Amietophrynus superciliaris superciliaris
|
publication ID |
https://doi.org/10.5281/zenodo.276862 |
|
DOI |
https://doi.org/10.5281/zenodo.6191918 |
|
persistent identifier |
https://treatment.plazi.org/id/0397721B-FFAD-BC03-D79C-FB4FFBFA6D6A |
|
treatment provided by |
Plazi |
|
scientific name |
Amietophrynus superciliaris superciliaris |
| status |
|
Amietophrynus superciliaris superciliaris View in CoL ( Boulenger, 1888 “1887”)
Figs. 1 View FIGURE 1 , 2 View FIGURE 2 b, 3a–g, 4b, 5, 6e–g, 7b–c.
Bufo superciliaris Boulenger, 1888 View in CoL “1887”, Proc. Zool. Soc. London 1887: 565
Bufo laevissimus Werner, 1897 View in CoL , Sitzungsber. Akad. Wiss. München 27: 212
Amietophrynus superciliaris View in CoL – Frost, Grant, Faivovich, Bain, Haas, Haddad, de Sa, Channing, Wilkinson, Donnellan, Raxworthy, Campbell, Blotto, Moler, Drewes, Nussbaum, Lynch, Green, & Wheeler, 2006, Bull. Am. Nat. Hist. 297: 363
Type locality. “Rio del Rey, Cameroons ”
Material examined. MHNG 757.61 (juvenile) Cameroon, Efulen; MHNG 917.99-100 ( 2 juveniles) Cameroon, Sangmelima, Foulassi; MHNG 955.88-89 (female + juvenile) Cameroon, Sangmelima; MHNG 1018.74-75, MHNG 1018.78, MHNG 1018.88, MHNG 1018.91 ( 2 males + 3 juveniles) Cameroon, Sangmelima; MHNG 1018.77 (female) Cameroon, Ambam (Ntem); MHNG 1390.94-96 (male + 2 females) Cameroon, Okola, Yaoundé; MHNG 1390.97 (juvenile) Cameroon, Kolmon Nga, Yaoundé; MHNG 1467.9 (female) Gabon, exact locality unknown; MHNG 2094.10 (female) Cameroon, Ongot; MHNG 2207.55-57 ( 2 females + juvenile) Gabon, Ogouée- Ivindo, Makokou; MHNG 2207.62 (female) Gabon, Loa Loa-Ivindo; MNHN 1946.157 (juvenile) Gabon, exact locality unknown; ZFMK 4578 (female) Cameroon, Kumba; ZFMK 8328 (female) Cameroon, Victoria; ZFMK 14905-6 ( 2 females) Cameroon, Mezam, Bafut; ZFMK 15990 (female) Cameroon, exact locality unknown; ZFMK 18682 (juvenile) Cameroon; ZFMK 57800 (female) Cameroon, Kumba; ZFMK 67268 (juvenile) Cameroon, Meked; ZFMK 73192 (juvenile) Gabon, Barrage de Kinguélé; ZFMK 90569 (female) Cameroon, Rumpi Hills, Mofako Balue; ZMB 3907 (female) Cameroon, exact locality unknown; ZMB 19919-20, ZMB 19924, ZMB 71139-40 (formerly part of ZMB 19920) ( 4 females + juvenile) Cameroon, Bipindi; ZMB 19921 (female) Cameroon, exact locality unknown; ZMB 19922-23 ( 2 females) Cameroon, Kribi; ZMB 19971-73 ( 3 juveniles) Cameroon, Bipindi; ZMB 20074 (juvenile) Cameroon, exact locality unknown; ZMB 20076 (juvenile) Cameroon, exact locality unknown; ZMB 20694 (juvenile) Cameroon, south Cameroon; ZMB 20718 (male) Cameroon, south Cameroon; ZMB 28775, ZMB 74317-8 (formerly part of ZMB 28775) (female + 2 juveniles) Cameroon, Bipindihof; ZMB 32037 (juvenile) Cameroon, Bipindi; ZMB 74319 (juvenile) Nigeria, Cross River; ZMB 74522 (juvenile) Cameroon, Nfakwo; ZSM 148/1989/1-2 ( syntypes of Bufo laevissimus ) ( 2 juveniles) Cameroon, exact locality unknown.
Problematic material. MNHN 1923.50 (juvenile) French Congo, locality unknown (today territories of Gabon, Central African Republic, Republic of the Congo); MNHN 1933.16 (adult) Equatorial Africa, locality unknown; MNHN 2002.0655 (adult) Central African Republic, La Maboké, Mboye. As MNHN 2002.0655 was completely dried out, the taxon specific characters are irrecognizable.
Diagnosis. Genetically the taxon belongs to the African toad genus Amietophrynus ( Frost et al. 2006) . As part of the Amietophrynus superciliaris -species complex, the taxon differs from all other members of the genus by a smooth dorsal skin in adult specimens, straight loreal region and large size.
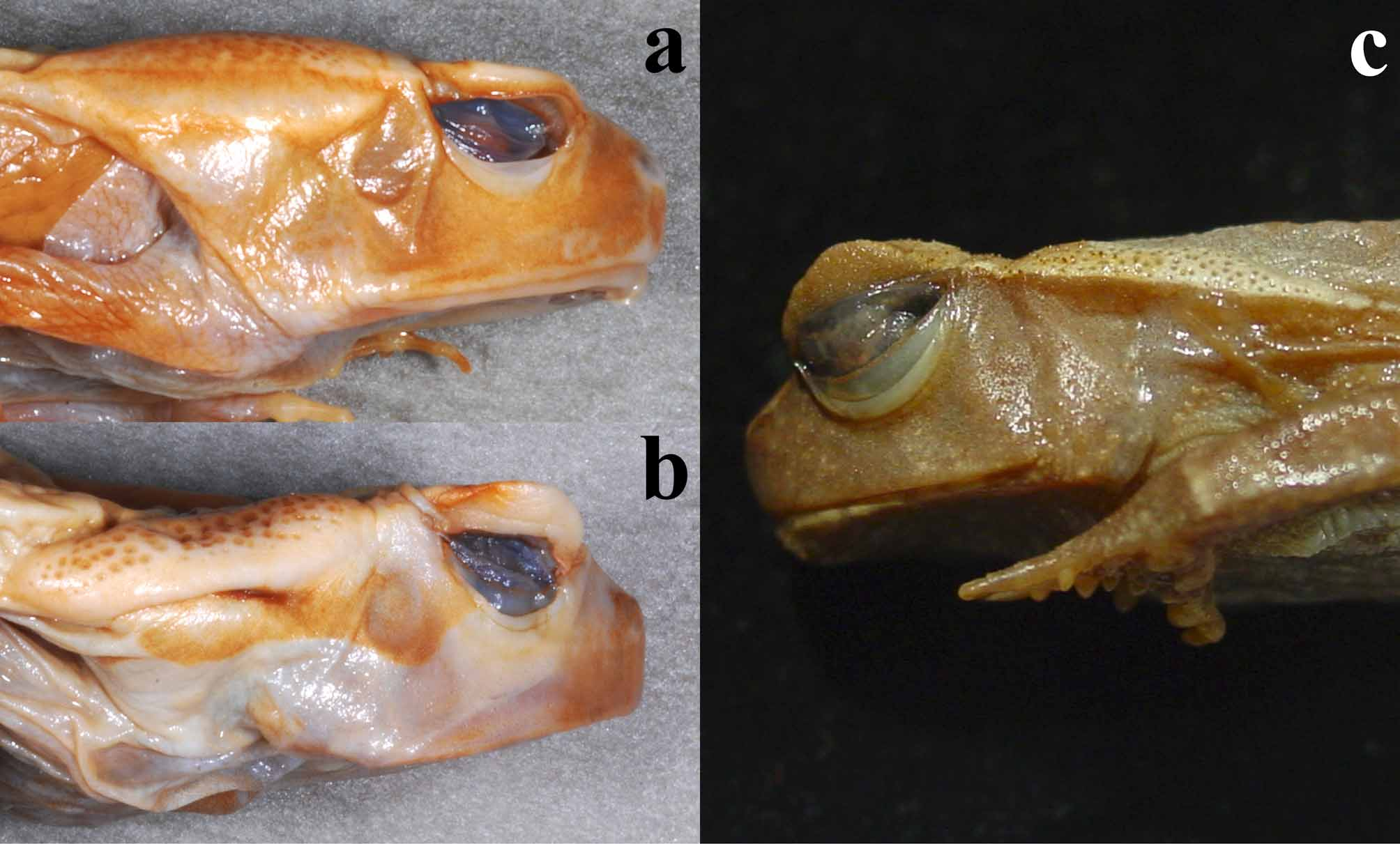
Large toad; dorsal skin smooth in adults, granular in juveniles; body shape very broad, ovoid; tympanum distinct, drop-shaped, smaller than eye diameter; parotids prominent, shape of parotids like slender drop, parotid glands distinctly converging caudally, posterior end of parotid glands pointed; eyelid triangular in dorsal view, enlarging from edge to middle of eyelid in lateral view, forming a triangular eyelid process ( Fig. 2 View FIGURE 2 b); flank colouration reddish-purple; posterior part of back coloured like anterior part of dorsum; pair of spots on abdominal region present; extremities slender; males with nuptial swelling on fingers I and II.
A. s. superciliaris can be distinguished from A. s. chevalieri and the new species A. channingi sp. nov. by colouration and body shape. For characters distinguishing between A. s. superciliaris and the two other taxa see below.
Description. Large sized Amietophrynus species, with very robust body shape; females distinctly larger than males (maximum snout-urostyle-length – SUL – in males: 116.0– 128.4 mm, in females: 108.1–153.4 mm); mean head width similar in both sexes, in males 38% of SUL, in females 39%; snout in lateral view short, slightly rounded; head without bony ridges; canthus rostralis distinct, angular; loreal region straight, slightly concave; distance eye-snout similar to eye diameter; nares closer to snout tip than to eye; eyelid in adult specimens of prominent triangular shape from dorsal and in lateral view, possessing a distinct prolongation in the middle; eyelid process in juveniles less distinct and almost absent in very small specimens; tympanum more or less distinct, positioned at a concave part of the cheeks; tympanum vertically prolonged, horizontal diameter smaller than eye diameter; dorsal skin smooth in adults, warty in juveniles; parotid glands very prominent, shaped like a slender drop, converging backwards, usually with a pointed tip (parotid length / parotid width ratio in males: 3.17, in females: 3.23); in some specimens posterior to parotids a glandular bulge extending to the groins as a dorsolateral fold; parotids distinctly bi-coloured: like flanks on lateral lower part and like back on dorsal surface; gland openings only on dorsal part of parotids (rarely extending onto the borderline of the colour transition); border between the two colours sharp; fingers and toes simple, not enlarged at the end; subarticular tubercles simple; relative length of fingers: III> I> II ≥ IV (mean length of finger I / length of finger III ratio in males: 88%, in females: 94%); manual webbing absent; femora short and slender; mean tibia length in both sexes 38% of SUL; inner and outer metatarsal tubercle present; relative toe length: IV> III> V> II> I; webbing rudimentary: 1 (0.5), 2 (1-0.5), 3 (2-1) or 3 (2- 1.25), 4 (2.75-2.75), 5 (1) or 5 (1.25); females growing larger than males; males with nuptial pads on fingers I and II. Boulenger (1888) mentions the presence of a tarsal fold in his description of the species, while Werner (1897) states that the tarsal fold is absent in Bufo laevissimus (current synonym of A. s. superciliaris , see below); a character also used within his bufonid key. A distinct tarsal fold was absent in all examined specimens, only an indistinct bulging of the skin has been observed in some specimens. In dehydrated specimens, this somehow concave skin might be mistaken for a tarsal fold.
Juveniles possess a granular skin till they reach a size of about 40 mm ( Amiet & Perret 1969). The largest specimen still showing minuscule warts on the dorsum measured 46 mm (MHNG 917.99), in few subadult and predominantly smooth specimens, single tiny bulges were recognizable on the eyelids. The eyelid projection can be very small and almost absent in juveniles of A. s. superciliaris , forming only a very indistinct “swelling” on the eyelid.
Colouration. Dorsum a pale yellowish colour or marbled orange yellow ( Figs. 3 View FIGURE 3 b, c, e–g), extending from the tip of the snout, along the upper side of the parotids backwards to the abdominal region; a pair of dark spots on posterior third of back ( Figs. 3 View FIGURE 3 a, c, e–g); additional smaller spots on anterior part of back or between eyes in few adult specimens ( Figs. 3 View FIGURE 3 c, e); intense red lateral colouration from tip of snout, continuing beneath the canthus rostralis and along entire flanks; sometimes parts of flank colouration darker, red-purple; ventral colouration ranging from light to intense reddish colour ( Fig. 3 View FIGURE 3 d); extremities dark purple; inguinal region rarely with small spots; upper hind limbs and feet with white transversal bars; anterior extremities dark purple ventrally; gular region coloured like venter; juveniles coloured like adults but with additional transversal bars on tibiofibula, usually less distinct in adults ( Figs. 3 View FIGURE 3 a–c, e); juveniles with more spots in addition to the abdominal pair on anterior part of back and in the interorbital region ( Figs. 3 View FIGURE 3 a; 4; 5), these dark spots of different structure than surrounding skin, “drier and softer”.
Interorbital markings of juvenile specimens usually consist of a pair of small blotches (sometimes dissolved into several smaller spots), caudally converging towards each other and barely reaching the eyelids ( Figs. 4 View FIGURE 4 b; 5). Exceptionally these blotches are fused ( Fig. 4 View FIGURE 4 b), a pattern also reported by Andersson (1905). Boulenger (1887) describes a “very fine lighter vertebral line” in some juveniles. According to Amiet & Perret (1969) juveniles possess a brown-black gular colouration and breast, while adults show an overall pale belly. Perret & Mertens (1957) comment on the absence of the pair of dark spots on the posterior part of the back in two specimens. Werner (1898) mentions a female from Cameroon (referred to as Bufo laevissimus ) with pale red-brown upper surface and chocolate brown flanks.
Colouration in preservation. Preserved specimens may loose their reddish colouration, which turns brownish. The typical reddish colouration was only observable where it remained covered between skin folds; in other specimens it completely disappeared. Nonetheless, in many specimens a clear differentiation between the lateral and dorsal colouration is recognizable. Very old specimens may be bleached out completely, lacking any colour pattern; e.g. even the pair of abdominal spots then may only be recognizable by a closer examination of the skin structure.
Natural history. Amietophrynus s. superciliaris inhabits the forest floor in primary forest vegetation along small rivers ( Frétey & Blanc 2001; Lasso et al. 2002; Figs. 6 View FIGURE 6 e–g). Akani et al. (2004) report the finding of the species in the rainy season in a pristine swamp site. But the species also inhabits secondary growth with dense vegetation or even plantations ( Amiet 1976a, 1986; Rödel et al. 2004). Occasionally the species can be seen in more open places searching for food ( Sanderson 1936a). Similarly, local farmers in western Cameroon reported to the senior author on occasional findings of the species in plantations. During the late rainy season ( September 2010) M. Hirschfeld and M.- O. Rödel ( pers. obs.) observed an adult male (SUL 124 mm) in a near pristine part of the Ebo forest, Cameroon, over a period of four days. During this time the toad only moved about 25 m, the largest daily distance being about 15 m. During two consecutive nights the male climbed onto the top of two different bare rocks of 50–60 cm height, respectively and seemed to stay there motionless for the night. The other days it was observed sitting under a smaller tree, turning its body only once into another direction. According to Amiet (1976a) breeding males possess slightly hypertrophied anterior extremities and black callosities on fingers I and II. A. s. superciliaris males lack a vocal sac ( Perret & Amiet 1971) and the species is assumed to utter no advertisement call ( Perret & Mertens 1957; Tandy & Keith 1972). A published sonogram of a A. s. superciliaris vocalization is not regarded to show an advertisement call ( Amiet 1976a). This vocalization, a dull buzzing noise, lasted for almost 1– 1.5 s, comprising a long series of pulses ( Amiet 1976a, b). Amiet only observed movements of the flanks during sound emission, but no movements of the gular region ( Amiet 1976a). Hence, Amiet doubts that this noise represents an advertisement call, as it can be heard on short distances only. However, it may function in short distance recognition ( Amiet 1976a, 1989). Clutches are deposited and tadpoles may develop in slowly running streams ( Amiet 1976a, 1986, 1989; Gossmann et al. 2002; Figs. 6 View FIGURE 6 e–f). Egg size ranges from 1.40–1.95 mm (N= 102, mean= 1.67, SD= 0.09). Amiet (1976a) assumes that reproduction takes place in the dry season (January – March) as most specimens have been found during that time and only at that time males show their nuptial dress. Juveniles have been found in January in Nigeria (M.- O. Rödel & A.B. Onadeko, pers. obs; Figs. 3 View FIGURE 3 a; 6e) and Cameroon ( Schmitz 1998). Amiet & Perret (1969) report on juveniles (< 40 mm) collected in February, May, June and July–October. According to Angel (1931) specimens in captivity feed on insects, snails, but also on frogs and tadpoles. Perret & Mertens (1957) mention beef meat as food in captivity. Affa’a & Amiet (1990) report on nyctotheres (Protozoa, Clevelandellida , Prosicuophora basoglui , Nyctotheroides ptychadenae ) found in adult A. s. superciliaris .
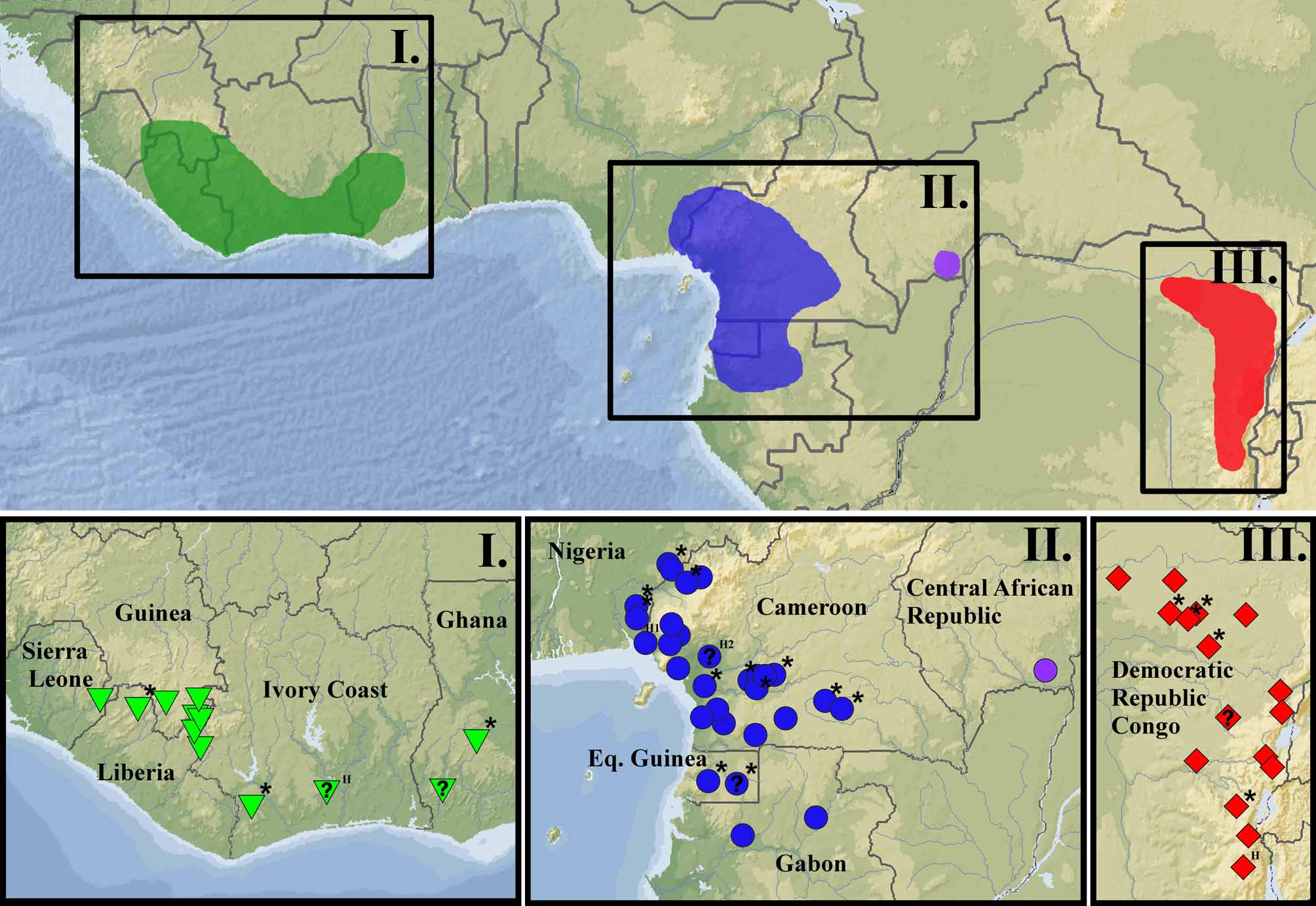
Distribution. Amietophrynus s. superciliaris inhabits the western Lower Guinean rain forest ( Fig. 1 View FIGURE 1 ). At present the taxon is known from localities in Nigeria (Boulenger in Perret & Mertens 1957; Schiøtz 1963; Akani et al. 2004; this paper), Cameroon (e.g. Müller 1910; Barbour 1911; Parker 1936; Sanderson 1936b; Mertens 1940; Perret 1966; Herrmann et al. 2005, this paper), Gabon ( Frétey & Dewynter 1998; Frétey & Blanc 2001; Lötters et al. 2001; Blanc & Frétey 2004; Pauwels & Rödel 2007), and Equatorial Guinea ( Nieden 1910; Lasso et al. 2002). According to Frétey & Blanc (2000) the species is also known from the Republic of Congo and the Central African Republic, but the authors do not provide localities. Joger (1990) lists A. s. superciliaris (MHNP 1968.331) for the Central African Republic (Oubangi-Chari = without specification), but the respective collection number is associated with a Ptychadena trinodis (mentioned with this number in the very publication). The only specimen of A. s. superciliaris with a similar collection number (MHNP 1968.359) refers to a specimen from “Afrique”. However, a specimen in very bad condition from the Central African Republic is deposited in the MNHN collection (MNHN 2002.0655; see remark above). The placement of MNHN 2002.0655 in the distribution map ( Fig. 1 View FIGURE 1 ) has been matched according to publications referring to the same locality ( Joger 1990; Rasmussen et al. 2000). Hence, we tentatively assign A. s. superciliaris to the herpetofauna of the western part of the Central African Republic, but suggest verifying the taxonomic status of this population as soon as new material becomes available. The wide distribution of A. s. superciliaris in western lowland rain forests of Central Africa ( Fig. 1 View FIGURE 1 ) makes it likely that this toad is indeed living in the south-western Central African Republic and possibly likewise in western Congo.
Taxonomy. Amietophrynus superciliaris was described based on a series of juvenile specimens from “Rio del Rey Cameroons ” (Boulenger 1887; Fig. 5 View FIGURE 5 ). The description of Bufo laevissimus was based on juveniles and one adult specimen from Cameroon ( Werner 1897), and in a later publication Werner (1898) describes a second adult toad. Werner (1897) distinguishes the two species on the size of the parotid glands and the lack of a dorsolateral fold in B. laevissimus . According to Frost (2010) B. laevissimus was synonymised with B. superciliaris by Andersson (1905). Indeed, Andersson (1905) discusses the consistency in morphometrics between juveniles of B. laevissimus and those of B. superciliaris and remarks that distinguishing characters presented by Werner (1897) are based on the presence of an adult specimen in his material only. He concluded that the differences in the descriptions may have been the result of allometric growth (Andersson 1905). Earlier, Boulenger (1900) remarks that “adult specimens [of A. s. superciliaris ] have been redescribed by Werner under the name of B. laevissimus , from Cameroons ”. Boulenger (1900) reports on a toad of about 120 mm and already lists B. laevissimus as a synonym of B. superciliaris . Andersson (1905) later provides a more detailed comparison and confirmed the conspecificity of the two species. He states that “therefore it can be that both Boulenger’s and Werner’s descriptions correspond with the case in this same species, that of the former with the young, the latter with the older ones”. He also cites Boulenger’s (1900) publication. Consequently, the synonymy of Bufo laevissimus Werner, 1897 with Amietophrynus s. superciliaris (Boulenger, 1887) , as defined in this work, is based on Boulenger (1900).
The syntypes of Bufo laevissimus Werner, 1897 are deposited in the ZSM collection. Two juvenile syntypes (ZSM 148/1989/1-2) are still present in the ZSM collection (and have been examined herein), while the only adult female syntype (ZSM 1113/0) is lost ( Glaw & Franzen 2006; M. Franzen 18.01. 2010 in litt.). Our examination of the two available syntypes revealed no characters distinguishing them from other juvenile A. s. superciliaris . The available specimens also correspond in the shape of the eyelid ( Figs. 7 View FIGURE 7 b, c) and we regard these specimens as conspecific with A. s. superciliaris . Amietophrynus s. superciliaris is morphologically most similar to A. channingi sp. nov., the second taxon from Central Africa. Amietophrynus s. superciliaris differs in colouration and morphology from A. s. chevalieri and A. channingi sp. nov. (see respective paragraphs and the key).
Ethnozoology. Perret & Mertens (1957) report that indigenous people fear the toad because of its venom. According to Herrmann et al. (2005) A. s. superciliaris plays an important role in traditional medicine for people in the Mt. Nlonako area, but they also report on the people’s fear that the toad transmits leprosy. This is similar to the belief in villages close to the Ebo forest, Cameroon, where people think that the saliva of the toad may damage human skin (M. Hirschfeld & M.- O. Rödel, unpubl. data). Lawson (1993) describes a local belief from western Cameroon that toad bones are used to treat poisoning and mentally deranged people. Traditional healers of the Bakossi people dry toads and pound them for native medicine (A. Schmitz, pers. obs.). In southern Cameroon Bakola-pygmees use the parotid secretion to envenom their arrowheads for hunting (Medjo, WWF-guide at Campo Ma’an NP, pers. comm.). Lawson (1993) reports the local belief in south-western Cameroon where people assume that these toads indicate the change of season by changing position into the opposite direction, while they otherwise rest for long times in the same place and position (see above). Local hunters in southern Cameroon reported to the senior author on large frogs sitting for months without movement in the forest.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Amietophrynus superciliaris superciliaris
| Barej, Michael F., Schmitz, Andreas, Menegon, Michele, Hillers, Annika, Hinkel, Harald, Böhme, Wolfgang & Rödel, Mark-Oliver 2011 |
Bufo laevissimus
| Werner 1897 |
Bufo superciliaris
| Boulenger 1888 |