Quadrivisio laleyei, Gnohossou & Piscart & Umr, 2019
|
publication ID |
https://doi.org/ 10.5852/ejt.2019.533 |
|
publication LSID |
lsid:zoobank.org:pub:E98D3A33-49A7-45A8-94BC-F2AC96A7622B |
|
persistent identifier |
https://treatment.plazi.org/id/AE890355-182D-4473-ABF2-A90DFE1E1208 |
|
taxon LSID |
lsid:zoobank.org:act:AE890355-182D-4473-ABF2-A90DFE1E1208 |
|
treatment provided by |
Plazi |
|
scientific name |
Quadrivisio laleyei |
| status |
sp. nov. |
Quadrivisio laleyei sp. nov.
urn:lsid:zoobank.org:act:AE890355-182D-4473-ABF2-A90DFE1E1208
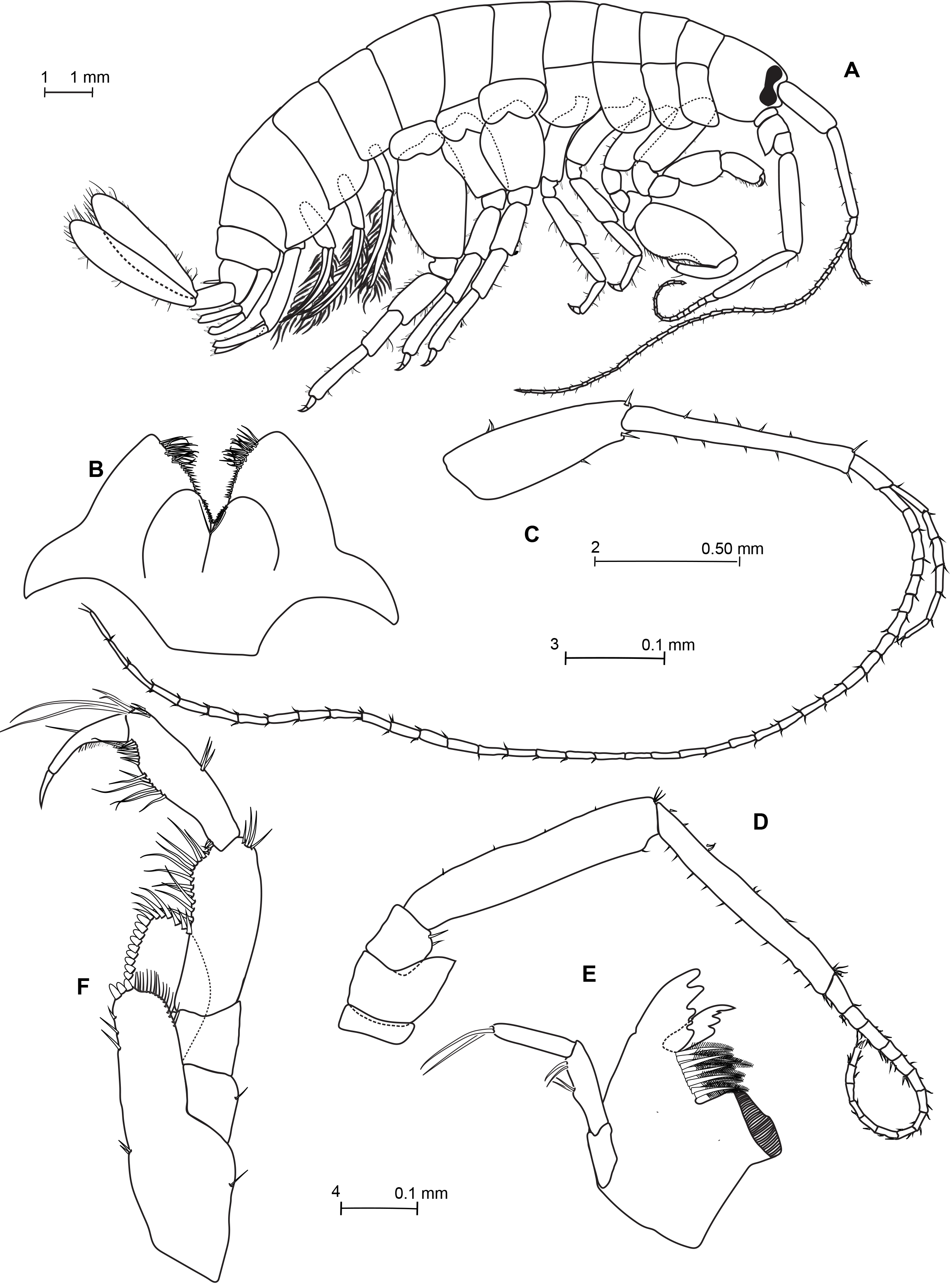
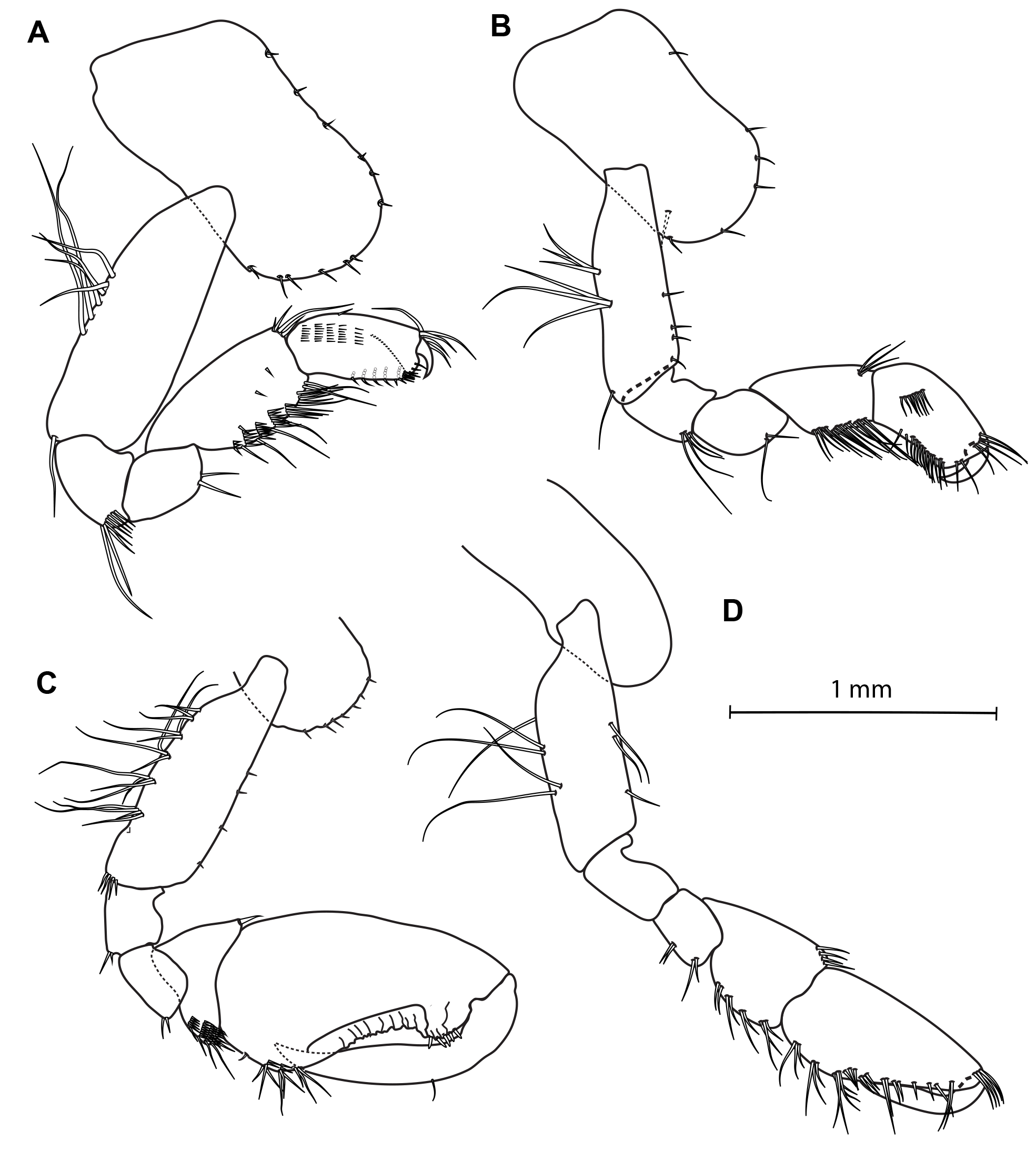
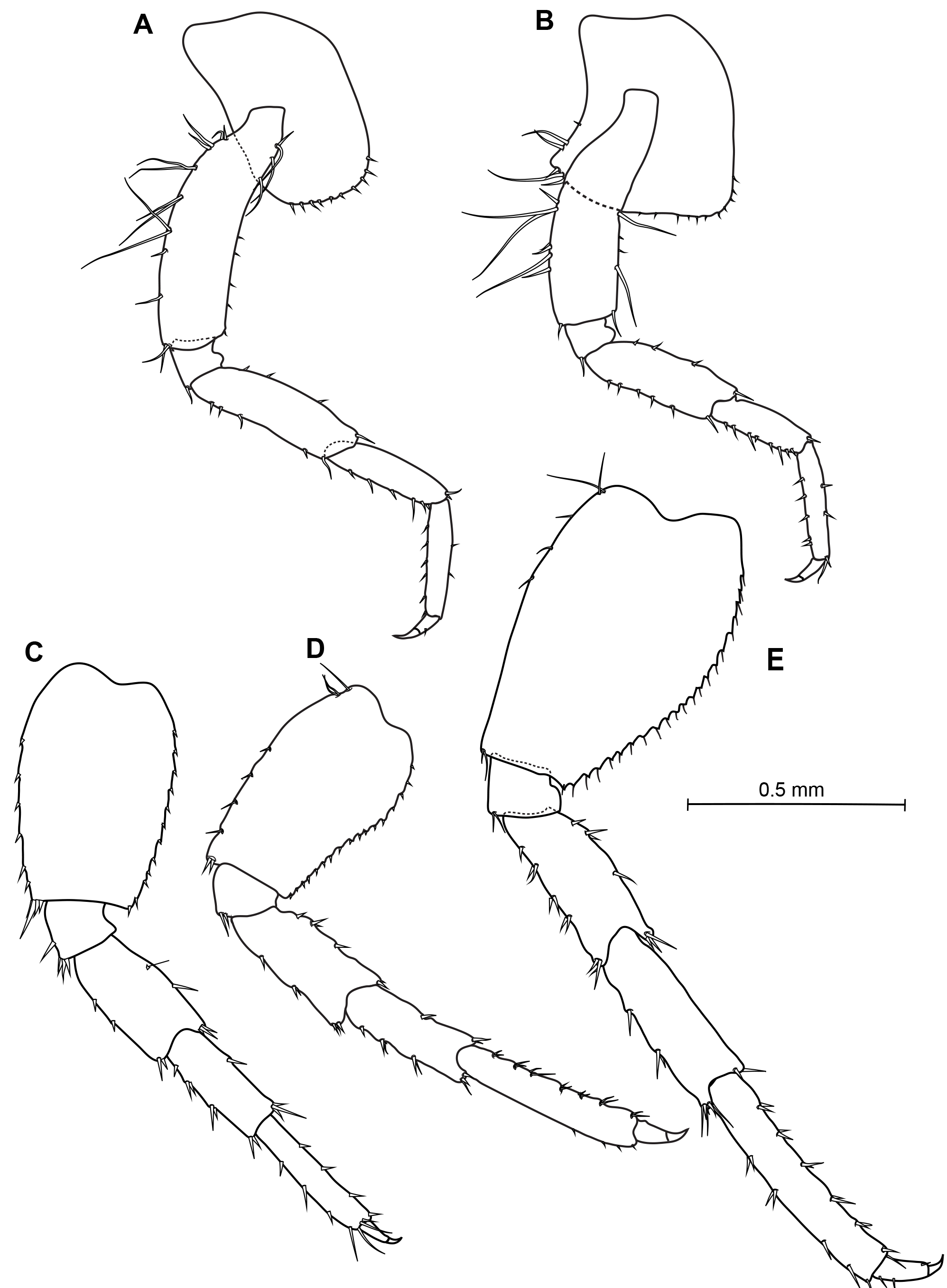
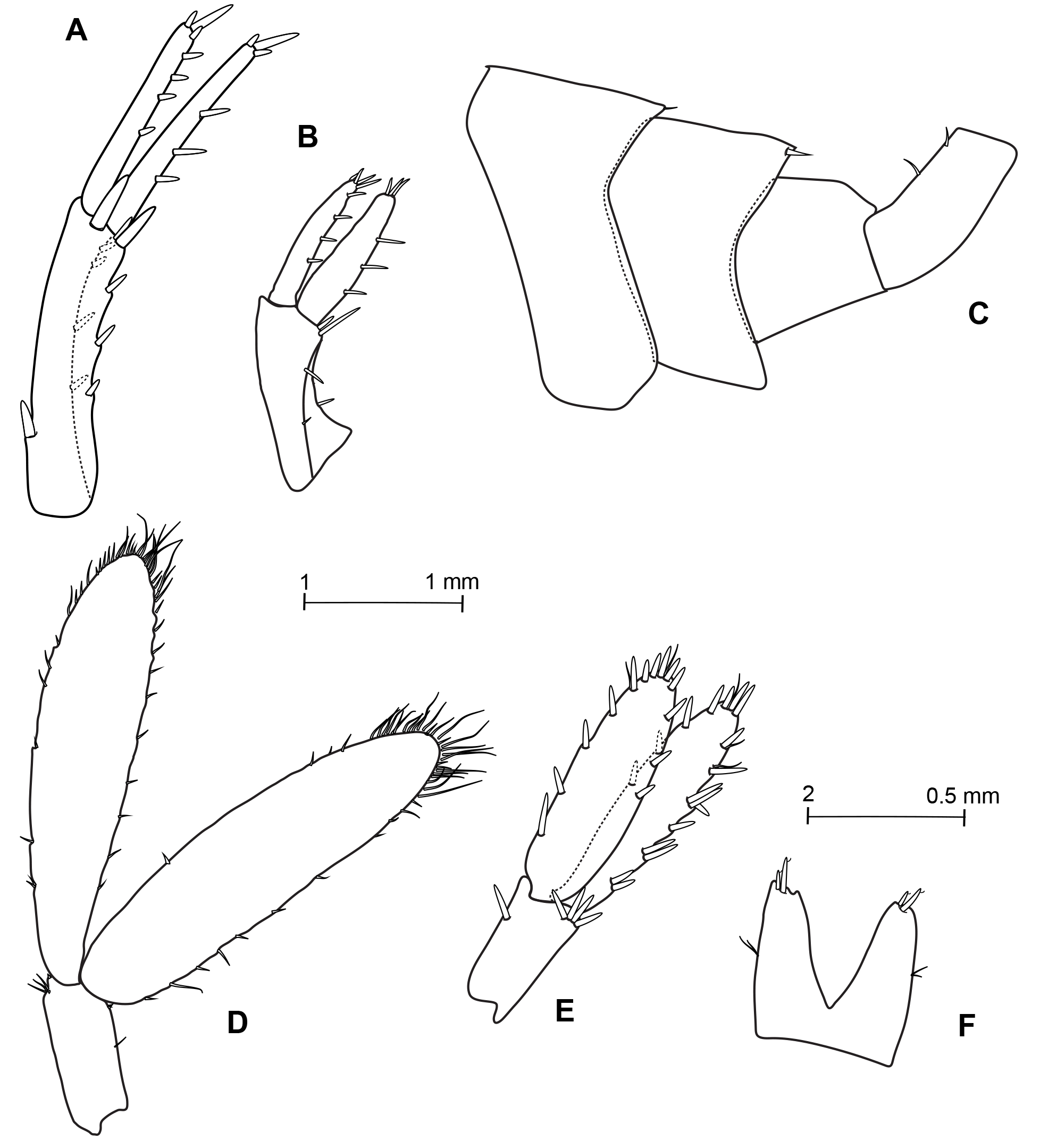
Figs 2–5 View Fig View Fig View Fig View Fig , Tables 1–2 View Table 1 View Table 2
Diagnosis
The new species belongs to the genus Quadrivisio , recognized by the shape of their eyes for which ommatidia are almost split into two pairs on each side of head, antenna 1 with a very long flagellum, propodus of male gnathopod 2 very large, uropod 3 with rami foliaceous. Accessory flagellum of antenna 1 with 4–6 articles. Article 3 of mandibular palp slightly shorter than article 2 and armed with two setae. Propodus of male gnathopod 1 almost rectangular. Gnathopod 2 sexually dimorphic. Epimeral plates 2–3 with a small tooth at posterodistal angle, but lacking supernumerary teeth on posterior margin. Pleonite and urosomites smooth. Urosomite 2 with posterodorsal robust setae (one on each side). Telson without spines or setae along inner margin of each lobe.
Etymology
The epithet laleyei refers to the name of the Professor Philippe A. Lalèyè who initiated the studies of macrofauna from lagoons in Benin. The name is a noun in the genitive case.
Material examined
Holotype
BENIN • ♂ (7.5 mm); Porto Novo city, Porto-Novo lagoon, 20–30 cm bottom depth, Kessounou station, muddy sediment; 06°28′05.5″ N, 02°36′56.2″ E; water temperature: 28° C; water salinity: 4 g. L- 1; Jun. 2007; collected with live macrophytes ( Paspalum vaginatum Sw. ), uprooted by hand; MNHN IU- 2017- 209. GoogleMaps
Paratypes
BENIN • 5 ♂♂; same data as for holotype; MNHN IU- 2017-210 GoogleMaps • 5 ♀♀; same data as for holotype; MNHN IU- 2017-211 GoogleMaps .
Other material examined
BENIN •Around 50 specimens; Porto Novo city, Porto-Novo lagoon , 20–30 cm bottom depth, Kessounou station , muddy sediment; 06°28′05.5″ N, 02°36′56.2″ E; water temperature: 28°C; water salinity: 4 g. L- 1; Jun. 2007; collected with live macrophytes ( Paspalum vaginatum Sw. ), uprooted by hand. GoogleMaps
Description
Male
CEPHALON. Lateral cephalic lobes broad, rounded, with anteroventral notch, anteroventral corner rounded. Eyes constricted in center, never divided into two parts ( Fig. 2A View Fig ). Antenna 1 longer and more slender than antenna 2, sparsely setose ( Fig. 2C View Fig ); peduncle much shorter than main flagellum, article 1 a shorter than article 2, article 3 very short (0.2 × length of the article 2); main flagellum with up to 38 articles unequal in length and in width; accessory flagellum well developed composed of six articles. Antenna 2 peduncle longer than the flagellum, article 4 and 5 similar in length ( Fig. 2D View Fig ); glandular cone on the peduncular article 2 prominent, reaching to end of peduncular article 3; flagellum with up to 15 articles. Lower lip with weakly-developed inner lobes, more pubescent outer lobes; mandibular lobes rather short and rounding ( Fig. 2B View Fig ). Mandibles asymmetrical. Left mandible incisor process dentate, with four chitinized teeth ( Fig. 2E View Fig ). Lacinia mobilis dentate, with four chitinized teeth, setal row with 6 plumose setae, molar process well developed and columnar, triturative. Palp article 1 shorter than article 2, article 2 slightly longer than article 3 with two setae on outer margin and two distal setae, article 3 rectolinear with two long terminal setae slightly longer than the article. Maxilla 1 and 2 do not present specific characteristics (not drawn). Maxilla 1 inner plate setose along entire inner margin and bearing a submarginal row of setae, outer plate bearing seven stout curved serrate setae, palp with distal article longer than proximal one, distal article with five terminal short stout setae and three subdistal slender setae on distal article. Maxilla 2 inner margin of inner plate of with double row of setae, unequal in length, and third oblique one on posterior face; outer plate with double rows of terminal and subterminal setae; inner and outer plates of maxilliped rather short ( Fig. 2F View Fig ); inner plate reaching to about middle of outer plate and bearing three terminal robust setae; outer plate reaching to about middle length of palp article 2, the ensemble of the distal margin and the distal part of the outer margin bear seven slender setae; palp article 3 slightly shorter than article 2, article 4 shorter than article 3 with a row of fine short setules on inner margin.
THORAX. Coxae 1–4 equal in depth and deeper than coxa 5. Coxa 1 anteroventral corner not produced forward, lower margin evenly rounded with few small setae and bearing a weak notch horned with one seta at hind corner. Coxae 2–3 lower margin evenly rounded with few small setae, with notch at hind corner; a seta is inserted in that notch. Coxa 4 posteriorly excavate so as to fit the front margin of coxa 5. Coxae 5–7 bilobed. Coxa 7 smallest. Coxal gills simples on each pereopod. Gnathopod 1 more slender than gnathopod 2, subchelate ( Fig. 3A View Fig ); basis subequal in length to the depth of coxa 1; carpus subequal to propodus, with hind margin furnished with transverse rows of setae; propodus subrectangular, slightly narrowing distally, palm nearly transverse, very convex and undefined; dactylus curved to fit palm, very stout at base but attenuated toward apex. Gnathopod 2 robust, subchelate; basis shorter than propodus ( Fig. 3C View Fig ); merus with quadrate posterodistal corner bearing two short setae; carpus compressed; propodus strong and stout, widest proximally, proximal hind margin with transverse group of setae, palm very oblique, defined proximally by a tuft of small and long setae and distally by a rounded process adjacent to dactylus hinge, bearing at least four stout short setae; dactylus stout, slightly curved proximally, apically subacute. Pereopods 3–4 subequal in length and morphologically similar ( Fig. 4A, B View Fig ), weakly setose, shorter than subsequent pereopods; basis not expanded. Pereopods 5–7 increasing length from P5 to P7; basis expanded with posterior margin convex and minutely serrate, with short setae, posterodistal corner subquadrate; merus, carpus and propodus armed with spines only ( Fig. 4C View Fig , D–E).
ABDOMEN. Epimeron 1 posterior and ventral margins smooth. Epimera 2–3 with a small tooth at posterodistal angle. Urosome posterodorsal margins smooth. Urosomite 1 with 1–2 short dorsolateral setules ( Fig. 5C View Fig ). Urosomite 2 with a dorsolateral robust setae (one on each side). Uropod 1 peduncle with a basofacial seta and a robust apical seta between rami, longer than rami, of which the inner is slightly the longer ( Fig. 5A View Fig ). Uropod 2 the shortest uropod; peduncle slightly longer than inner ramus; inner ramus slightly longer than outer ramus ( Fig. 5B View Fig ). Uropod 3 peduncle much shorter than rami (around ⅓ and ¼ of rami), reaching to end of inner ramus of uropod 2, rami subequal in length, broad, flat, edged with few short setae, terminally rounding and adorned with longer setae ( Fig. 5D View Fig ). Telson slightly shorter than uropod 3 peduncle, almost cleft to base, lobes widely divergent, apex truncated, with 2–3 short stout flagellated spines, outer margins with two sensorial setules, inner margins without setae ( Fig. 5F View Fig ).
Female
ABDOMEN. Gnathopod 2 sexually dimorphic ( Fig. 3C, D View Fig ); propodus comparatively much smaller; palm evenly and slightly convex, without either the protuberance near the hinge or the membranous cushion. Uropod 3 proportionally much shorter than males but much more spinose ( Fig. 5D, E View Fig ). Oostegites narrow and present from gnathopods to pereopod 6.
Differential diagnosis
The genus Quadrivisio currently contains eight species: Q. aviceps (K.H. Barnard, 1940) ; Q. bengalensis Stebbing, 1907 ; Q. bousfieldi Karaman & Barnard, 1979 ; Q. chevreuxi Gordon & Monod, 1968 ; Q. lobata Asari, 1983 ; Q. lutzi ( Shoemaker, 1933) ; Q. meufong Hughes & Kaji, 2016 and Q. sarina Lowry & Springthorpe, 2005 .
Quadrivisio lalaeyei differs from most of the known species by several characteristics (see updated key of the genus after Hughes & Kaji 2016): the lack of carina on pleonites or urosome; the setation of the mandibular palp ( Q. bousfieldi ) and telson ( Q. bengalensis , Q. lobata and Q. sarina ); the shape of pereopod 7 ( Q. sarina ), by the number of article on the accessory flagellum of antenna 1 ( Q. aviceps , Q. meufong ). Quadrivisio lalaeyei closely resembles to Q. lutzi but differs by several characters such as the lack of carina on urosomite 2 in females or juveniles, the presence of the dorsolateral robust setae on urosomite 2, the shape of the eyes, the article 2 of the mandibular palp slightly longer than the third.
Identification key to known species of Quadrivisio (updated after Hughes & Kaji 2016)
1. Carina absent on pleonites or urosome in males and females .......................................................... 2 – Carina present on pleonites or urosome in males and/or females .................................................... 6
2. Male gnathopod 2 propodus palm without posterodistal shelf ........................................................... ................................................................................................ Q. chevreuxi Gordon & Monod, 1968
– Male gnathopod 2 propodus palm with posterodistal shelf .............................................................. 3
3. Male gnathopod 2 dactylus short, not reaching end of palm .......... Q. aviceps (K.H. Barnard, 1940) – Male gnathopod 2 dactylus subequal to palm length ........................................................................ 4
4. Urosome and telson covered in dense setae; male gnathopod 2 propodus anterior margin produced into lobe ............................................................................................................. Q. lobata Asari, 1983
– Urosome and telson without dense setae; male gnathopod 2 anterior margin not produced............ 5
5. Posterior margin of pereopod 7 basis regularly convex, telson with robust setae along inner margins of each lobe .......................................................................... Q. sarina Lowry & Springthorpe, 2005
– Posterior margin of pereopod 7 basis not convex, telson without robust setae along inner margins of each lobe ................................................................................................................. Q. laleyei sp. nov.
6. Telson with robust setae along inner margins of each lobe ............... Q. bengalensis Stebbing, 1907 – Telson without setae along inner margin of each lobe ...................................................................... 7
7. Epimeron 3 margins smooth.................................................................... Q. lutzi ( Shoemaker, 1933) – Epimeron 3 margins with several teeth ............................................................................................. 8
8. Accessory flagellum with 7 articles; mandibular palp articles 2–3 without setae .............................. ............................................................................................. Q. bousfieldi Karaman & Barnard, 1979
– Accessory flagellum with 9–10 articles; mandibular palp articles 2–3 with setae ............................. ....................................................................................................... Q. meufong Hughes & Haji, 2016
Ecology and distribution
In the Nokoué lagoon, Q. laleyei was sampled periodically at all five sampling sites visited during this study, down to a water depth of at least 1.8 m ( Tables 1–2 View Table 1 View Table 2 ). Its potential habitat probably extends to the entire lagoon, with a preference for sandy bottoms located near to the entrance of the northern Ouémé and Sô rivers. The levels of abundance observed were highly variable, and associated with the sampling methods ( Table 2 View Table 2 ). The basket method showed the greatest sampling efficiency, principally when collectors were filled with local sediment (see Gnohossou 2006), demonstrating the close relationship of the species to the substratum (endo-/epibenthic behaviour). Its highest abundance (133 ind. m-2) was registered with this method at station Embouchure during the flood event of September 2002 (salinity: 0.0 g. L-1; oxygen concentration: 3.8 mg. L-1; depth: 1.8 m; sandy bottom). The few specimens collected with bundles (immersed 30 cm below the lagoon surface) suggest this benthic species can also swim in the water column searching for food and new favourable habitats. Within the lagoon, this euryhaline amphipod was found to live in a wide range of salinities (between 0 and 22 g. L-1), disappearing in southern high salinity areas during the low-water season. Furthermore, it shows some tolerance for pollution, as demonstrated by its presence in areas experiencing low oxygen concentration in the water column (down to 2.5 mg. L-1 at station Ganvié in September 2002). It was also observed to have high abundance in the Ahémé and Porto-Novo lagoons during the flooding period (samples collected with Surber net and the macrophytes method).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |