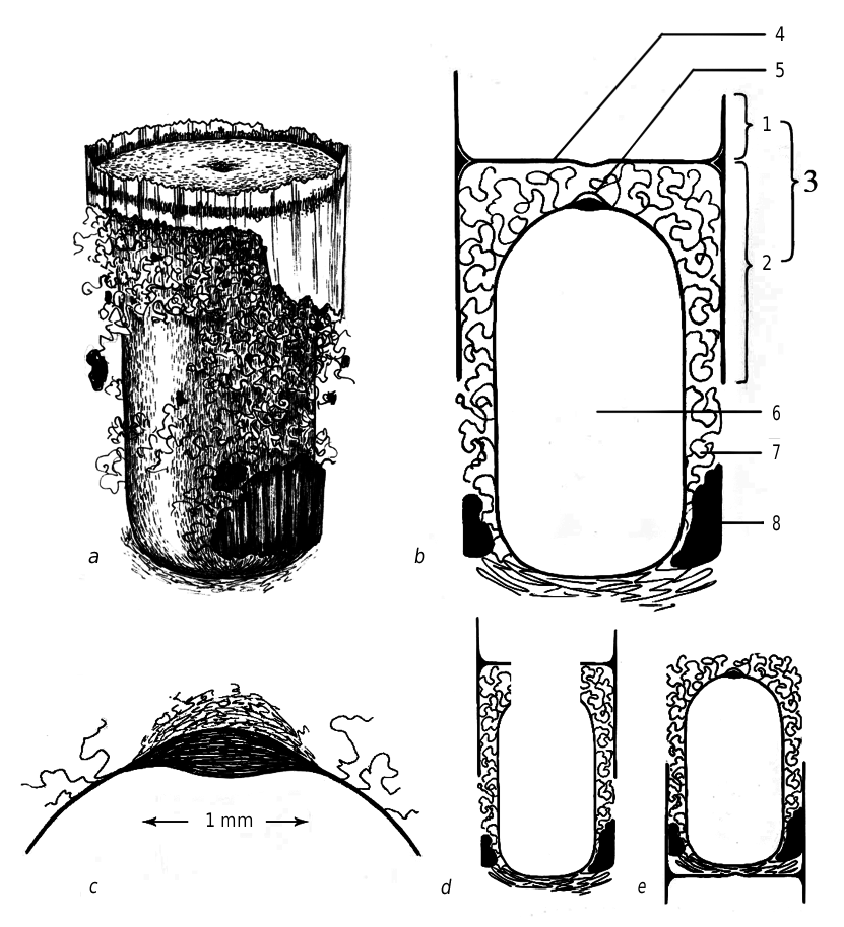
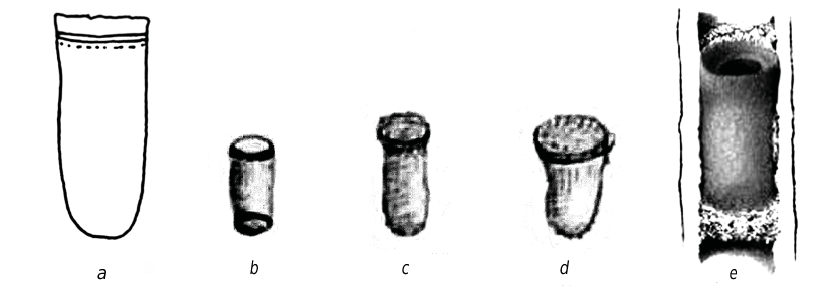
The cocoon of O. sculpticollis looks like a brownish truncated cylinder with rounded posterior end ( fig. 7 View Fig ). Its length can vary from 9 to 11 mm. The cocoon has a complex structure and consists of two parts, which differ in structure and functions.
The apical part consists of a transverse septum, made across the boring of the stem, and a circular band attached to it; the septum can include the remainder of intercellar partitions (constructed by P. fuscipennis female) on the periphery. All these structures resemble an “arch with collar” at the cross section of the cocoon (terminology by A. N. Kilimnik, pers. comm.). The collar is formed of the remainder of cell facing. The septum is made of opaque, rather thin, but quite dense yellowish material. A small depression is observed in the center of the septum. The circular band is much thinner, semitransparent, light-brown and it is often torn off during the removal of the cocoon. Functionally the arch serves as a plug or barrier, which separates the chrysidid larva from the mass of aphids and protects it from the superfluous humidity (Kilimnik, pers. comm.). The septum is not always attached to the remainder of cell partitions. When the cocoon is located out of relation to nest structure (in the middle of the cell or partially in one cell and partially in other), its arch could be less distinct, having more rounded forms, but still it preserves the functions.
The cocoon proper is thin-walled, varnished, light-brown, cylindrical with rounded ends (oval in cross section). Its apical part is inserted into the arch. The dense thick pubescence observed provides the junction of the cocoon and the circular band of the arch, which in turn fixes all the structures in the stem. The surface of the cocoon bears an abundant pubescence: it consists of thick brownish fibers all around the cocoon, except for the posterior end of the cocoon, where it is quite thin and whitish. According to Kilimnik (pers. comm.), the function of the pubescence is a shock-absorbing fixation of the cocoon to the nest walls. It should be noted, that the small inclusions (possibly excrements and chitinous remainder of host bodies) are observed within the pubescence all over. Excrements are black in color. Sometimes they can be described as the compact dense mass, arranged along the posterior part of the cocoon, otherwise they look like small round pellets laying more roughly. The apical part of the cocoon bears the nipplelike structure about 1 mm in diameter ( fig. 7 View Fig , c). The latter is well visible and distinctly differs from the other semitransparent brownish parts of cocoon walls by being whitish and opaque. The minute examination of the nipple showed that it has a double-layer structure in cross section. The innermost lenticular layer is formed of very thin brownish fibers laying in parallel and compactly; instead the outermost one consists of more thick white fibers, laying loosely.
The emerging in linear nests forces the imagoes of chrysidids to get out of cocoons through their apical parts — by making holes first in the “nipple area” of the cocoon, and then in the transverse septum of the arch ( fig. 7 View Fig , d).
Sometimes the cocoons of O. sculpticollis have the inverted structure: the apical part bearing the nipple is not inserted into the arch, instead the bare nipple faces the entrance of the nest tube; additionally the arch is attached to the bottom end of the cocoon proper ( fig. 7 View Fig , e). Though such structure appeared to be reasonable, as it was revealed in the cases when the mass of aphids was situated behind the posterior part of the cocoon.
Analyzing the variation of O. sculpticollis cocoons, we can note the following: the wall thickness, size and coloration can vary considerably; the shape and the structure of the cocoon, the look and colour of the excrements, the presence of “nipple” and “arch” could be considered as stable features.
On the base of literature data (du Buysson, 1891; Maréchal, 1925; Grandi, 1959, 1961; Danks, 1971; Krombein, 1967) we can assume that the similar cocoon structure, as given above, is common for all the species in the genus Omalus Panzer, 1801 sensu Linsenmaier ( fig. 8 View Fig ). Though the cocoon of O. aeneus (Fabricius, 1787) , which is stored in the collection of the second author, differs from the cocoon of O. sculpticollis by having much more distinct nipple, which has also the membranous cap at the top, and by being smaller in size (length — 7 mm). According to detailed descriptions given by Grandi (1959, 1961) and Marechal (1925) the cocoon of O. auratus (Linnaeus, 1758) differs by the white to pale yellow color, the absence of distinct nipple (instead the wall of cocoon proper under arch is more dense, matt and opaque) and the smaller size too (length — 5 mm).
T h e l a r v a l f e a t u r e s
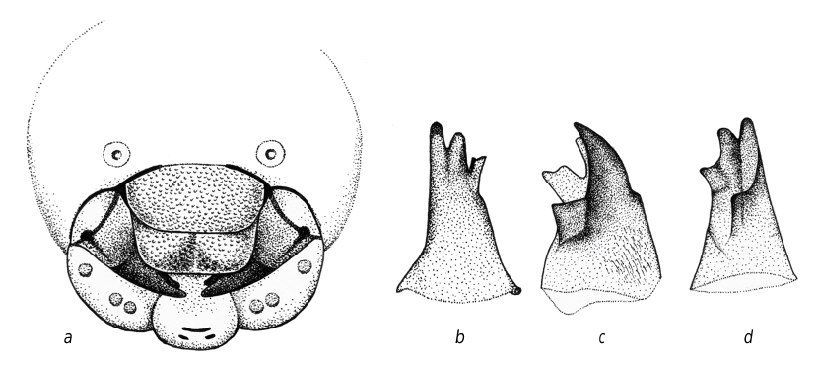
The cocoons of O. sculpticollis contained the cast off skins of the last instar larvae. The level of sclerotization at mouthparts was high, and it was possible to identify some features of the mature larvae ( fig. 9 View Fig ): coronal suture and parietal bands present; antennal orbits circular, located below the middle of head; labrum with depression in the middle and with almost strait apical margin; labrum and clypeus punctulate; mandibles fourdentate (symmetrical); maxillae and clypeus with strong, pigmented bands.
As the last instar larva of O. auratus was described in detail ( Enslin, 1929; Giordani Soika, 1934; Grandi, 1959, 1961), it could be distinguished from that of O. sculpticollis at least by following: the labrum with six (3 + 3) sensillae in the anterior part, distinctly cutout in the middle; clypeus is trapezoidal. The mature larva of O. aeneus was also minutely described ( Tormos et al., 1999). We can propose to differ it by emarginate labrum with six setae and six protuberant marginal sensillae and bidentate mandibles. The same is for O. biaccinctus (Buysson, 1891) , which was studied by Tormos et al. (1996): its labrum is emarginate, with 10 short setae and six marginal sensillae, mandibles are tridentate. The drawings of head capsules given by Kilimnik (1993) provide the opportunity to differ the larvae of O. pusillus (Fabricius, 1804) at least by widely cutout labrum and the clypeus much bigger than labrum having the convex posterior margin; of O. politus (Buysson, 1887) by the presence of eight (4 + 4) sensillae on labrum and rugae on the lateral parts of clypeus (labrum is distinctly smaller than clypeus).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.