Anopheles (Nyssorhynchus) oswaldoi ( Peryassú, 1922 ), Peryassu, 1922
|
publication ID |
https://doi.org/ 10.5281/zenodo.178520 |
|
DOI |
https://doi.org/10.5281/zenodo.5613746 |
|
persistent identifier |
https://treatment.plazi.org/id/0396983B-FFAC-FFA3-FF17-F884FBB4FC0D |
|
treatment provided by |
Plazi |
|
scientific name |
Anopheles (Nyssorhynchus) oswaldoi ( Peryassú, 1922 ) |
| status |
|
Anopheles (Nyssorhynchus) oswaldoi ( Peryassú, 1922) View in CoL
Cellia oswaldoi Peryassú, 1922: 179 . Syntypes: adult male and female. Vale do Rio Doce, State of Espírito Santo and Baixada Fluminense, State of Rio de Janeiro, Brazil [Museu Nacional do Rio de Janeiro – MNRJ]. LECTOTYPE (hereby designated): Adult male with associated male genitalia dissected and mounted on a microscope slide by one of the authors (CFM), BRAZIL, State of Espírito Santo, Vale do Rio Doce, AG Peryassú coll., 4 - XI -1922.
Anopheles (Nys.) View in CoL tarsimaculatus var. aquacaelestis Curry (1932) : 566-571. Syntypes: male with genitalia slide, female and larva. Atlantic side of Canal Zone, Panama, no type locality cited. Synonymy with An. oswaldoi by Senevet & Abonnenc 1938: 487 -493.
Anopheles (Nys.) View in CoL oswaldoi of Belkin et al. 1971: 6 (type info); Faran 1980: 55 (M*, F*, P*, L*); Lounibos et al. 1997: 136 -155 (scanning electron micro photo, E*).
Description. Female. Integument light brown with grayish pollinosity. Head: interocular space with a frontal tuft of long, pale-yellow setae and decumbent, curved, white spatulate scales along ocular margin, vertex immediately posterior to frontal tuft with erect, white spatulate scales and few long pale-yellow setae; remain- der of vertex and occiput with erect, dark-brown spatulate scales; postgena with tuft of dark-brown spatulate scales and few semi-erect, white spatulate scales at junction of eyes; clypeus bare. Pedicel of antenna dark brown with decumbent, white spatulate scales on dorsal surface; flagellomere 1 with semi-erect white scales on medial and lateral surface, and decumbent white scales at base of dorsal surface. Proboscis dark, length 2.24-2.30 mm (mean=2.27±0.03) (n=4) (Espírito Santo, ES), 2.13–2.93 mm (mean=2.41±0.29) (n=6) (São Paulo, SP), 1.40–1.46 (mean=1.44±0.03) (n=3) (ES), 1.43–1.51 (mean=1.46±0.03) (n=5) (SP) length of forefemur, 1.40–1.46 (mean=1.44±0.03) (n=3) (ES), 1.43–1.51 (mean=1.46±0.03) (n=5) (SP) length of maxillary palpus. Maxillary palpomere 1 dark, with erect scales on dorsal surface; maxillary palpomere 2 dark, with few pale scales at apex of dorsal surface; maxillary palpomere 3 dark-scaled, with a subapical pale patch on dorsal surface; maxillary palpomere 4 white-scaled, with dark scales at base, apex and lateral surface; maxillary palpomere 5 white-scaled, with dark scales at base; length of palpomere 2 / length of maxillary palpus 0.26–0.28 (mean=0.27±0.01) (n=4) (ES); 0.27–0.28 (mean=0.28±0.06) (n=2) (SP); length of palpomere 3/ length of maxillary palpus 0.37–0.39 (mean=0.37±0.01) (n=4) (ES), 0.37–0.38 (mean=0.37±0.01) (n=2) (SP); length of palpomere 4 / length of maxillary palpus 0.18–0.19 (mean=0.185±0.005) (n=4) (ES), 0.15–0.18 (mean=0.17±0.02) (n=2) (SP); length of palpomere 5 / length of maxillary palpus 0.14 (n=3) (ES), 0.1 (n=2) (SP). Thorax: integument with darker area between dorsocentral area and lateral margin, on posterior edge of scutal fossa and posteriorly on prescutellar area; pale, spatulate, decumbent scales on acrostichal and dorsocentral areas, scutal fossa and anteriorly on prescutellar area; supraalar and antealar areas with white, spatulate, decumbent scales; elongate, narrow and erect white scales along lateral margin of antealar area extending posteriorly onto supraalar area; scutum bare anteriorly between acrostichal and dorsocentral areas, posteriorly to scutal fossa, and posteriorly on prescutellar area; anterior promontory with erect, piliform, white scales; lateral anterior end of dorsocentral area with erect, piliform scales; anterior lateral margin of scutum with erect, spatulate scales, white scales dorsally and dark scales ventrally. Scutellum with a few yellowish spatulate scales, posterior margin with long dark setae. Mesopostnotum bare. Antepronotum with dark setae, and a patch of spatulate scales, white dorsally and dark ventrally on upper area, remainder of antepronotum without scales, with scattered setae. Patch of white scales on lower prealar, prespiracular and lower mesokatepisternum; patch of setae on upper prealar, lower mesokatepisternum and proepisternum. Wing: length 3.39–3.63 mm (mean=3.52±0.12) (n=4) (ES), 3.54–3.89 mm (mean=3.70±0.14) (n=4) (SP); wing spot measurements in Table 1 View TABLE 1 ; veins with dark areas and spots of pale yellow scales as follow: costa always with basal pale, prehumeral dark, humeral pale, humeral dark, presector pale, presector dark, distal sector dark, subcostal pale, preapical dark, preapical pale and apical dark; sector pale, proximal sector dark and accessory sector pale present in 25% (ES) and 50% (SP) of wings examined; remigium pale; 0.75–0.79 mm (mean=0.77±0.01) (n=4) (ES), 0.71–0.79 mm (mean=0.75±0.03) (n=5) (SP) distance between basal pale and sector pale spots of vein R; R S with a patch of pale scales at junction of R4+5, and few pale scales on basal 0.5; R2+3 with a patch of pale scales along middle region and few dark scales at apex before furcation of R2 and R3; R4+5 mostly pale, with small patch of dark scales at proximal 0.2 and distal end; vein M variable, mostly dark, with pale scales from basal end to middle region, or mostly pale with dark spots in middle region and at distal end at furcation of M1+2 and M3+4; CuA mostly pale, with small dark spot at distal 0.2 before furcation of CuA1 and CuA2; CuA1 mostly pale, with 2 separate dark spots at basal 0.5 and a small dark spot at distal end; pale fringe spots at apices of veins R2, R4+5,M1+2, M3+4, CuA1, CuA2 and 1A. Halter : pale ventrally and dorsally with proximal and distal dark spots on dorsal surface, capitellum dark ventrally. Legs: anterior surface of forecoxa with a patch of white spatulate scales and long, dark setae, posterolateral surface with white spatulate scales, posterior surface with dark spatulate scales; outer surface of midcoxa with white spatulate scales, proximal area with semi-erect scales, posterior and anterior surface with a patch of white spatulate scales; posterior surface of hindcoxa with a small apical patch of white spatulate scales, distal posterior surface with white spatulate scales. Fore-, mid- and hindtrochanters dark-scaled on anterior and inner surfaces, lateral surface with a few dark scales at base, white scales on posterolateral surface, posterior surface with dark scales at base and white scales distally. Foretarsomere 1 with pale scales at apex; dark base of foretarsomere 2 0.64–0.72 (mean=0.69±0.04) (n=6) (ES), 0.67–0.71 (mean=0.69±0.03) (n=2) (SP) length of tarsomere, dark base of foretarsomere 3 0.16–0.24 (mean=0.20±0.04) (n=6) (ES), 0.34–0.48 (mean=0.41±0.10) (n=2) (SP) length of tarsomere, foretarsomeres 4 and 5 dark-scaled with pale scales at apices, sometimes foretarsomere 5 with a subapical pale patch; midtarsomere 2 with an apical ring of pale scales, dark base of midtarsomere 2 0.87–1.00 (mean=0.92±0.06) (n=6) (ES), 0.83–0.89 (mean=0.86±0.04) (n=2) (SP) length of tarsomere, midtarsomere 3 with a few apical pale scales, dark base of midtarsomere 3 0.77–0.96 (mean=0.89±0.06) (n=6) (ES), 0.95–0.96 (mean=0.96±0.00) (n=2) (SP) length of tarsomere, midtarsomere 4 dark, sometimes with a few pale scales at apex, midtarsomere 5 with pale scales at about apical 0.5; dark base of hindtarsomere 2 0.08–0.15 (mean=0.13±0.03) (n=6) (ES), 0.13–0.24 (mean=0.19±0.05) (n=4) (SP) length of tarsomere, dark base of hindtarsomere 5 0.39–0.42 (mean=0.40±0.01) (n=4) (ES), 0.41–0.51 (mean=0.46±0.04) (n=4) (SP) length of tarsomere, remainder of hindtarsomeres 2, 3, 4 and 5 white-scaled. Abdomen: integument light to dark brown; terga II-IV with pale scales, most scales disposed in a sub-triangular pattern on segments II-V, segments VI-VII more equally covered with scales; dark posterolateral scale-tufts large, present on terga II-VII.
Male. Similar to female except for sexual differences. Maxillary palpus pale and dark-scaled; scales semierect on basal 0.5 of palpomere 2, decumbent on remainder of palpomere 2 and palpomeres 3-5; palpomere 2 dark-scaled with pale scales at apex, dorsal surface with a pale spot on basal 0.5; palpomere 3 with pale scales at base, dorsally with a pale spot on medial surface, long setae at apex; palpomere 4 with pale scales on ventral and dorsal surface, dark-scaled at apex and base; palpomere 5 with dark setae along ventral surface, pale scales on dorsal surface, dark scales on ventral surface and base.
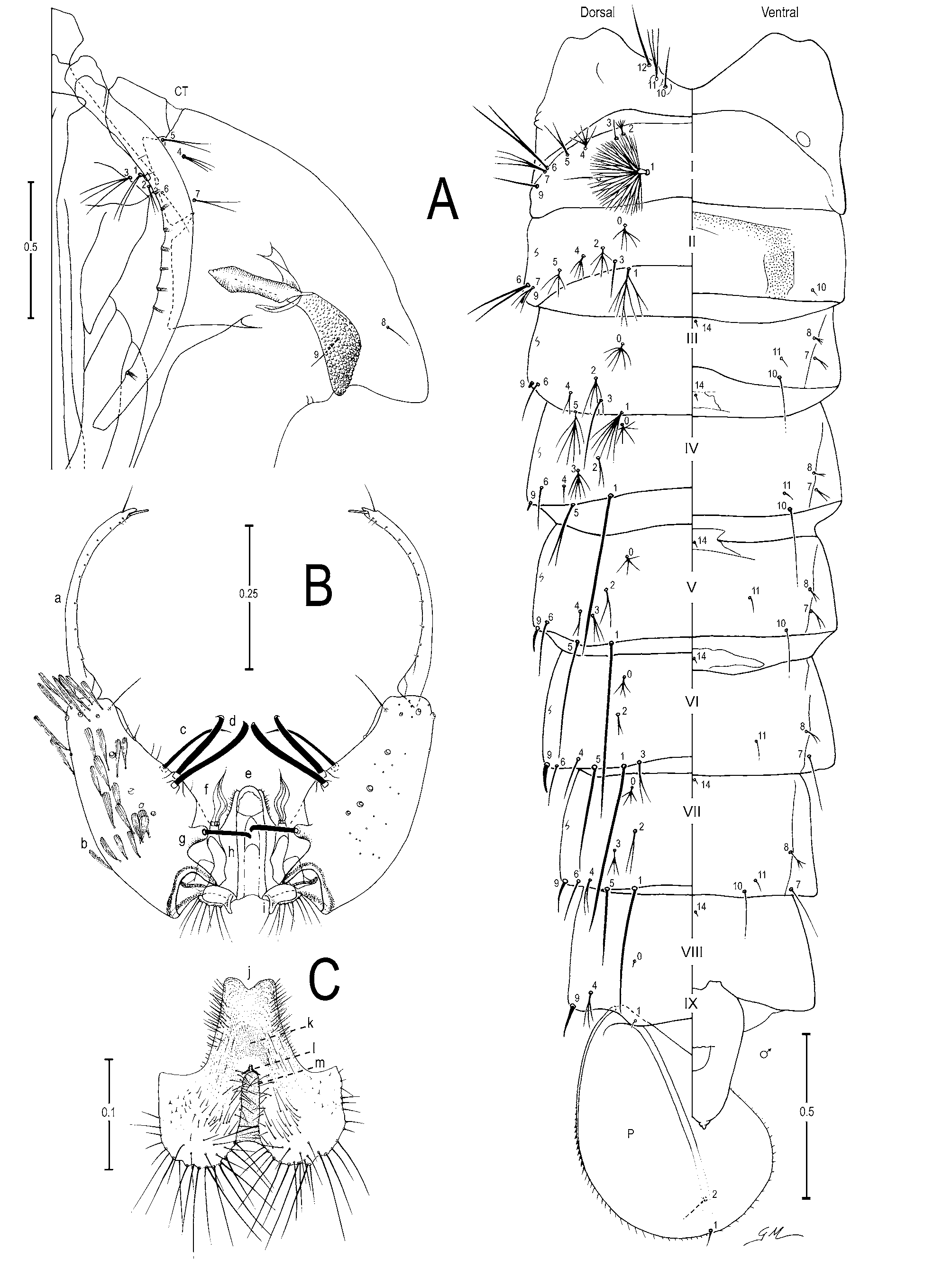
Male genitalia ( Figures 1 View FIGURE 1 A,C; 2B,C). Segment VIII: tergum and sternum narrow, with broad spatulate scales and long setae, scales slightly broader on sternum than on tergum. Segment IX: anterior apodeme subtrapezoidal; gonocoxite elongate; tergal surface with 4 or 5 setae and subapical surface with 1 or 2 setae; tubercle of parabasal spine large, 0.4 length of parabasal spine; dorsomedian rim 0.11–0.14 (mean=0.12±0.01) (n=2) (ES), 0.13 (n=1) (SP) length of gonocoxite; accessory spines 0.33–0.40 (mean=0.37±0.02) (n=3) (ES), 0.38 (n=1) (SP) length of gonocoxite; internal spine retrorse apically; gonostylar claw spiniform, thin and long. Dorsal claspette: pedicel narrow, base rounded, leaflets broad, internally expanded on median area. Ve n - tral claspette ( Figures 1 View FIGURE 1 C; 2C): spiculate, with spicules extending to apex, 0.44–0.55 (mean=0.48±0.04) (n=3) (ES), 0.45 (n=1) (SP) length of gonocoxite, lateral margin tapered toward a narrow apex, basal lobule expanded laterally with long setae on basal margin, setae about 2.0 width of aedeagus, lateral margin of apex rounded and median sulcus shallow, sulcus with sloping sides, preapical plate large, a weakly sclerotized, transparent membranous area basal to preapical plate, refringent structure in shape of an inverted horseshoe or V; aedeagus without subapical leaflets, apex longer than wide, somewhat rounded ( Figure 1 View FIGURE 1 A).
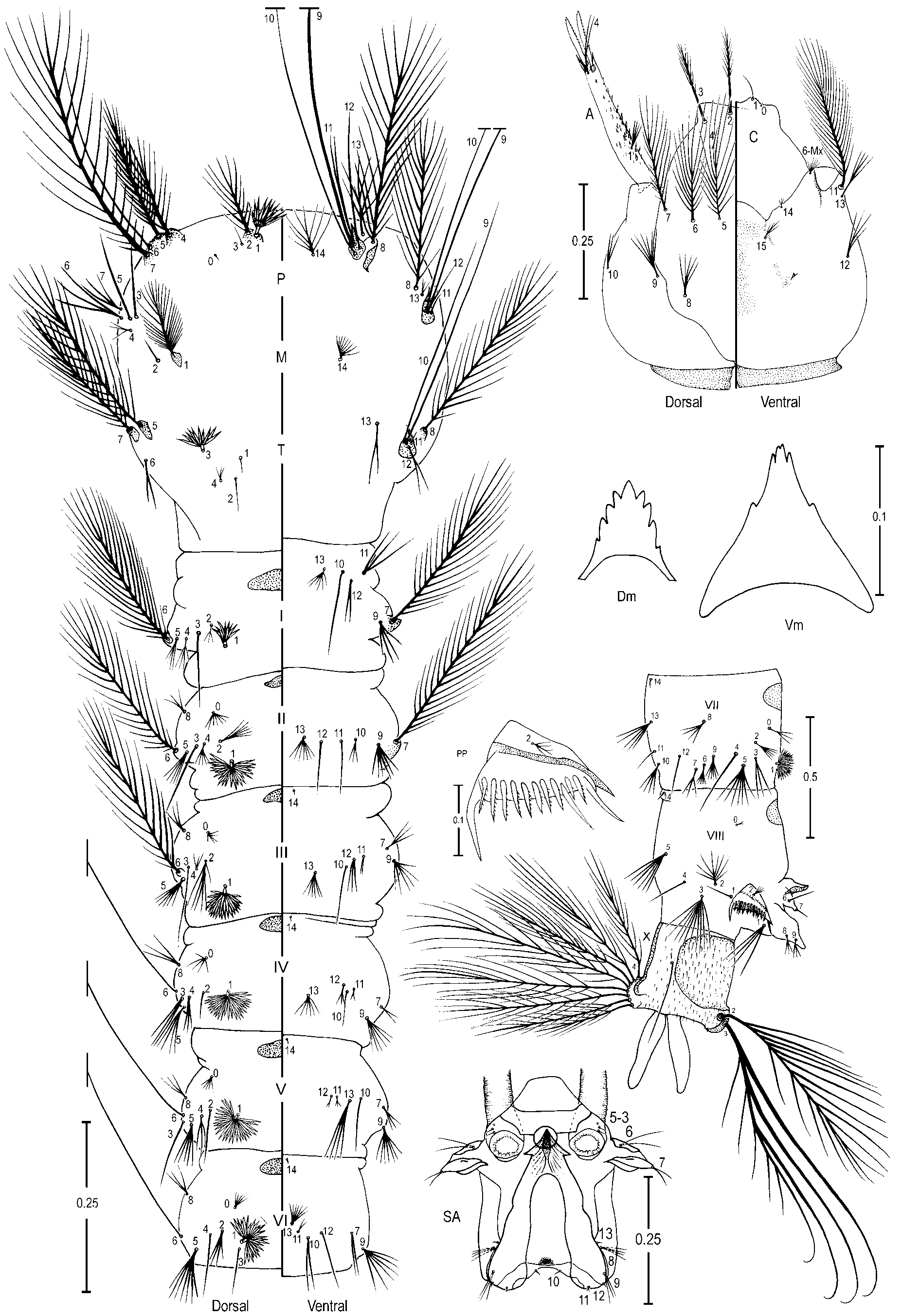
Pupa ( Figure 2 View FIGURE 2 A). Position and development of setae as figured; range, number and mode of branches in Table 2 View TABLE 2 . All measurements from 19 or 20 specimens, unless otherwise indicated. Cephalothorax: integument yellowish with dark areas in legs and dorsal part, without a pattern of dark areas; trumpet length 0.36–0.43 mm (mean=0.41±0.02) (n=17) (ES), 0.39–0.41 mm (mean=0.41±0.01) (n=3) (SP), pinna moderately to heavily pigmented, light to dark brown, 3.06–4.55 (mean=3.84±0.41) (n=12) (ES), 3.59–4.39 (mean=3.98±0.36) (n=3) (SP) length of meatus, trumpet appearing truncate and flared apically in lateral Female
Wing spot Range Mean SD(±) n=
(ES) (SP) (ES) (SP) (ES) (SP) (ES) (SP) Male
aspect; seta 2-CT slightly shorter than 1,3-CT, 10-CT usually single (1-2), shorter than 11-CT, 12-CT with 1-4 branches, 1.33–2.00 (mean=1.67±0.24) (n=10) (ES), 1.60–1.87 (mean=1.76±0.13) (n=2) (SP) length of 10- CT. Abdomen: integument yellowish with ventral dark areas in intersegmental membrane and laterally on segment II, length 2.58–2.90 mm (mean=2.75±0.09) (n=17) (ES), 2.82–2.88 mm (mean=2.84±0.03) (n=3) (SP); 1-II,III with median branches longer than lateral; 1-IV-VII single and long, slightly longer than following segment; 2-I with 3-9 branches, dendritic, long; 3-I 0.60–1.00 (mean=0.79±0.13) (n=12) (ES), 0.75–1.00 (mean=0.87±0.13) (n=2) (SP) length of 2-I, 3-V normally triple (1-4 branches); 5-IV with 1-4 branches, longer and more accentuated than 5-III, 5-V-VII normally single; 6-I single or double, long, 6-II generally double (1-4 branches), 7-I with 2-5 branches, shorter than 6-I, 7-III-V short, with 5 or fewer branches, generally with 2 or 3 branches, 7-VII single and long; 9-II small and unpigmented, 9-III short, 1.20–2.00 (mean=1.76±0.25) (n=14) (ES), 1.33–1.60 (mean=1.51±0.15) (n=2) (SP) length of 9-II, weakly pigmented, 9- IV 1.25–2.33 (mean=1.69±0.28) (n=17) (ES), 2.00–2.50 (mean=2.24±0.26) (n=3) (SP) length of 9-III, 9-V strong, 1.57–3.29 (mean=2.07±0.30) (n=17) (ES), 1.63–1.90 (mean=1.77±0.09) (n=3) (SP) length of 9-IV, 9- VI strong 0.78–1.36 (mean=1.16±0.11) (n=17) (ES), 1.07–1.43 (mean=1.16±0.14) (n=3) (SP) length of 9-V, 9-VII strong, weakly curved, 0.71–1.57 (mean=1.19±0.16) (n=16) (ES), 1.27–1.82 (mean=1.47±0.24) (n=3) (SP) length of 9-VI, 9-VIII 0.75–1.5 (mean=0.96±0.15) (n=17) (ES), 0.82–1.15 (mean=0.94±0.13) (n=3) (SP) length of 9-VII, 9-II 0.04–0.06 (mean=0.04±0.01) (n=14) (ES), 0.05–0.07 (mean=0.05±0.01) (n=2) (SP) length of segment II, 9-III 0.04–0.06 (mean=0.05±0.01) (n=17) (ES), 0.05–0.06 (mean=0.06±0.01) (n=3) (SP) length of segment III, 9-IV 0.07–0.12 (mean=0.09±0.01) (n=17) (ES), 0.10–0.15 (mean=0.12±0.02) (n=3) (SP) length of segment IV, 9-V 0.13–0.31 (mean=0.18±0.03) (n=17) (ES), 0.17–0.26 (mean=0.21±0.04) (n=3) (SP) length of segment V, 9-VI 0.15–0.28 (mean=0.21±0.03) (n=17) (ES), 0.17–0.28 (mean=0.24±0.05) (n=3) (SP) length of segment VI, 9-VII 0.15–0.29 (mean=0.23±0.03) (n=17) (ES), 0.21–0.48 (mean=0.34±0.11) (n=3) (SP) length of segment VII, 9-VIII 0.19–0.28 (mean= 0.22±0.02) (n=16) (ES), 0.23–0.44 (mean=0.33±0.09) (n=3) (SP) length of segment VIII; 10-VI absent. Paddle longer than wide, length 0.72– 0.82 mm (mean=0.77±0.03) (n=17) (ES), 0.79–0.81 mm (mean=0.80±0.01) (n=1) (SP), width 0.32–0.40 mm (mean=0.36±0.02) (n=17) (ES), 0.33–0.37 (mean=0.35±0.01) (n=3) (SP); refractile index 0.65–0.73 (mean=0.69±0.02) (n=17) (ES), 0.71 (n=1) (SP); subapical area of paddle weakly emarginate at insertion of setae 1-P; midrib distinct basally, indistinct distally; external buttress 0.62–0.76 (mean=0.69±0.04) (n=17) (ES), 0.63 (n=1) (SP) length of paddle; seta 1,2-P single.
Fourth-instar larva ( Figure 3 View FIGURE 3 ). Position and development of setae as figured; range and modes of branches in Table 3 View TABLE 3 . Measurements from 19 or 20 specimens unless otherwise indicated. Head: length 0.58– 0.65 mm (mean=0.63±0.02) (n=18) (ES); width 0.56–0.57 mm (mean=0.57±0.01) (n=2) (SP); integument weakly sclerotized, somewhat yellowish with dark spots on posterior region of dorsal apotome and lateralia along dorsal ecdysial line, posterior area of lateralia, ventral area of lateralia, labiogula and along of hypocranial ecdysial line; dorsomentum strongly sclerotized, blackish, median tooth broad, pointed, stronger than lateral teeth. Seta 2-C single, distinctly brushlike on 0.5 distal, 0.95–1.33 (mean=1.10±0.08) (n=17) (ES), 0.80– 1.05 (mean=0.96±0.11) (n=2) (SP) length of 3-C; 0.04–0.05 mm (mean=0.04±0.03) (n=18) (ES), 0.05 mm (±0.01) (n=2) (SP) distance between bases of 2-C; 3-C visibly brushlike on 0.5 distal, 0.75–1.06 (mean=0.90±0.07) (n=17) (ES), 0.95–1.25 (mean=1.06±0.14) (n=2) (SP) length of 2-C; clypeal index (distance between bases of 2-C and 3-C one side / distance between bases of 2-C) 1.10–1.63 (mean=1.39±0.11) (n=18) (ES), 1.20–1.30 (mean=1.24±0.08) (n=2) (SP). Seta 4-C with 2-8 short branches, extending half distance to base of 2-C. Collar dark brown, strongly pigmented, dorsomesal region 0.05–0.06 mm (mean=0.05±0.003) (n=17) (ES), 0.05 mm (n=2) (SP). Antenna: 0.27–0.31 mm (mean=0.29±0.01) (n=17) (ES), 0.30–0.32 mm (mean=0.31±0.01) (n=2) (SP), darker pigmented than head capsule, mesal margin with numerous, long spicules; 1-A with 4-9 branches, inserted 0.06–0.10 mm (mean=0.08±0.01) (n=17) (ES), 0.09–0.10 mm (mean=0.09±0.01) (n=2) (SP) distance from base. Thorax: dark granules under integument, seta 1,2-P on separate tubercles, 1-P with 9-15 narrow, pointed leaflets, 2-P with 11-21 branches, 2.59–4.44 (mean=3.38±0.50) (n=12) (ES), 3.60 (n=1) (SP) length of 1P; 14-P with 7-11 branches, flattened stalk and lateral branches shorter than median branches; 1-M strongly plumose, 22-35 branches; 3-T with 9-15 somewhat transparent leaflets. Abdomen: integument similar to that of thorax; seta 0-II-VII usually large; 1-I-VII palmate, 1-I with 8-16 long, narrow, both pointed and truncate leaflets; 2-II with 3-10 large branches, 2-III with 3-8 large branches, 2-IV single; 5-I with 3,4 branches, inserted on lateral margin of abdomen, 13-IV with 4-11 large branches, 13-V with 3-6 branches larger than 13-IV. Pecten plate with 2-5 long spines, 10-16 short spines, long spines 2.00–3.00 (mean=2.40±0.24) (n=12) (ES), 2.20–3.00 (mean=2.59±0.40) (n=2) (SP) length of short spines. Segment X: covered with fine spicules except anteriorly, spicules stronger posteriorly; seta 1- X as long as saddle, inserted on ventral margin of saddle.
Molecular characterization. The ITS2 region was sequenced for 12 individuals, 10 from Espírito Santo and two from São Paulo (GenBank accession numbers EF457228 View Materials – EF457239 View Materials ; ES= EF457228 View Materials -37; SP= EF457238 View Materials -9). The amplicon length was consistent at 530-bp (including primers), and the 12 sequences revealed a single haplotype. The ITS2 haplotype comprised the following bases: 20.6% T, 28.1% A, 27.2% C, and 24.1% G.
The ITS2 sequences of An. oswaldoi s.l. are available in GenBank from Brazil (Acre AF055068 View Materials , Amapá AF056318 View Materials , Amazonas AF056317 View Materials , Rond ȏ nia AF055069 View Materials ; Marrelli et al. 1999b), Colombia (Putumayo, AY679149 View Materials -55; Ruiz et al. 2005), Peru (Yurimaguas, AF055071 View Materials ; Marrelli et al. 1999b), Venezuela (Ocama, AF055070 View Materials ; Marrelli et al. 1999b) and from unlisted localities ( U92352 View Materials -3, U92344 View Materials ; Danoff-Burg & Conn direct submission). A FASTA search revealed that the ITS2 sequences of An. oswaldoi s.s. (n=12) are unique with regard to those already in GenBank. Anopheles oswaldoi s.l. from Rondônia, Brazil ( AF055069 View Materials ) shares highest sequence similarity at 97.9% ( Figure 4). Along a 486 bp alignment (CCGCGG and GGTACCC removed from 5’ and 3’ end of AF055069 View Materials , respectively), 10 bases varied, including three 2-bp indels (bases 312-3, 354-5, and 415-6) and 4 singleton polymorphic sites (260, 282, 315, 405) ( Figure 4).
Diagnosis. The Oswaldoi Group of Nyssorhynchus Blanchard mosquitoes includes 15 formally recognized species divided between two subgroups: the Oswaldoi Subgroup (12 species: An. anomalophyllus Komp , An. aquasalis Curry , An. dunhami Causey , An. evansae (Brèthes) , An. galvaoi Causey , An. ininii Senevet & Abonnenc , An. konderi Galvão & Damasceno , An. nuneztovari Gabaldón (cytotypes A and B/C, Conn et al. 1993), An. oswaldoi , An. rangeli Gabaldón, Cova Garcia & Lopez , An. sanctielii Senevet & Abonnec , and An. trinkae Faran ; and the Strodei Subgroup (3 species: An. benarrochi Gabaldón , An. rondoni (Neiva & Pinto) and An. strodei Root ) ( Harbach 2004). Six of these species are actively involved in malaria transmission, and at least four, An. aquasalis ( Conn et al. 1993; Maldonado et al. 1997), An. benarrochi ( Ruiz et al. 2005) , An. oswaldoi ( Marrelli et al. 1999b), and An. nuneztovari ( Conn et al. 1993; Sierra et al. 2004), are known to comprise complexes of morphologically indistinct species that exhibit differences in genetics, behavior and vector competence. Herein, the terminology regarding groups, subgroups and complexes follows Harbach (2004): the Oswaldoi Series (= Oswaldoi Group of Faran 1980), Oswaldoi Group (= Oswaldoi Subgroup of Faran 1980), and Oswaldoi Subgroup (= Oswaldoi Complex of Faran 1980). Herein we use the term Oswaldoi Complex to indicate a group of hitherto undifferentiated taxa close to An. oswaldoi s.s.
Adult females of the Oswaldoi Series are recognized by having palpomere 4 with the mid-lateral surface pale-scaled, abdominal segment II with posterolateral tuft of dark scales, foretarsomere 5 either entirely palescaled or with at least the apex pale-scaled and basally dark. However, in An. strodei and An. triannulatus the foretarsomere 5 is either entirely pale-scaled or with at least the apex pale-scaled and basally dark or entirely dark-scaled. Based on characters of the male genitalia, members of the Oswaldoi Series are recognized by having sternum IX large, either sub-trapezoidal or sub-triangular, the ventral claspette with or without spicules and the apex expanded forming large auricular lateral lobes. In the fourth-instar larva, seta 4-C is normally small, except in An. trinkae and An. nuneztovari where it is long, seta 1-P palmate, seta 9-P,T single, seta 13- I,III,IV varying from small to moderately large, and seta 11-I large with 5-7 branches.
Most species of the Oswaldoi Group can be recognized in the adult female by having palpomere 4 mostly pale-scaled, the absence of an anterior mesepimeral scale- patch, foretarsomere 4 without an apical pale band, except An. nuneztovari and An. ininii in which foretarsomere 5 is either entirely pale or pale and dark-scaled, and some individuals of An. strodei in which foretarsomere 5 is sometimes entirely dark. Additionally, member species possess setae on the basal lobe of the ventral claspette of the male genitalia; the fourth-instar larvae have seta 1-P palmate, usually with 16 narrow or broad branches, seta 14-P with a short or moderately long axis, seta 11-I large with 2-4 branches, seta 13-I short to large, normally triple, seta 13-III normally small with multiple branches, the lateral arm of spiracular apparatus varying from short to moderately long, except for An. ininii that has a moderately long lateral arm ( Faran 1980).
The Oswaldoi Subgroup is recognized by having the male genitalia with spicules extending toward the apex of the ventral claspette or extending to the apical margin of the preapical plate, the apex of the ventral claspette not laterally expanded, and the parabasal spines short. Based on characters of the male genitalia, Faran (1980) suggested two phyletic lines within the Oswaldoi Subgroup; one formed by An. oswaldoi , An. galvaoi , An. evansae (as An. noroestensis ), An. aquasalis , An. ininii , and possibly An. anomalophyllus , and with An. trinkae , An. nuneztovari , and An. rangeli in the other.
Anopheles oswaldoi s.l. is morphologically more similar to An. konderi , An. galvaoi , and An. ininii than to any other species of the Oswaldoi Subgroup. However, these four species can be distinguished based on male genitalia and a few adult female characters. Faran (1980) should be consulted for details to separate An. oswaldoi s.l. and An. konderi from An. galvaoi and An. ininii . Briefly, in An. oswaldoi s.s., setae of the ventral claspette along the basal margin of the basal lobules are about twice as wide as the aedeagus, the preapical plate is moderately sclerotized, and the apex of the aedeagus is longer than broad, whereas in An. galvaoi the setae of the ventral claspette along the basal margin of the basal lobules are very long, about three times the width of the aedeagus, the preapical plate is strongly sclerotized and, the apex of the aedeagus is broader than long. Anopheles ininii can be recognized by the shape of the ventral claspette, which is somewhat conic, with a very small median sulcus at the apex, and spicules that extends to the apex, whereas in An. galvaoi the ventral claspette is somewhat trapezoidal, with a well-developed median sulcus and spicules extending to the apex. Additionally, characters of the female of An. oswaldoi s.s. can be distinguished from An. ininii by having midtarsomere 4 all dark, foretarsomere 2 pale in the apical 0.20-0.45, foretarsomere 3 pale in the apical 0.50-0.85, dark basal bands on foretarsomeres 3-5 almost completely encircling each segment, foretarsomere 4 dark to least the basal third. In An. ininii , midtarsomere 4 has a pale band in the apical 0.15-0.25, foretarsomere 2 pale in the apical 0.35-0.55, foretarsomere 3 pale in the apical 0.70-0.86, foretarsomeres 3-5 predominantly cream-colored to white, foretarsomere 4 all pale to rarely more than the basal third dark.
Lounibos et al. (1997) found no differences to separate An. oswaldoi s.l. (from Coca, Napo Province and Sucumbios Province, Ecuador, Capanema, State of Pará, Brazil and Brokopondo, Suriname) and An. konderi (from Alto Linhares, Cochabamba Dept., Bolivia) using scanning electron micrographs of the egg exocorion and this, along with lack of morphological or morphometric differences in the pupal stage, was later verified by Flores-Mendoza et al. (2004b). However, they reported differences in the dark spots of foretarsomere 2 and hindtarsomere 2, and wing spots that can be used to distinguish the adult females of An. oswaldoi s.l. and An. konderi . More importantly, these two species can be easily distinguished based on the shape of the apex of aedeagus of the male, which is usually longer than broad and somewhat ovate in An. oswaldoi s.s. ( Figure 1 View FIGURE 1 A), and is broader than long and more conical in An. konderi ( Figure 1 View FIGURE 1 B). Additionally, the lateral surface of the aedeagus is curved into a small lateral projection in An. konderi ( Figure 1 View FIGURE 1 B), whereas it is straight in An. oswaldoi s.s. ( Figure 1 View FIGURE 1 A). The ventral claspette is indistinguishable in these species ( Figures 1 View FIGURE 1 C, D).
continued.
*8 pairs 2233333444 6811155011 0 223545556 OSWALDOI AA--C--A-- AF055069 View Materials TCCCTCGGGC State of Brazil References
Acre (AC) Deane et al., 1948; Natal et al., 1992; Branquinho et al., 1993; Branquinho et al., 1996; Marrelli et al., 1998; Marrelli et al., 1999a; Marrelli et al., 1999b; Scarpassa, 2005; Scarpassa & Conn, 2006
Amapá (AP) Deane et al., 1948; Steiner et al., 1982; Marrelli et al., 1999b; Póvoa et al., 2001
Amazonas (AM) Deane et al., 1948; Arruda et al., 1986; Lourenço-de-Oliveira & Luz, 1996; Marrelli et al.,
1999b; Tadei et al., 1998; Póvoa et al., 2001; Scarpassa, 2005; Scarpassa & Conn, 2006
Espírito Santo (ES) Peryassú, 1922; Marrelli et al., 1999b, herein
Goiás (GO) Naves et al., 1996; Guimarães et al., 2004
Maranhão (MA) Deane et al., 1948; Rebêlo et al., 1997
Pará (PA) Deane et al., 1948; Arruda et al., 1986; Tadei et al., 1998; Póvoa et al., 2003; Santos et al.,
2005; Silva et al., 2006; Scarpassa & Conn, 2006
Paraná (PR) Lopes & Lozovei, 1995; Lopes et al., 2002
Piauí (PI) Deane et al., 1948
Rio de Janiero (RJ) Peryassú, 1922
Rondônia (RO) Deane et al., 1948; Tadei et al., 1988; Lourenço-de-Oliveira et al., 1989; Oliveira-Ferreira et al., 1990; Klein & Lima, 1990; Klein et al., 1991a; Klein et al., 1991b; Perera et al., 1998; Tadei et al., 1998; Marrelli et al., 1999b; Marrelli et al., 1999a; Scarpassa, 2005; Scarpassa & Conn, 2006
Roraima (RR) Deane et al., 1948; Póvoa et al., 2006
São Paulo (SP) Fonseca & Fonseca, 1942; Forattini et al., 1993; Guimarães et al., 2000; herein
*The underlined references are cited for vector competence.
Adult females of An. oswaldoi s.l. can be identified by the following characters: foretarsomeres 2 and 3 pale-scaled on 0.28-0.33 and 0.52-0.84 of the apex, respectively, foretarsomere 4 dark-scaled with pale scales at the apex, midtarsomere 4 dark, pale at the apex, hindtarsomere 2 dark-scaled in less than the basal 0.25; basal lobe of the ventral claspette large, laterally expanded, with long setae on the basal margin, twice as long as the width of the aedeagus, preapical plate of the ventral claspette large, poorly sclerotized, aedeagus without apicolateral leaflets; pinna of the pupal trumpet 3.06-4.55 length of the meatus, trumpet truncate and broad at the apex, seta 9-V-VIII short, seta 6-II as long as or longer than 7-II, seta 2-I with 3-9 long branches, dendritic; in the fourth-instar larva, seta 2-C are broadly spaced, clypeal index 1.1-1.6, seta 4-C short, distance between the insertions of 4-C and 2-C equal or longer than distance between insertions of 2-C and 3-C, setae 8,9-C long, 0.17-0.21 distance between 8-C and 5-C, seta 1-X arises from the ventral margin of the saddle, and the anal papillae long, longer than segment X.
Distribution of An. oswaldoi s.s. Based on a comparison of ITS2 sequence data in GenBank (Danoff- Burg & Conn direct submissions; Marrelli et al. 1999b; Ruiz et al. 2005; Quiñones et al. 2006), An. oswaldoi s.s. has only been recorded with certainty in Espírito Santo ( Peryassú 1922; herein), Rio de Janeiro ( Peryassú 1922), and São Paulo (herein).
Anopheles oswaldoi View in CoL s.l. has been reported throughout South America east of the Andes and as far south as the northern provinces of Argentina ( Faran 1980; Faran & Linthicum 1981). It has been reported from Argentina ( Faran 1980), Bolivia ( Peyton et al. 1983), Brazil (see Table 4), Colombia ( Ruiz et al. 2005; Quiñones et al. 2006), Costa Rica ( Faran 1980), Ecuador ( San Sebastián et al. 2000), Guianas ( Rambajanl 1987; Laubach et al. 2001), Panama ( Simmons 1979), Paraguay ( Faran 1980), Peru ( Hayes et al. 1987; Flores-Mendoza et al. 2004a), Suriname ( Lounibos & Conn 2000), Trinidad (Rozenboom 1942; Chadee & Beier 1996), and Venezuela ( Rubio-Palis & Curtis 1992; Grillet 2000).
Bionomics of An. oswaldoi View in CoL s.s. Due to the restricted knowledge of the true distribution of An. oswaldoi View in CoL s.s. ( Peryassú 1922; herein), little is known of the behavior or vector competence of this specie. In this study, An. oswaldoi View in CoL s.s. was collected in a well-preserved forested area in the Vale do Rio Doce, Espírito Santo, Brazil, using a Shannon trap with both light and human attractants, between 18:00-21:00h and accepted a blood meal at 02:00h. As only two females were collected, it is not possible infer species behavior from our data.
Immature stages of An. oswaldoi have been collected from permanent or temporary freshwater habitats situated in the interior or at the edges of tropical forests ( Faran 1980). In the most detailed study of larval habitats of An. oswaldoi to date, Grillet (2000) reported that An. oswaldoi was relatively rare, but was the most abundant anopheline at the end of the dry season in Sucre State, northeastern Venezuela. Larvae were found in permanent freshwater (ponds, canals, swamp forests and clear-cut marsh forests) and both the occurrence and abundance of An. oswaldoi was closely correlated with high levels of dissolved oxygen. In coastal brackish marshes in Venezuela, Berti et al. (1993) found An. oswaldoi larvae in the rainy season when salinity was low.
According to most authors, An. oswaldoi is typically exophilic and zoophilic ( Deane et al. 1948; Faran 1980; Consoli & Lourenço-de-Oliveira 1994; Lourenço-de-Oliveira & Luz 1996), but there have also been reports of females being captured biting humans, both indoors and out ( Rubio-Palis & Curtis 1992; Branquinho et al. 1996; Quiñones et al. 2006), and reports of involvement in malaria transmission indicate bionomic plasticity. In Brazil, An. oswaldoi has been collected inside primary tropical forest in Balbina, State of Amazonas (Lourenço-de-Oliveira & Luz 1996), in a forested region near hydroelectric dams in Serra da Mesa, State of Goias ( Guimarães et al. 2004), and in a well-preserved forested area in the Vale do Rio Doce, State of Espírito Santo (herein). In contrast, a study in western Venezuela reported that 42% of 1,000 An. oswaldoi were captured on human bait stationed inside an experimental hut ( Rubio-Palis & Curtis 1992), whereas in the Purus River Basin, State of Acre, Brazil, An. oswaldoi was collected in both open areas and in the peri-domiciliary environment ( Natal et al. 1992). In Colombia, An. oswaldoi was reported resting indoors, with a preferred resting height of> 1.5m on walls ( Quiñones & Suarez 1990). Klein & Lima (1990) suggested that An. konderi is often mistaken for An. oswaldoi , proposing that An. konderi is present in human impacted environments, whereas An. oswaldoi is restricted to forested areas. Peak biting activity has been reported between 18:00-19:00h ( Deane et al. 1948), and between 18:00-20:00h in Rondônia, Brazil, ceasing after 21:00h ( Tadei et al. 1998). However, in intra-domiciliary environments in Venezuela, the species continues to bite until around midnight ( Rubio-Palis & Curtis 1992; Rubio-Palis et al. 1994).
Vector competence of An. oswaldoi s.s. No natural infectivity studies appear to have been carried out in Espírito Santo, thus the role of An. oswaldoi s.s. in malaria transmission remains unknown. Populations of An. oswaldoi s.l. from the State of São Paulo have been shown to be susceptible to infection with P. vivax and P. falciparum in the laboratory ( Fonseca & Fonseca 1942) but laboratory susceptibility does not necessarily indicate that the species is a malaria vector under natural conditions. Sequence data generated in our laboratory suggests that there is another closely related species in sympatry with An. oswaldoi s.s. in São Paulo. Whether this species belongs to the Oswaldoi Complex or is a closely related species, e.g. An. ininii or An. galvaoi , remains to be determined.
Whereas An. oswaldoi View in CoL seems to be involved in the dynamics of malaria transmission in South America, its perceived importance varies geographically. Although the females have been found naturally infected in Colombia ( Quiñones et al. 2006), Peru ( Hayes et al. 1987) and Venezuela ( Rubio-Palis & Curtis 1992), it is not regarded to be a major vector in these countries due to its low densities. Many studies have detected natural Plasmodium infections in An. oswaldoi View in CoL in Brazil ( Table 4), yet it is regarded as a primary vector in some regions, and a secondary or unimportant vector in others. In the Brazilian State of Acre, An. oswaldoi View in CoL is found indoors and in peri-domiciliary areas where it is reportedly the most anthropophilic species and acts as an efficient vector ( Branquinho et al. 1993; Branquinho et al. 1996; Marrelli et al. 1999a). More than 7% (190/2610) of specimens tested by ELISA were positive: 3.41% for P. falciparum , 2.26% for P. vivax VK210, 1.22% for P. vivax VK247, and 0.42% for P. m a l a r i a e ( Branquinho et al. 1993). In a later study in the same area, 2.9% of specimens (1/34) were found positive by dissection of guts and salivary glands ( Branquinho et al. 1996). In the State of Pará, Arruda et al. (1986) assayed 442 An. oswaldoi View in CoL (of 962 collected) and reported 10 ELISA positive for P. falciparum . Later specimens from the same population were found to be infected by P. vivax by Immuno Radio Metric Assay (IRMA) ( Oliveira-Ferreira et al. 1990). ELISA tests on the salivary glands of 417 Anopheles View in CoL from the State of Pará showed two specimens of An. oswaldoi View in CoL to be infected, one with P. v i v a x VK247 and the other with P. m a l a r i a e ( Santos et al. 2005). In Costa Marques (State of Rondônia, Brazil), Klein et al. (1991a, b) reported low natural infection rates with P. v i v a x and low numbers of sporozoites in the salivary glands of wild-caught An. oswaldoi View in CoL . Under laboratory conditions, it was shown that populations of An. oswaldoi View in CoL from Trinidad were susceptible to infection with P. vivax ( Rozeboom 1942) and those from São Paulo, Brazil could be infected with P. v i v a x and P. falciparum ( Fonseca & Fonseca 1942) . In a monkey malaria area in the Amazon, An. oswaldoi View in CoL was found at both canopy and ground levels, and circumstantially incriminated as a potential vector of P. brasilianum to monkeys (Lourenço-de-Oliveira & Luz 1996).
Systematics. Anopheles oswaldoi View in CoL is considered a species complex based on differences in DNA sequences, bionomics, and vector competence. Herein, the nominotypical member of the complex, An. oswaldoi View in CoL s.s., is redescribed using morphology and DNA sequence data to fix its identity and form a solid taxonomic foundation from which to describe all other members of the Oswaldoi View in CoL Complex. A lectotype is designated herein from Peryassú’s original series of syntypes. The internal systematics of the Oswaldoi View in CoL Complex in South America is currently being studied in our laboratory and the results will be published elsewhere.
Material examined. TYPE SPECIMENS. LECTOTYPE: adult male with dissected male genitalia mounted on a microscope slide, BRAZIL, State of Espírito Santo, Vale do Rio Doce, AG Peryassú coll., 4-XI- 1922. PARALECTOTYPES: two females, same collection data as the lectotype; one female, State of Rio de Janeiro, Magé, AG Peryassú coll. The lectotype and two paralectotypes from Espírito Santo reside in the same glass vial, in which there is a paper label and the number 13.206 on the cork in the top of the vial. The specimen collected in Rio de Janeiro, Magé, is placed in a separate vial. The lectotype and three paralectotypes are deposited in Museu Nacional do Rio de Janeiro, Brazil ( MNRJ); all are in poor condition.
Non-type specimens. BRAZIL, State of Espírito Santo, Jaguaré municipality, Fazenda Marianelli, Lagoa do Macuco (19º02'5.36''S 39º56'54.54''W), Natal et al., coll., 18-I-2006, progeny broods from females collected landing on human bait, ES08(11)-1 MG [E-12903 at FSP/ USP]; ES08(11)-2 FLePe [E-12884 at FSP/ USP]; ES08(11)-3 MLePe [E-12885 at FSP/ USP]; ES08(11)-4 MLePe [E-12886 at FSP/ USP]; ES08(11)-5 MLePe [E-12887 at FSP/ USP]; ES08(11)-6 MLePe [E-12888 at FSP/ USP]; ES08(11)-7 LePe [E-12889 at FSP/ USP]; ES08(11)-8 LePe [E-12890 at FSP/ USP]; ES08(11)-9 MGLePe [E-12904 at FSP/ USP]; ES08(11)- 10 FLe [E-12891 at FSP/ USP]; ES08(11)-11 FLePe [E-12892 at FSP/ USP]; ES08(11)-12 F [E-12905 at FSP/ USP]; ES08(11)-13 MGLePe [E-12907 at FSP/ USP]; ES08(11)-14 FLePe [E-12894 at FSP/ USP]; ES08(20)- 1 LePe [E-12895 at FSP/ USP]; ES08(20)-2 Le [E-12896 at FSP/ USP]; ES08(20)-10 Pe [E-12897 at FSP/ USP]; ES08(20)-11 LePe [E-12906 at FSP/ USP]; ES08(20)-13 FLePe [E-12898 at FSP/ USP]; ES08(20)-14 MLePe [E-12899 at FSP/ USP]; ES08(20)-15 LePe [E-12893 at FSP/ USP]; ES08(20)-16 MLePe [E-12900 at FSP/ USP]; ES08(20)-17 FLePe [E-12901 at FSP/ USP]; ES08(20)-18 FLePe [E-12902 at FSP/ USP]; ES08(20)-19 MLePe [E-12883 at FSP/ USP]; ES08(20) Le [E-12908 at FSP/ USP]; Linhares municipality, Reserva Florestal de Sooretama (19º13'00''S, 40º08'00''W), C Flores-Mendoza & CB Santos coll., IV-1996, progenies from females collected on animal bait, 1M, 2F, 1MLePe, 2FLePe, 1MG, 1FG. State of São Paulo, Pariquera-Açu, P. Mirim, AC Gomes coll., 22-II-1988, E-8351FPe [at FSP/ USP]; E-8352MGLePe [at FSP/ USP]; E-8353FLePe [at FSP/ USP]; State of São Paulo, Pariquera-Açú, Experimental Station, OP Forattini coll., Shannon trap, 25-II-1992, E-9461F [at FSP/ USP]; State of São Paulo, Iguape, Pq. Fontes, Sucen SR2 coll., 5-IV-1994, human landing, E-11020F [at FSP/ USP]; E-11021F [at FSP/ USP]. State of São Paulo, Pariquera-Açu municipality, Pariquera-Mirim district (24° 43' 60S 47° 49' 0W), Sallum coll.,SP03-4F; SP03- 5F. Newly collected specimens from Espírito Santo used in this study are deposited in the Entomological Collections of the Faculdade de Saúde Pública, Universidade de São Paulo, Brazil.
TABLE 1. Wing spot measurements (in mm) for adult male and female of Anopheles oswaldoi collected in Jaguaré municipality, Vale do Rio Doce, State of Espírito Santo, Brazil (ES) and Vale do Ribeira, Mata Atlântica, southern State of São Paulo, Brazil.
| Basal pale + prehumeral pale | 0.19-0.25 0.14-0.26 | 0.22 0.21 | 0.02 0.04 | 4 5 |
|---|---|---|---|---|
| Prehumeral dark Humeral pale | 0.05-0.11 0.06-0.12 0.21-0.25 0.18-0.27 | 0.08 0.08 0.24 0.23 | 0.03 0.03 0.02 0.04 | 4 5 4 5 |
| Humeral dark | 0.09-0.12 0.06-0.14 | 0.10 0.12 | 0.01 0.03 | 4 5 |
| Presector pale Presector dark | 0.10-0.13 0.11-0.17 0.38-0.49 0.36-0.46 | 0.10 0.12 0.44 0.39 | 0.01 0.02 0.05 0.04 | 4 5 4 3 |
| Sector pale | 0.06 0.08-0.28 | - 0.15 | - 0.11 | 1 3 |
| Proximal sector pale Accessory sector pale | 0.09 0.09-0.10 0.10 0.13 | - 0.09 - 0.13 | - 0.01 - - | 1 2 1 2 |
| Distal sector dark | 0.75-0.84 0.71-0.85 | 0.79 0.78 | 0.04 0.07 | 4 5 |
| Subcostal pale Preapical dark | 0.13-0.24 0.21-0.36 0.73-0.82 0.69-0.83 | 0.17 0.30 0.78 0.75 | 0.05 0.06 0.04 0.06 | 4 5 4 5 |
| Preapical pale | 0.24-0.27 0.22-0.35 | 0.25 0.75 | 0.01 0.06 | 4 5 |
| Apical dark | 0.12-0.13 0.09-0.13 | 0.12 0.11 | 0.01 0.01 | 4 5 |
| continued. |
TABLE 2. Number and range (mode) of setal branches of the pupa of Anopheles oswaldoi. (n = 17, ES; n = 3, SP) (n. c. = not counted).
| Seta | Cephalothorax | Abdominal Segments | Paddle | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | CT | I | II | III | IV V VI | VII | VIII | IX | P |
| 0 | - | - | 3–8 (5) | 4–9 (6) | 4–9 (6) 3–7 (4) 3–5 (4) | 2–5 (4) | 1–2 (1) | - | - |
| 1 | 1–3 (2) | n.c. | 5–15 (8) | 4–10 (6) | 1 1 1 | 1 | - | 1 | 1 |
| 2 | 1–3 (2) | 3–9 (5) | 4–12 (5) | 4–8 (4) | 1–4 (2) 1–4 (2) 1–3 (2) | 1–3 (2) | - | - | 1–4 (2) |
| 3 | 2–4 (3) | 1 | 1 | 1 | 2–8 (6) 1–4 (3) 1–3 (2) | 2–5 (3) | - | - | - |
| 4 | 1–5 (3) | 3–7 (5) | 1–6 (4) | 1–7 (2) | 1–4 (1) 1–4 (2) 1–3 (1) | 1–3 (2) | 1–5 (3) | - | - |
| 5 | 1–3 (2) | 1–4 (2) | 2–7 (4) | 3–8 (6) | 1–4 (2) 1–2 (1) 1–2 (1) | 1 | - | - | - |
| 6 | 1–4 (1) | 1–2 (2) | 1–4 (2) | 1–4 (1) | 1–2 (1) 1–2 (1) 1–2 (1) | 1–3 (1) | - | - | - |
| 7 | 1–3 (2) | 2–5 (3) | 2–5 (3) | 1–4 (2) | 1–5 (2) 1–3 (2) 1 | 1 | - | - | - |
| 8 | 1 | - | - | 1–5 (3) | 1–4 (2) 1–3 (2) 1–3 (2) | 2–5 (3) | - | - | - |
| 9 | 1–3 (2) | 1–2 (1) | 1 | 1 | 1 1 1 | 1 | 1 | - | - |
| 10 | 1–2 (1) | - | 1 | 1–3 (1) | 1 1 - | 1–2 (1) | - | - | - |
| 11 | 2–7 (3) | - | - | 1–4 (1) | 1–3 (1) 1–3 (1) 1–2 (1) | 1–2 (1) | - | - | - |
| 12 | 1–4 (2) | - | - | - | - - - | - | - | - | - |
| 13 | - | - | - | - | - - - | - | - | - | - |
| 14 | - | - | - | 1 | 1 1 1 | 1 | 1 | - | - |
TABLE 3. Number and range (mode) of setal branches of the fourth-instar larva of Anopheles oswaldoi (n = 18, ES; n = 2, SP) (n. c. = not counted).
| Head | Thorax | ||
|---|---|---|---|
| No C | P | M | T |
| 0 1 | n. c. | - | - |
| 1 1 | 9–15 (10) | 22–35 (27) | 1 |
| 2 9–15 (12) | 11–21 (16) | 1–4 (1) | 1–2 (1) |
| 3 9–14 (10) | 1 | 1 | 9–15 (10) |
| 4 2–8 (5) | 14–20 (19) | 2–5 (3) | 1–4 (3) |
| 5 13–19 (15) | 26–37 (29) | 1 | 28–37 (32) |
| 6 10–18 (14) | 1 | 2–3 (2) | 1–3 (2) |
| 7 13–23 (17) | 21–35 (31) | 2–4 (3) | 24–38 (29) |
| 8 4–7 (5) | 23–33 (30) | 20–29 (23) | 25–35 (30) |
| 9 3–8 (6) | 1 | 1 | 1 |
| 10 1–3 (3) | 1 | 1 | 1 |
| 11 n.c. | 1–3 (2) | 1–2 (1) | n. c. |
| 12 2–6 (4) | 1 | 1 | 1–2 (2) |
| 13 2–6 (4) | 3–6 (5) | 3–9 (5) | 2–3 (2) |
| 14 1–3 (2) | 7–11 (8) | 6–13 (9) | - |
| 15 2–5 (3) | - | - | - |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Anopheles (Nyssorhynchus) oswaldoi ( Peryassú, 1922 )
| Motoki, Maysa Tiemi, Linton, Yvonne-Marie, Ruiz, Freddy, Flores-Mendoza, Carmen & Sallum, Maria Anice Mureb 2007 |
Anopheles
| Lounibos 1997: 136 |
| Faran 1980: 55 |
| Belkin 1971: 6 |
Anopheles
| Senevet 1938: 487 |
| Curry 1932: 566 |
Cellia oswaldoi Peryassú, 1922 : 179
| Peryassu 1922: 179 |