Paraba multicolor ( Graff, 1899 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4362.1.5 |
|
publication LSID |
lsid:zoobank.org:pub:F72F750D-563E-4960-BAB3-CBEE139A288C |
|
DOI |
https://doi.org/10.5281/zenodo.6042426 |
|
persistent identifier |
https://treatment.plazi.org/id/0397BB6C-FFEC-FFD6-BDBE-972ECCADF8C5 |
|
treatment provided by |
Plazi |
|
scientific name |
Paraba multicolor ( Graff, 1899 ) |
| status |
|
Paraba multicolor ( Graff, 1899) View in CoL
( Figs. 9–11 View FIGURE 9 View FIGURE 10 View FIGURE 11 , Tables 3, 4)
Material examined. MLP – He 6471-3, San Antonio town, 6 November 2010; cephalic and anterior region at the level of the ovaries: sagittal sections on 52 slides; pre-pharyngeal region: transverse sections on 5 slides; pharynx and copulatory apparatus: sagittal sections on 52 slides . MLP – He 6471-2, San Antonio town, 6 November 2010; cephalic and anterior region at the level of the ovaries: sagittal sections on 60 slides; pre-pharyngeal region: transverse sections on 10 slides; pharynx and copulatory apparatus: sagittal sections on 40 slides . MLP – He 6471-1, San Antonio town, 6 November 2010; preserved in ethanol.
Locality. San Antonio town (26°03’26.63’’S, 53°44’07.91’’W), Misiones province, Argentina ( Fig. 1 View FIGURE 1 ). GoogleMaps
Description. External morphology. Body lanceolate, with the anterior region gradually narrowing, and the posterior body region ending abruptly ( Fig. 9A, B View FIGURE 9 ). Dorsum graphite black with a wide yellow orange median band (~30% of body width) along the entire body except a small portion in the cephalic region ( Fig. 9A, B View FIGURE 9 ). A thin red orange stripe runs throughout the median band, which is a bit thicker at the level of the pharynx and copulatory apparatus ( Fig. 9A, B View FIGURE 9 ). The pigment bordering the median band is more concentrated, forming jet black paramedian stripes, darker than the background colour. After fixation, the dorsal pigment becomes slightly faded and the para-median stripes as well as the red orange median stripe are better discernible ( Fig. 9B View FIGURE 9 ). The ventral surface is cream. The eyes, with clear halos, are uniserial around the anterior tip and up to 2mm on the body margins; posteriorly, they remain marginal and extend in two and three irregular rows for 4–6mm. Then, they spread dorsally and pluriserially, forming 10–12 irregular rows for 10–12mm, being posteriorly more isolated and less numerous. At the pre-pharyngeal level, they form 6–8 rows of eyes, and posteriorly remain dorsal, reaching the posterior end ( Fig. 9A, B View FIGURE 9 ). When crawling, maximum length was ~ 50mm. After fixation, maximum length was 35.3–42.5mm and maximum width was 4.6–5.6mm. The position of the mouth and gonopore relative to body length varies from 63% to 67% and from 74% to 82% respectively.
Internal morphology. The dorsal epidermis (30–35µm high), with abundant rhabdites, receives abundant fine granular xanthophil secretion, and scarce fine granular erythrophil and cyanophil secretions ( Fig. 9C–E View FIGURE 9 ). The ventral epidermis (35–40µm high), ciliated on the creeping sole (~100% of body width), is filled with small rhabdites which occupy the apex of epidermal cells, and abundant fine granular cyanophil secretion. The glandular margin contains abundant coarse xanthophil granules and scarce fine cyanophil granules ( Fig. 9C, D View FIGURE 9 ). The sensory pits are simple invaginations (30–50µm deep) distributed in a single row along the cephalic region and extending up to ~500µm from the anterior tip. The cutaneous musculature, organized with the three typical layers of geoplaninids, is almost twice thicker ventrally than dorsally. Its thickness relative to body height at the prepharyngeal region is 4–7% ( Table 3). The parenchymal musculature is organized with three layers: a dorsal layer with decussate diagonal fibres, a supra-intestinal layer with transverse fibres, and a sub-intestinal layer with transverse fibres. Its thickness relative to body height at the pre-pharyngeal region is 12–13% ( Table 3).
Pharynx cylindrical (2.3–2.6mm long, 6% of body length), with the dorsal insertion posteriorly displaced (300–350µm), and mouth located in the distal third of the pharyngeal pouch (3–3.8mm long) ( Fig. 9G View FIGURE 9 ). Pharyngeal stroma traversed by abundant fine granular erythrophil and cyanophil secretions, whose cell bodies are placed in the surrounding parenchyma anterior to the pharynx and pierce the pharyngeal tip. Pharynx lined by ciliated cuboidal epithelium, followed by a thin layer of longitudinal muscle fibres (2.5µm thick) and a subjacent circular muscle layer (5–10µm thick). The epithelium of the pharyngeal lumen is columnar and ciliated, surrounded by a thick muscle layer consisting of circular fibres with some longitudinal fibres interspersed (35–60µm thick). An oesophagus is present (22–24% of pharyngeal length).
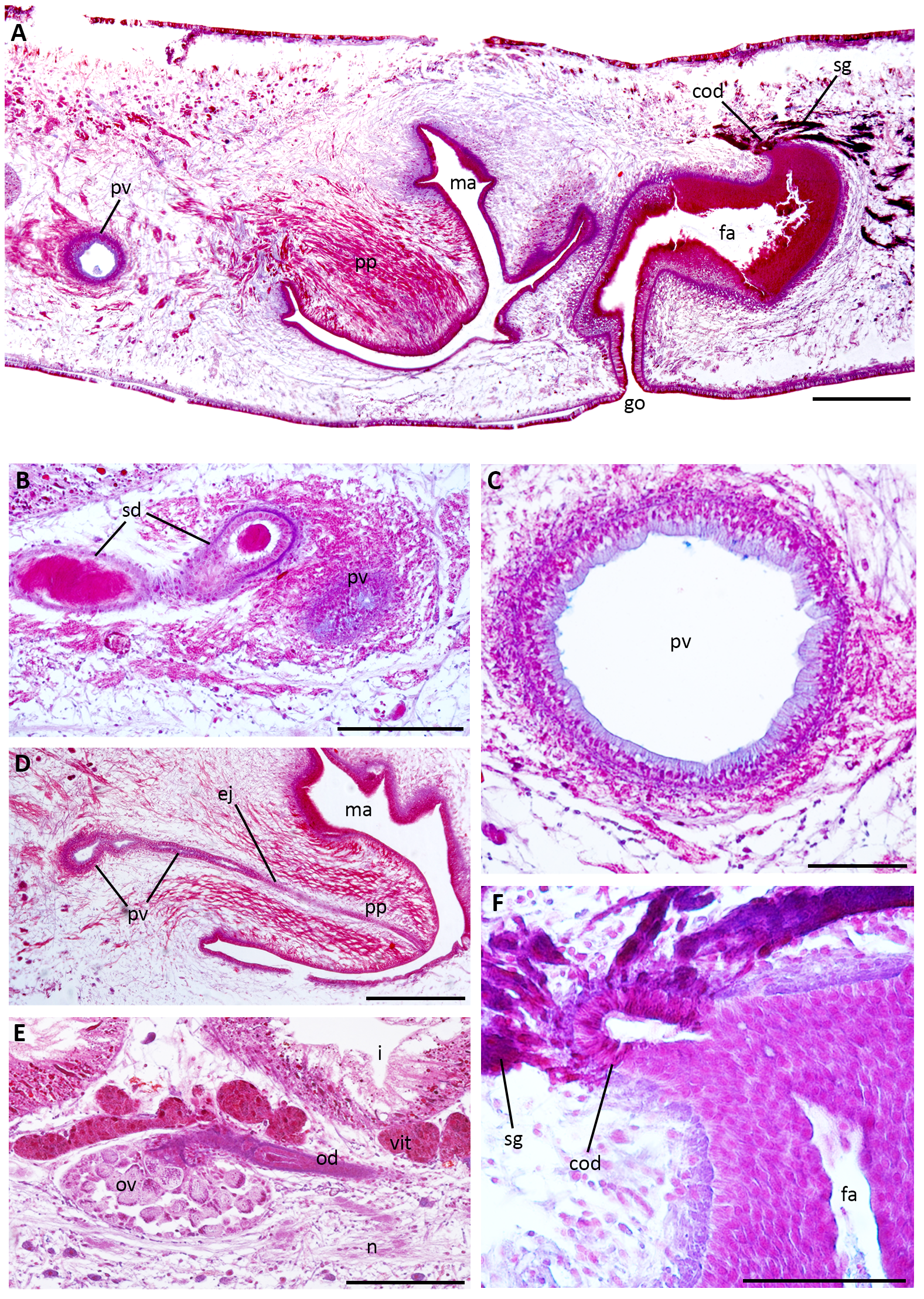
Dorsal testes, rounded, located between the supra-intestinal parenchymal muscle layer and intestinal branches ( Fig. 9C, E View FIGURE 9 ). They form three irregular rows on each side of the body and extend behind the ovaries to near the pharyngeal root (16–18% and 52–60% of body length from the anterior end, respectively). At pre-pharyngeal level, sperm ducts, which are located among fibres of the sub-intestinal parenchymal muscle layer, are dorsal and medially displaced to the ovovitelline ducts ( Fig. 9F View FIGURE 9 ). Behind the pharynx, they are expanded and full of spermatozoa, forming spermiducal vesicles, and open laterally into the paired portions of the prostatic vesicle ( Figs. 10 View FIGURE 10 , 11B View FIGURE 11 ). The extrabulbar prostatic vesicle is composed of proximal globose paired portions and a distal unpaired portion, which is tubular ( Figs. 10 View FIGURE 10 , 11A, C, D View FIGURE 11 ). The latter (0.7–1.1mm long) runs sinuously, traverses the penis bulb and, after a short intrabulbar tract, communicates with the ejaculatory duct, which is slightly ventrally displaced inside the penis papilla ( Figs. 10 View FIGURE 10 , 11D View FIGURE 11 ). The slightly assymetrical penis papilla (0.8–0.9mm long) occupies most of the male atrium (1.5mm long). Folds of the dorsal wall of the male atrium separate this from the female atrium ( Figs. 10 View FIGURE 10 , 11A View FIGURE 11 ). Sperm ducts are lined with cuboidal epithelium, while distally (spermiducal vesicles) they are lined with columnar epithelium ( Fig. 11B View FIGURE 11 ). The two portions of the prostatic vesicle are lined with non-ciliated columnar epithelium that receives apically cyanophil secretion as well as abundant fine granular erythrophil secretion, which is more abundant in the paired portions ( Fig. 11A–D View FIGURE 11 ). The cell bodies of these erythrophil glands are located in the surrounding parenchyma, anterior to the copulatory apparatus. The musculature of the prostatic vesicle, composed of a circular layer with some interspersed longitudinal fibres, is thicker in the paired portions (20–25µm thick) than in the unpaired portion (5–10µm thick). The epithelial lining of the ejaculatory duct is cuboidal and ciliated, followed by a thin circular muscle layer (2.5µm thick). The penis papilla is lined by a cuboidal epithelium, pierced by highly abundant fine granular erythrophil secretion ( Fig. 11A, D View FIGURE 11 ). Also, amorphous erythrophil secretion and cyanophil granules discharge mainly at the level of penis insertions ( Fig. 11A, D View FIGURE 11 ). The musculature of the penis is composed of circular fibres (5–10µm thick). The male atrium is lined with a columnar epithelium filled with erythrophil and cyanophil granules. The muscularis of the male atrium is composed of a circular layer with interspersed longitudinal fibres (5–15µm thick).
The ovaries, ovoid in shape, are located between the sub-intestinal parenchymal muscle layer and the nervous plate ( Fig. 11E View FIGURE 11 ). The ovovitelline ducts arise from the mid-dorsal third of the ovaries ( Fig. 11E View FIGURE 11 ). Vitelline follicles, well developed, are located among intestine branches and discharge along the ovovitelline ducts ( Figs. 9C, E, F View FIGURE 9 , 11E View FIGURE 11 ). At the level of the gonopore, the ovovitelline ducts ascend and run to the middle plane, joining each other in a short common duct (~100µm long) located above the posterior region of the female atrium ( Figs. 10 View FIGURE 10 , 11A, F View FIGURE 11 ). The common ovovitelline duct is horizontal and, distally, it is ventrally flexed to open into the female atrium, which is almost tubular in shape ( Figs. 10 View FIGURE 10 , 11A, F View FIGURE 11 ). Histologically, the female atrium can be differentiated in two portions (see below). The ental part of the proximal portion is vertical, with a narrow lumen, and continues almost horizontally and more spacious, while the distal portion curves ventrally before opening in the gonopore ( Figs. 10 View FIGURE 10 , 11A View FIGURE 11 ). The ovovitelline ducts are lined with cuboidal ciliated epithelium followed by a circular muscle layer (2.5µm thick). Their distal ascending portions receive abundant secretion from shell glands as the common ovovitelline duct ( Fig. 11A, F View FIGURE 11 ). The latter is lined by ciliated columnar epithelium surrounded by circular muscle fibres (10– 15µm thick). The epithelial lining of the proximal region of the female is columnar and non-ciliated with stratified appearance (180–250µm high), while the distal portion is lined with columnar ciliated epithelium (60–100µm high) ( Figs. 10 View FIGURE 10 , 11A, F View FIGURE 11 ). The female atrium receives abundant fine granular erythrophil secretion, more abundant in the proximal portion, and less abundant fine cyanophil granules. Its muscularis is composed of a circular subepithelial layer (15–25µm thick) and a subjacent longitudinal layer (10–15µm thick). A common muscle coat, composed of longitudinal and oblique fibres, well organized around the male atrium (50–70µm thick), is less conspicuous around the female atrium.
Remarks and comparative discussion. Paraba multicolor ( Graff, 1899) has been reported in some localities of Brazil (states of Paraná, Rio de Janeiro, Rio Grande do Sul, and São Paulo) ( Graff 1899; Marcus 1951; Froehlich 1955b, 1956a, 1956b, Leal-Zanchet & Matos 2011) ( Fig. 1 View FIGURE 1 ), and in Germany (Hamburg) as an introduced species ( Arndt 1934). The external appearance of P. multicolor exhibits high variability since the dorsal ground colour can be dark brown, dark grey or black, with a median band pigmented from pale yellowish, yellow, ochre, orange to reddish, flanked by white or black para-median stripes ( Graff 1899; Marcus 1951; Froehlich 1956a; Leal-Zanchet & Matos 2011). Also, in some specimens, the occurrence of a narrow stripe of a rust-red pigment along the median band can be observed ( Leal-Zanchet & Matos 2011). The specimens from Argentina fit with the description of the holotype from São Paulo, with a yellow orange median band (‘fulvo-ferrugineus’ according to Graff) with a narrow red orange median stripe, although the para-median stripes in the Argentinean specimens are black and those in the specimen studied by Graff (1899) are whitish. Flatworms from Rio Grande do Sul also agree in general with Argentinean specimens, but do not exhibit the rust-red pigment along the median band ( Leal-Zanchet & Matos 2011). Regarding body size, specimens from Argentina (45–50mm long in maximum extension) are intermediate between those from Rio Grande do Sul (~ 35mm long) ( Leal-Zanchet & Matos 2011) and those from São Paulo (70mm long) ( Marcus 1951). Once fixed, the relative distance of the mouth and gonopore in relation to the anterior end is similar to that observed in specimens from all localities from Brazil. Although Graff (1899) described only the external aspect of P. multicolor, Marcus (1951) and Leal-Zanchet & Matos (2011) provided more comprehensive descriptions of both the external and internal anatomy of this species. Froehlich (1955b) also included some notes regarding internal features of the musculature and glandular secretions of the pre-pharyngeal region. The presence of a glandular margin, with abundant coarse granular xanthophil secretion, and the thickness of the cutaneous musculature relative to body height at the pre-pharyngeal region (~6% on average) are congruent with specimens from Rio Grande do Sul and Rio de Janeiro ( Froehlich 1955b; Leal-Zanchet & Matos 2011). Also, the cylindrical pharynx with the dorsal insertion a bit posteriorly displaced, short oesophagus and mouth opening in the distal part of the pharyngeal pouch agree with descriptions given by Marcus (1951) and Leal-Zanchet & Matos (2011). Regarding both the general morphology and histology of the copulatory apparatus, the Argentinean specimens are similar to those from Rio Grande do Sul and São Paulo ( Marcus 1951; Leal-Zanchet & Matos 2011). Leal-Zanchet & Matos (2011) re-analysed material studied by Marcus (1951) and interpreted that the female canal considered by Marcus corresponds to the most proximal portion of the female atrium, whose lumen is narrow. We agree with this opinion, based on observations of the Argentinean specimens. Also, Leal-Zanchet & Matos (2011) referred to a vagina (i.e. female canal) that connects the short common ovovitelline duct with the ental portion of the female atrium. However, histologically, we did not distinguish a female canal in specimens from Argentina.
Like Geoplana quagga and other geoplaninids, Paraba multicolor View in CoL is common in anthropized areas (backyards, vacant lots) ( Froehlich 1955a, 1956a, 1958), although it is not restricted to these disturbed environments since it has been also found in native forests ( Leal-Zanchet & Matos 2011). Likewise, specimens from San Antonio ( Argentina) were found below flower plots in a backyard, probably being an accidental introduction through the trade of ornamental plants.
| MLP |
Museo de La Plata |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Paraba multicolor ( Graff, 1899 )
| Negrete, Lisandro & Brusa, Francisco 2017 |
Geoplana quagga
| Negrete & Brusa 2017 |
Paraba multicolor
| , Marcus 1951 |