Cylapini sensu Gorczyca (2000)
|
publication ID |
https://doi.org/10.11646/zootaxa.5074.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:7B3C6765-F0D2-4846-BB95-200258ECC0E1 |
|
DOI |
https://doi.org/10.5281/zenodo.5784429 |
|
persistent identifier |
https://treatment.plazi.org/id/039587FB-AE75-FF90-FF51-15F9437CFEFC |
|
treatment provided by |
Plazi (2021-12-06 08:45:40, last updated 2024-11-28 20:55:07) |
|
scientific name |
Cylapini sensu Gorczyca (2000) |
| status |
|
Tribe Cylapini sensu Gorczyca (2000)
Diagnosis. For details see Gorczyca (2000) and Wolski (2017).
Remarks. The current phylogenetic analysis rendered the tribe Cylapini paraphyletic with respect to the vanniines. The tribe Vanniini , as currently understood, is composed of the Vannius complex sensu Cassis et al. (2003) and the genus Palaucoris Carvalho ( Cassis & Schuh 2012; Namyatova et al. 2016). Distant (1883) first noticed the similarity between Vannius and Valdasus , which are currently classified in the tribes Vanniini and Cylapini , respectively. The taxa of Cylapini and Vanniini were united in one tribe, the Cylapini , by Poppius (1909), based on the long antenna and by Carvalho (1952a, 1955, 1957) based on the vertical head. Gorczyca (1997) excluded the Vannius complex from Cylapinae and moved it to the controversial subfamily Palaucorinae (with a single genus Palaucoris Carvalho ) based mainly on the flattened parempodia found in both taxa and he erected two tribes: the Vanniini (for Vannius complex) and Palaucorini (for Palaucoris ). Cassis et al. (2003) refuted this concept, and the Vannius complex was assigned again to Cylapinae as incertae sedis. Consequently, Gorczyca (2006a) treated these genera again within Cylapinae but placed them among Cylapini . The Vannius complex was given a tribal status within Cylapinae by Cassis & Schuh (2012). The present analysis, showing vanniines nested well within Cylapini , provides a strong argument for treatment of vanniines within Cylapini , corroborating the classification of Gorczyca (2006a). On the other hand, given the uncertainties concerning the results of the current analysis (see discussion above) I believe it is premature to relegate the vanniines to Cylapini , and I suggest retaining the concept of Cylapini in its current composition pending studies with larger sampling and emphasis on the molecular data.
Notes on the morphological characters in Cylapini . The Cylapini in its present concept includes the genera listed in the Tab. 2 View TABLE 2 . Herein, I present the discussion of the main characters found in Cylapini taking into account their diversity within the group and comparison with other tribes.
Body shape. In Cylapini body is usually elongate to elongate-oval. In some species of Carvalhoma ( C. ovatum Namyatova & Cassis , C. parvum Namyatova & Cassis or C. taplini Namyatova & Cassis ) and in Schizopteromiris the body is ovate ( Schuh 1986; Namyatova & Cassis 2016).
Head. The head in Cylapini is always hypognathous, varying from weakly wider than high or as high as wide with frons sloping, not perpendicular to vertex ( Carvalhoma , Corcovadocola Carvalho , Cylapinus Carvalho , Cylapoides Carvalho , Dariella Namyatova et Cassis , Labriella Namyatova et Cassis , Mangalcoris Murphy et Polhemus , Schizopteromiris Schuh ) ( Figs 8a, b View FIGURE 8 ; Namyatova & Cassis 2016: figs 2A, C, 3C, 4A, C, 2021: figs 3A, C, 5A, C) to much higher than wide in anterior view with frons strongly flattened, perpendicular to vertex ( Cylapomorpha , Cylapus complex) ( Figs 8c–i View FIGURE 8 , 9a, g View FIGURE 9 ; Yasunaga 2000: fig. 2, 3; Wolski 2017: figs 9, 92; Wolski et al. 2020: fig. 28). In all Cylapini , Vanniini and Bothriomirini the antennal fossa is placed more or less above the suture between the maxillary and mandibular plates ( Namyatova & Cassis 2016: figs 2C, 4C, 2021: figs 3A, 5A), and the mandibular plate is separated from the remainder of the head by a suture ( Figs 9a–d View FIGURE 9 ; Namyatova & Cassis 2016: fig. 2C). In contrast, in all fulviines and rhinomirines the antennal insertion is continuous with the suture between maxillary and mandibular plates ( Namyatova & Cassis 2019: figs 6G, 10E, 22C) and the mandibular plate is not separated from the remainder of head by any suture ( Figs 9e, f View FIGURE 9 ; Wolski & Henry 2012: fig. 74; Wolski et al. 2018: fig. 11). Within Cylapini the antennal fossa is either situated close to the suture between maxillary and mandibular plates (all Cylapini except Cylapus complex) (8a, b, i, 9b; Namyatova & Cassis 2016: figs 2C, 4C, 2021: figs 3A, 5A) or distinctly removed from it ( Figs 8c–h View FIGURE 8 , 9a, g View FIGURE 9 ), and the suture behind the mandibular plate can be faint ( Fig. 9b View FIGURE 9 ; Namyatova & Cassis 2016: 2 C) or strongly depressed ( Figs 9a, g View FIGURE 9 ). All members of Cylapini , Vanniini , and many bothriomirines have the ventral margin of the eyes removed from the ventral margin of head ( Figs 9a–d View FIGURE 9 ; Namyatova & Cassis 2016: fig. 2C, 2021: 3A; Namyatova et al. 2019: figs 5C, 10D, 11B) and the base of clypeus is positioned below the ventral margin of the eyes ( Figs 9g –i View FIGURE 9 ; Namyatova & Cassis 2016: fig. 3A, 2021: figs 3C, 5C; Namyatova et al. 2019: figs 5A, 10A, 11A). In most Fulviini and Rhinomirini the ventral margins of eyes are reaching or almost reaching the ventral margin of the head ( Figs 9e, f View FIGURE 9 , Wolski & Henry 2012: fig. 73; Wolski 2013: fig. 73) and the base of clypeus is situated above the ventral margin of the eyes ( Figs 8l, m View FIGURE 8 ; Wolski 2013: figs 33–36; Namyatova et al. 2016: fig. 3H; Namyatova & Cassis 2019: figs 10A, 13C, 16C). Within Cylapini the eyes are situated either relatively close to the ventral margin of head ( Figs 9b View FIGURE 9 ; Namyatova & Cassis 2021: figs 3A, 5A) or are strongly removed in the dorsal direction ( Fig. 9a,c View FIGURE 9 ; Wolski 2017: figs 9, 95). The base of clypeus is situated close to the ventral margin of the eyes ( Fig. 9h View FIGURE 9 , 8a, b, i View FIGURE 8 ; Yasunaga 2000: fig. 2; Namyatova & Cassis 2021: 3 C, 5C) or is strongly removed from the eyes’ ventral margin ( Figs 8c–h View FIGURE 8 , 9g View FIGURE 9 ). The vertex in Cylapini is either carinate posteriorly ( Carvalhoma , Corcovadocola , Cylapoides , Dariella , Labriella ) ( Figs 9h View FIGURE 9 , 11e View FIGURE 11 ; Namyatova & Cassis 2016, 2021: figs 3b, 5b) or devoid of carina ( Cylapinus , Cylapomorpha , Mangalcoris , Cylapus complex) ( Figs 11a, g View FIGURE 11 ; Wolski 2017: fig. 33). The vertex in most Cylapini possesses a more or less developed depression along the midline. The depression is either faint ( Figs 9h View FIGURE 9 , 11a View FIGURE 11 ; Namyatova & Cassis 2016; Wolski 2017: 33) or strongly developed so the head is V-shaped in anterior view ( Cylapus complex) ( Figs 8c–h View FIGURE 8 , 9g View FIGURE 9 ). The eyes are either embedded into the head, with the dorsal margin situated at the same plane as vertex ( Carvalhoma , Corcovadocola , Cylapoides , Dariella , Labriella , Mangalcoris , Schizopteromiris ) ( Figs 8b View FIGURE 8 , 9h View FIGURE 9 ; Namyatova & Cassis 2016: fig. 2B, 2021: 3B, 5B) or the eyes are pedunculate ( Cylapomorpha , Cylapinus , Cylapus complex) ( Figs 8a, c–i View FIGURE 8 , 9g View FIGURE 9 ). The latter condition is unique within Cylapinae .
Antennae. The shape of antennae is variable among the genera of Cylapini . In Carvalhoma , Corcovadocola , Cylapinus , Cylapoides , Dariella , Labriella and Schizopteromiris the antenna is relatively short, shorter than body length and the segments III and IV are not thread-like ( Figs 3a, b, d View FIGURE 3 ; Namyatova & Cassis 2016: fig. 1, 2021: fig. 1). In Mangalcoris , Cylapomorpha and Cylapus complex the antennae are longer than the body length and segments III and IV are thread-like (e.g., Figs 4b, c View FIGURE 4 ; Wolski 2017: 40, 46, 48, 51; Yasunaga 2000: fig. 1; Gorczyca 2006a: fig. 5; Murphy & Polhemus 2012: figs 1A, B, D). The latter type of antenna is also found in Vanniini (e.g., Gorczyca 2006a: fig. 7).
Labium. In all Cylapini , Vanniini , and Bothriomirini the labium is stout and rather short, the apex usually reaching the hind coxae or barely beyond it, and the segments I and II are not subdivided ( Figs 9j, k View FIGURE 9 ; Namyatova & Cassis 2016: figs 5D, 6E, 2021: 3D, 5D; Namyatova et al. 2016: figs 9F, G, 2019: 5E, 9J). In contrast, in Fulviini and Rhinomirini the labium is thin and long, sharply pointed, the apex reaching beyond the middle of the abdomen, and segments I and II are subdivided ( Fig. 9l View FIGURE 9 ; Namyatova et al. 2016: figs 9J, 10A; Namyatova & Cassis 2019: figs 10 R, 13J, 22D).
Pronotum. In Cylapini the pronotum is either impunctate ( Carvalhoma , Corcovadocola , Cylapoides , Cylapomorpha , Labriella , Mangalcoris , Schizopteromiris ) ( Figs 9b View FIGURE 9 , 11e View FIGURE 11 ) or punctate ( Cylapinus , Dariella , Cylapus complex) ( Figs 9a View FIGURE 9 , 10a View FIGURE 10 , 11a, g View FIGURE 11 ). The lateral margin is always ecarinate ( Figs 9a, b View FIGURE 9 , 10a View FIGURE 10 , 11b View FIGURE 11 ). The collar in Cylapini is always present, and in lateral view is situated anteriorly to the propleural suture ( Fig. 9a View FIGURE 9 ).
Thoracic pleura. The mesepimeral spiracle in Cylapini is slit-like ( Figs 10b–e View FIGURE 10 , 11c, f View FIGURE 11 ), in members of the Cylapus complex it is covered with evaporative bodies ( Figs 10b–e View FIGURE 10 ). In most Cylapini taxa except for the Cylapus complex, the metepisternum is narrow and rectangular ( Figs 11c, f View FIGURE 11 ; Namyatova & Cassis 2016: fig. 2K, 2021: figs 3F, 5H). In the Cylapus complex the metepisternum is broad and almost square ( Figs 10b–e View FIGURE 10 ). In most Cylapini genera the metepisternum is carinate posteriorly ( Figs 10b–e View FIGURE 10 , 11c, f, i View FIGURE 11 ). The metepisternal evaporative areas are either relatively narrow, straight posteriorly and not extended onto anterior margin of metepisternum ( Carvalhoma , Corcovadocola , Cylapinus , Cylapoides , Dariella , Labriella , Cylapomorpha ) ( Figs 11c View FIGURE 11 ; Namyatova & Cassis 2016: fig. 2K, 2021: figs 3F, 5H; Wolski 2017: fig. 35) or are broad, rounded, extended posteriorly, and extended well onto anterior margin of metepisternum ( Cylapus complex) ( Figs 10b–e View FIGURE 10 ). In many genera of the Cylapini the metepimeron is well exposed, not obscured by second abdominal segment ( Figs 10b–e View FIGURE 10 , 11c, f View FIGURE 11 ). The ostiolar peritreme is usually oval ( Figs 10d–e View FIGURE 10 , 11c, f View FIGURE 11 ), rarely the peritreme is narrow and ear-like ( Amapacylapus ) ( Fig. 10b View FIGURE 10 ) or strongly protruding, thin and arcuate and sharply pointed ( Cylapus ) ( Fig. 10c View FIGURE 10 ).
Hemelytron. The hemelytron in Cylapini is either impunctate ( Corcovadocola , Cylapoides , Labriella , Cylapomorpha ) ( Fig. 11e View FIGURE 11 ; Wolski 2017: fig. 33; Namyatova & Cassis 2021: fig. 1) or covered with deep and dense punctation ( Carvalhoma , Cylapinus , Dariella , Cylapus complex) ( Figs 11a, g, m View FIGURE 11 ; Namyatova & Cassis 2016: fig. 1, 2021: fig. 1). Most Cylapini genera are macropterous, and in such genera as Carvalhoma , Corcovadocola and Mangalcoris the hemelytron is further modified (see Wolski & Gorczyca 2014 and Namyatova & Cassis 2019 for detailed discussion on the wing modification in Cylapinae ).
Legs. In most Cylapini the legs are not modified; only in Phyllocylapus lutheri Poppius, 1913 the fore tibiae are strongly broadened and leaf-like ( Gorczyca 2000: fig. 15). Most Cylapini possess moderately elongated legs. In the Cylapus complex the legs, especially hind legs, are strongly elongated ( Figs 4c View FIGURE 4 , 6a View FIGURE 6 ; Wolski 2017: figs 37, 41, 55). In all Cylapini the tarsus is three-segmented. Tarsal segment I is usually shorter than II and III combined ( Figs 10j View FIGURE 10 , 11d, j View FIGURE 11 ; Namyatova & Cassis 2021: fig. 3J, 5G), rarely I segment is as long as or longer than segments II and III combined ( Carvalhoma , Cylapus ) ( Fig.10i View FIGURE 10 ; Namyatova & Cassis 2016: fig. 2I).
Male genitalia. The pygophore in Cylapini has no supragenital bridge sensu Konstantinov 2003, instead the ventral wall is much longer than the dorsal wall, and the genital opening is directed upward ( Yasunaga 2000: fig. 5; Namyatova & Cassis 2016: figs 7C, 8C, 2021: figs 4C, 6C; Wolski 2017: figs 17, 134). The parameres in Cylapini are similar in size, the left paramere is usually C-shaped, and the right paramere is usually sickle-shaped and frequently with more or less shortened apical process ( Figs 12b, c, h, i, m, n View FIGURE 12 ; Yasunaga 2000: fig. 5; Namyatova & Cassis 2016: figs 7D, E, 8D, E, 2021: figs 4D–G, 6C–G). The aedeagus is moderately voluminous, not subdivided into the vesica and conjunctiva. The phallotheca is moderately sclerotized. Ductus seminis relatively short and thick ( Figs 12a, g, j View FIGURE 12 , 13m View FIGURE 13 ; Namyatova & Cassis 2016: figs 7A, B, 8A, B, 2021: figs 4A, B, 6A,B; Wolski 2017: figs 12, 66; Wolski et al. 2020: fig. 40, 48). The endosoma in Cylapini is either furnished with sclerites ( Fig. 14r View FIGURE 14 ; Wolski 2017: figs 12, 66; Wolski et al. 2020: fig. 48; Namyatova & Cassis 2021: fig. 6) or devoid of spiculi ( Figs 12j, p View FIGURE 12 , 13a, g, m View FIGURE 13 ; Wolski et al. 2020: fig. 40). Sometimes the endosoma is composed of strongly inflated sclerotized lobes ( Fig. 12g View FIGURE 12 ; Yasunaga 2000: fig. 6).
Female genitalia. The ovipositor in the Cylapini is reminiscent of that found in Vanniini and Bothriomirini . Its first gonapophyses have the ventral margin more or less arcuate, dorsal margin sinuate, strongly convex subapically ( Figs 21e–i View FIGURE 21 ; Yasunaga & Miyamoto 2006: fig. 4B; Wolski et al. 2020: figs 55, 59, 67, 69). The second gonapophyses are ventrally arcuate, with the dorsal margin weakly sinuate and strongly serrate ( Figs 21m –q View FIGURE 21 , 22i, j View FIGURE 22 ; Wolski et al. 2020: figs 56, 60, 68, 70). In contrast, in most known Fulviini the gonapophyses are rounded, without tooth-like structures or with weakly developed denticles ( Figs 22k, l View FIGURE 22 ; Sadowska-Woda et al. 2006: figs 2, 3, 4, 12, 13; Kim et al. 2019: figs 2D, 4D; Gorczyca et al. 2020: figs 5c, d). The first gonapophyses are often connected by well-developed, membranous structure (e.g., Sadowska-Woda et al. 2006: figs 2, 3, 12, 13; Gorczyca et al. 2020: figs 5c, d) and together with the second gonapophyses they form a dilatable ovipositing tube ( Schmitz & Štys 1973). The latter authors suggested that such a shape of the ovipositor allow fulviines to deposit eggs into the cavities tree bark where they live, indicating that they are predators. This hypothesis may be supported by the fact that most fulviines (unlike Cylapini , Vanniini , and Bothriomirini ) have long, sharply pointed labium with subdivisions on segments I and II (which may make it highly flexible (van Doesburg 1985)), allowing these bugs for effective hunting. Moreover, direct evidence for predation exists only for fulviines and not for the representatives of the other tribes (see Introduction).
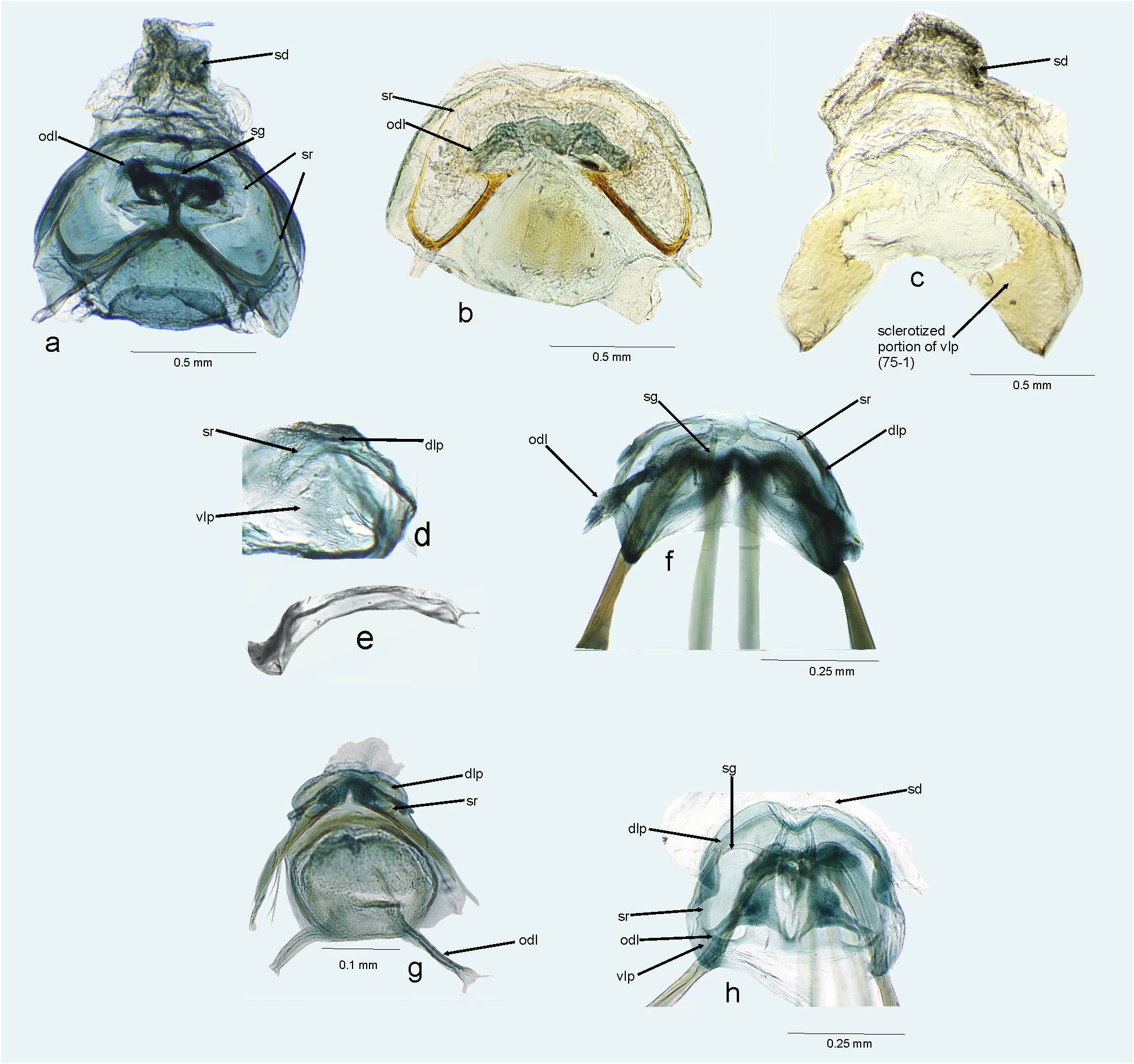
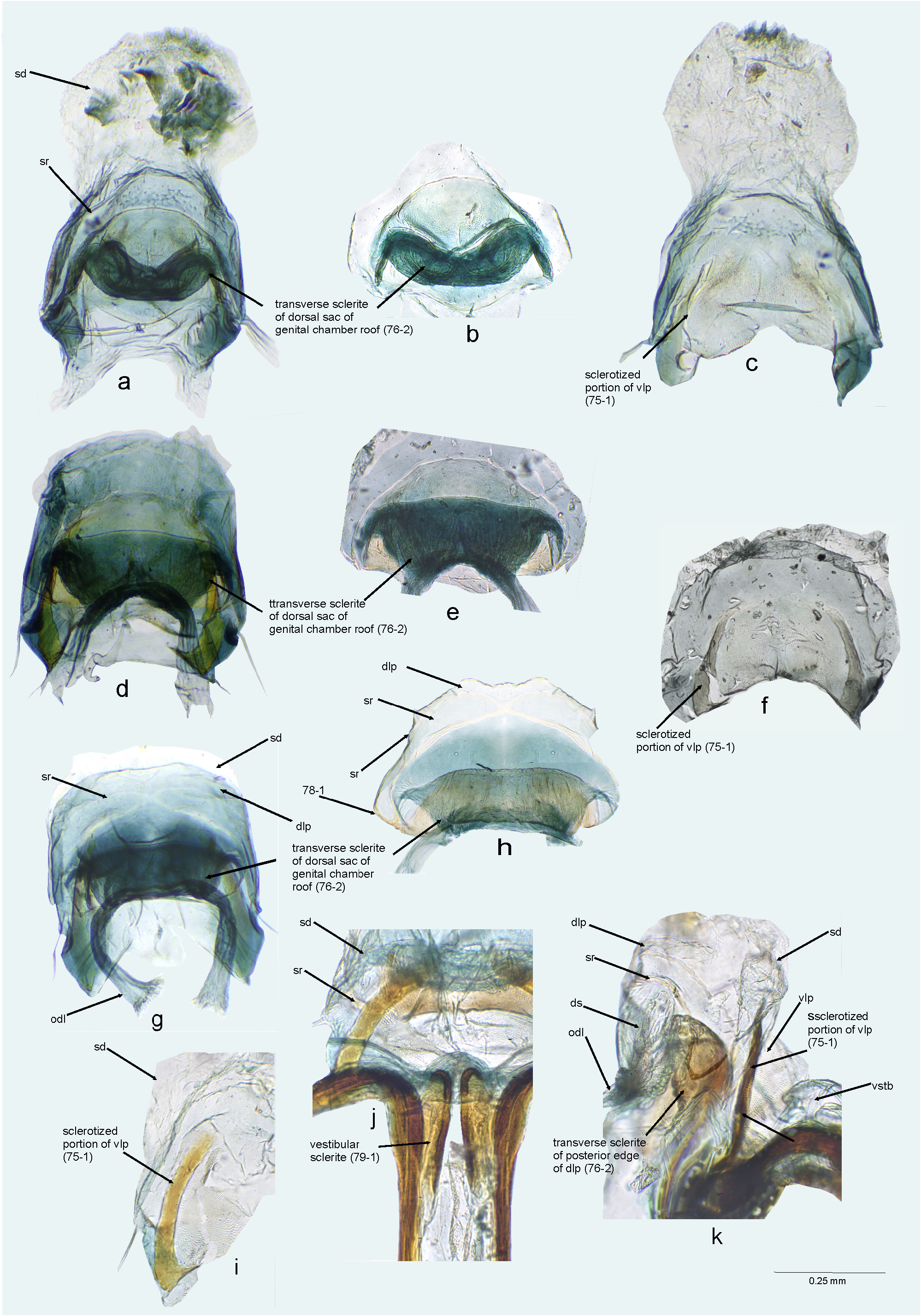
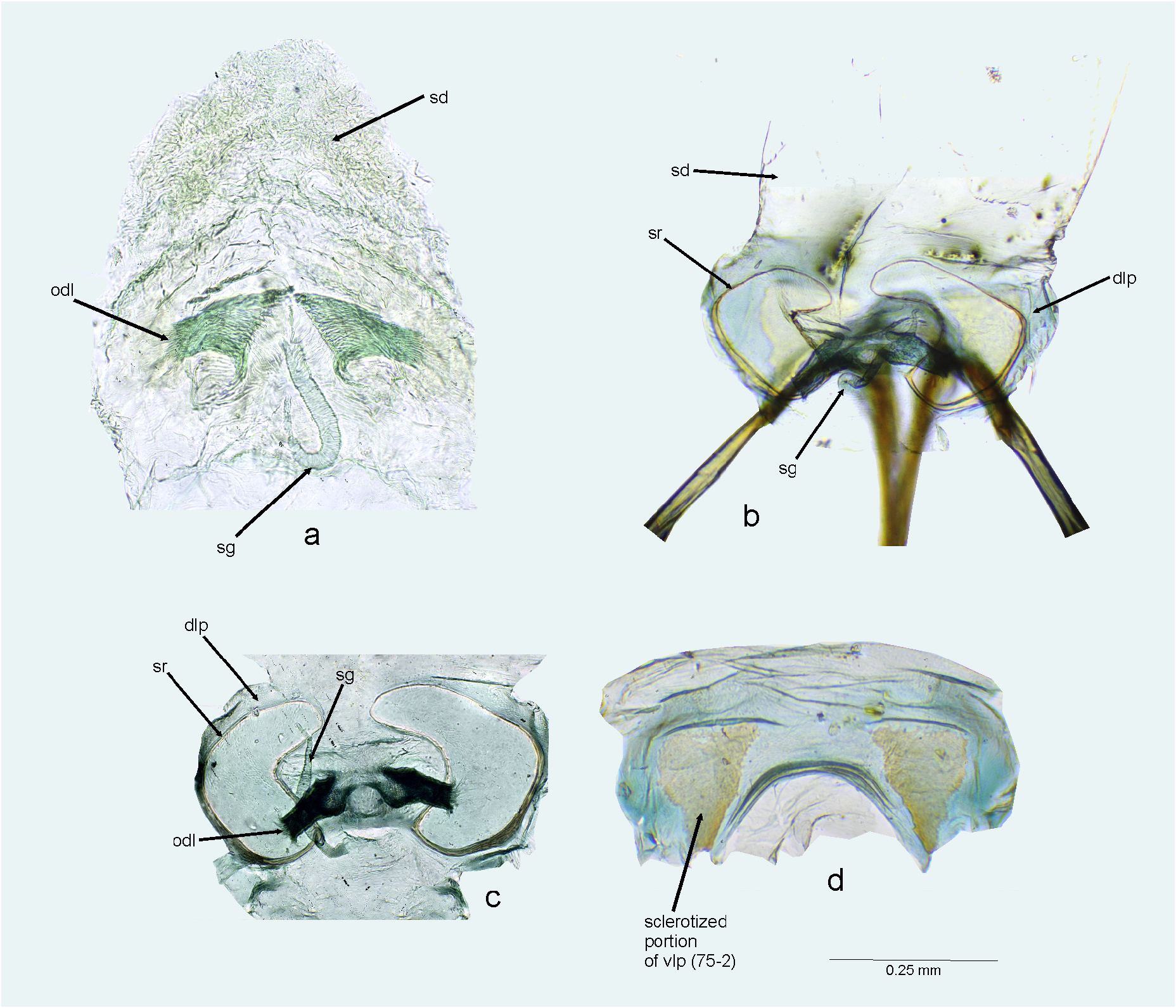
The vestibulum in Cylapini is either membranous and without any structures encircling vulva ( Figs 20b, c View FIGURE 20 ), or it possesses a well-developed, elongated sclerite adjacent to the base of the first gonapophyses ( Figs 20a, d–h View FIGURE 20 ).
The bursa copulatrix in Cylapini is voluminous, laterally extending beyond first gonapophyses ( Figs 15a, b, f, h View FIGURE 15 ; 16a, d, g View FIGURE 16 , 17j View FIGURE 17 , 18b View FIGURE 18 , 19a View FIGURE 19 ; Namyatova & Cassis 2016: 7 F, 9F; Wolski et al. 2020: fig. 66), and with the anterior margin strongly removed from the first gonapophyses in many cases ( Cylapus complex) ( Figs 15a, b, f, h View FIGURE 15 ; 16a, d, g View FIGURE 16 , 17j View FIGURE 17 , 18b View FIGURE 18 , 19a View FIGURE 19 ; Wolski et al. 2020: fig. 66). The posterior wall of the genital chamber is simple and membranous ( Figs 19f, g View FIGURE 19 ; Namyatova & Cassis 2019: figs 7G, 9G, 2021: fig. 7c; Wolski et al. 2020: fig. 54). The shape and position of the sclerotized rings are variable within the Cylapini . They can be paired ( Figs 15f, g, h View FIGURE 15 , 16g View FIGURE 16 , 18c View FIGURE 18 , 19a, d View FIGURE 19 ) or unpaired ( Figs 15a, b View FIGURE 15 , 16a, d View FIGURE 16 , 17a, d, h, k View FIGURE 17 ). In some genera they can be minute, occupying a small portion of the dorsal wall (roof) of the genital chamber ( Cylapoides , Labriella ) ( Fig. 15g View FIGURE 15 ; Namyatova & Cassis 2021: fig. 7A) while in other taxa the rings embrace anterior and lateral portions of the genital chamber ( Cylapus , some species of Valdasus ) ( Figs 16a, d, g View FIGURE 16 , 17a, d, h, k View FIGURE 17 ; Wolski et al. 2020: Figs 57, 63, 65) or occupies most of it ( Amapacylapus , Peltidocylapus , some Valdasus ) ( Figs 15a, b View FIGURE 15 , 18c View FIGURE 18 , 19b, e View FIGURE 19 ). The lateral oviducts are situated centrally on the roof of the genital chamber roof. They are usually contiguous and, rarely they are separated ( Cylapinus minusculus ) ( Fig. 15f View FIGURE 15 ). The lateral oviducts are either wide ( Cylapus complex) ( Figs 15a View FIGURE 15 , 16d, g View FIGURE 16 , 18a, b View FIGURE 18 , 19b, e View FIGURE 19 ) or thin ( Cylapinus minusculus , Cylapomorpha , Cylapoides unicolor ) ( Figs 15f, g, h View FIGURE 15 ; Yasunaga 2000: Fig. 7 View FIGURE 7 ). They can be short (as in Amapacylapus , Peltidocylapus , and some species of Valdasus ) ( Figs 15a, b View FIGURE 15 , 18c View FIGURE 18 , 19b, e View FIGURE 19 ) or long, reaching beyond lateral or posterior portion of the genital chamber ( Cylapus , some species of Valdasus , Cylapomorpha , Cylapinus , Cylapoides ) ( Figs 15f, g View FIGURE 15 , 16d, g View FIGURE 16 , 17d, k View FIGURE 17 ; Yasunaga 2000: fig. 7). The spermathecal gland opens centrally in the genital chamber, between the lateral oviducts ( Figs 15a, b, f, h View FIGURE 15 , 18a View FIGURE 18 , 19b, e View FIGURE 19 ; Wolski et al. 2020: 52, 57), or it rarely it opens in the posterior area of the bursa copulatrix (some species of Cylapus ) ( Figs 16d View FIGURE 16 , 17d View FIGURE 17 ). The dorsal sac sensu Kullenberg (1947) is absent in most studied Cylapini . The representative of Cylapomorpha investigated herein possesses the medio-longitudinal, U-shaped, membranous, structure ( Fig. 15h View FIGURE 15 ), similar to that observed in Orthotylini (e.g., Pluot-Sigwalt & Matocq 2017: figs 5, 6). In all investigated species of Cylapus and some Valdasus ( V. henryi Wolski, Chérot et Carpintero , V. schoenherri Stål ) the posterior portion of bursa copulatrix is furnished with a more or less developed, transverse sclerotization connecting the posterior portion(s) of the sclerotized ring(s) ( Figs 16b, e, h View FIGURE 16 , 17f, h, k, l View FIGURE 17 ; Wolski et al. 2020: figs 63, 64, 65). In anterior view this structure clearly forms a pouch or pocket, and it is reminiscent of the pouch-like structures found in Hallodapini ( Pluot-Sigwalt & Matocq 2017: fig. 12). It is noteworthy that species investigated here also possess the endosoma furnished with at least three sclerites ( Wolski 2017: figs 66, 71, 80, 97, 102, 135, 14). At the same time, all studied Cylapus complex species without dorsal sac have the endosoma without sclerites ( Peltidocylapus ) ( Figs 12j View FIGURE 12 , 13a, m View FIGURE 13 , 14a, f, e, l View FIGURE 14 ) or with a single, weakly developed sclerotized appendage ( Amapacylapus ) ( Wolski 2017: figs 12, 18). This observation may support the hypothesis of Pluot-Sigwalt & Matocq (2017) who suggested the role of the dorsal sac during copulation when some portions of aedeagus and parameres penetrate the gynatrium and probably enter and anchor into the cavity of the dorsal sack sac.
Bergroth, E. (1922) New Neotropical Miridae (Hem.). Arkiv for Zoologi, 14 (21), 1 - 14. https: // doi. org / 10.5962 / bhl. part. 7726
Carvalho, J. C. M. (1952 a) Neotropical Miridae, 56: Description of three new genera and five new species from Brazil and British Honduras (Hemiptera). Revista Brasileira de Biologia, 12, 265 - 273.
Carvalho, J. C. M. (1955) Keys to the genera of Miridae of the world (Hemiptera). Boletim do Museu Paraense Emilio Goeldi, 11, 1 - 151.
Carvalho, J. C. M. (1957) Catalogue of the Miridae of the World. Part I. Subfamilies Cylapinae, Deraeocorinae and Bryocorinae. Arquivos do Museu Nacional Rio de Janeiro, 44, 1 - 158.
Carvalho, J. C. M. & Fontes, A. V. (1968) Mirideos neotropicais, CI: Revisao do complexo Cylapus Say, com descricoes de gene- ros e especies novos (Hemiptera). Revista Brasileira de Biologia, 28, 273 - 282.
Carvalho, J. C. M. (1986) Mirideos neotropicais, CCLVI: Dois generos e seis especies novos da tribo Cylapini (Hemiptera). Acta Amazonica, 16 / 17, 589 - 598. https: // doi. org / 10.1590 / 1809 - 43921987171598
Carvalho, J. C. M. (1989) Mirideos neotropicais, CCCVI: novos generos e especies da tribo Cylapini Kirkaldy (Hemiptera). Boletim do Museu Paraense Emilio Goeldi, Zoologia, 5, 79 - 94.
Cassis, G., Schwartz, M. D. & Moulds, T. (2003) Systematics and new taxa of the Vannius complex (Hemiptera: Miridae: Cylapinae) from the Australian Region. Memoirs of the Queensland Museum, 49, 123 - 151.
Cassis, G. & Schuh, R. T. (2012) Systematics, biodiversity, biogeography, and host associations of Miridae (Insecta: Hemiptera: Heteroptera: Cimicomorpha). Annual Review of Entomology, 57, 377 - 404. https: // doi. org / 10.1146 / annurev-ento- 121510 - 133533
Distant, W. L. (1883) Insecta. Rhynchota. Hemiptera-Heteroptera. Biologia Centrali Americana, 1, 225 - 264.
Distant, W. L. (1893) Insecta. Rhynchota. Hemiptera-Heteroptera. Biologia Centrali Americana, Supplement, i - xx + 329 - 462.
Gorczyca, J. (1997) Revision on Vannius - complex and its subfamily placement (Hemiptera: Heteroptera: Miridae). Genus, 3, 517 - 533.
Gorczyca, J. (2000) A systematic study on Cylapinae with a revision of the Afrotropical Region (Heteroptera, Miridae). Wydawnictwo Uniwersytetu Slaskiego, Katowice, 174 pp.
Gorczyca, J. (2006 a) The catalogue of the subfamily Cylapinae Kirkaldy, 1903 of the World (Hemiptera, Heteroptera, Miridae). Monographs of the Upper Silesian Museum. Vol. 5. Department of Natural History, Upper Silesian Museum, Bytom, 100 pp.
Gorczyca, J., Wolski, A. & Taszakowski, A. (2020) The first record of the genus Fulvius Stal, 1862 (Heteroptera: Miridae: Cylapinae) from continental China with description of a new species. Bonn zoological Bulletin, 69 (1), 123 - 130. https: // doi. org / 10.20363 / bzb- 2020.69.1.123
Kim, J., Lim, J. & Jung, S. (2019) A taxonomic review of the fungal-inhabiting plant bugs (Hemiptera: Heteroptera: Miridae: Cylapinae) from the Korean Peninsula. Journal of Asia - Pacific Biodiversity, 12 (2019), 249 - 256. https: // doi. org / 10.1016 / j. japb. 2019.01.006
Konstantinov, F. V. (2003) Male genitalia in Miridae (Heteroptera) and their significance for suprageneric classification of the family. Part I: general review, Isometopinae and Psallopinae. Belgian Journal of Entomology, 5, 3 - 36.
Konstantinov, F. V. (2012) A new species of Palaucoris from Sulawesi. Entomologica Americana, 118, 121 - 129. https: // doi. org / 10.1664 / 12 - RA- 013.1
Kullenberg, B. (1947) Uber Morphologie und Funktion des Kopulationsapparats der Capsiden und Nabiden. Zoologiska Bidrag fran Uppsala, 24, 217 - 248.
Murphy, D. H. & Polhemus, D. A. (2012) A new genus of micropterous Miridae from Singapore mangroves (Insecta: Hemiptera: Heteroptera). The Raffles Bulletin of Zoology, 60, 109 - 115.
Namyatova, A. A. & Cassis, G. (2016) Revision of the staphylinoid and ground-dwelling genus Carvalhoma Slater and Gross (Insecta: Heteroptera: Miridae: Cylapinae) of Australia. European Journal of Taxonomy, 253, 1 - 27. https: // doi. org / 10.5852 / ejt. 2016.253
Namyatova, A. A. & Cassis, G. (2019) Total-evidence phylogeny of the Rhinomirini, taxonomic review of its subgroupings (Insecta: Heteroptera: Miridae: Cylapinae) and description of new Australian taxa. Zoological Journal of the Linnean Society, 187 (4), 1196 - 1252. https: // doi. org / 10.1093 / zoolinnean / zlz 058
Namyatova, A. A. & Cassis, G. (2021) Five new genera of the subfamily Cylapinae (Insecta, Heteroptera, Miridae) from Australia. ZooKeys, 1012, 95 - 134. https: // doi. org / 10.3897 / zookeys. 1012.57172
Pluot-Sigwalt, D. & Matocq, A. (2017) An investigation of the roof of the genital chamber in female plant-bugs with special emphasis on the dorsal sac (Hemiptera: Heteroptera: Miridae). Annales de la Societe entomologique de France, New Series, 1 - 16. https: // doi. org / 10.1080 / 00379271.2017.1285723
Poppius, B. (1909) Zur Kenntnis der Miriden-Unterfamilie Cylapina Reut. Acta Societatis Scientiarum Fennicae, 37 (4), 1 - 46. https: // doi. org / 10.5962 / bhl. title. 9441
Sadowska-Woda, I., Cherot, F. & Gorczyca, J. (2006) Contribution to the study of the female genitalia of twelve Fulvius species (Heteroptera, Miridae, Cylapinae). In: Rabitsch W. (Ed.), Hug the bug - For love of true bugs. Festschrift zum 70. Geburtstag von Ernst Heiss. Denisia, 19, pp. 617 - 636.
Schmitz, G. & Stys, P. (1973) Howefulvius elytratus gen. n. sp. n. (Heteroptera, Miridae, Fulviinae) from Lord Howe Island in the Tasman Sea. Acta Entomologica Bohemoslovaka, 70, 400 - 407.
Schuh, R. T. (1986) Schizopteromiris, a new genus and four new species of coleopteroid cylapine Miridae from the Australian Region (Heteroptera). Annales de la Societe Entomologique de France, 22, 241 - 246.
Wolski, A. & Henry, T. J. (2012) Revision of the New World Species of Peritropis Uhler (Heteroptera: Miridae: Cylapinae). Insect Systematics and Evolution, 43, 213 - 270. https: // doi. org / 10.1163 / 1876312 X- 04303002
Wolski, A. (2013) Revision of the plant bug genus Cylapocoris (Hemiptera: Heteroptera: Miridae: Cylapinae), with descriptions of seven new species from Costa Rica, Brazil, Ecuador, and Venezuela. Zootaxa, 3721 (6), 501 - 528. https: // doi. org / 10.11646 / zootaxa. 3721.6.1
Wolski, A. & Gorczyca, J. (2014) Revision of the plant bug genus Xenocylapidius (Hemiptera, Heteroptera, Miridae, Cylapinae), with descriptions of five new species from Australia and New Caledonia. ZooKeys, 459, 73 - 94. https: // doi. org / 10.3897 / zookeys. 459.8015
Wolski, A. (2017) Taxonomic review of the plant bug genera Amapacylapus and Cylapus with descriptions of two new species and a key to the genera of Cylapini (Hemiptera: Heteroptera: Miridae). Acta Entomologica Musei Nationalis Pragae, 57 (2), 399 - 455. https: // doi. org / 10.1515 / aemnp- 2017 - 0084
Wolski, A., Gorczyca, J. & Yasunaga, T. (2018) Taxonomic review of the bifenestratus species group of the genus Fulvius Stal with descriptions of two new species (Hemiptera, Heteroptera, Miridae, Cylapinae). ZooKeys, 796, 107 - 129. https: // doi. org / 10.3897 / zookeys. 796.21293
Wolski, A., Cherot, F. & Carpintero, L. C. (2020) Review of the genus Valdasus Stal, 1860 (Heteroptera, Miridae, Cylapinae), with descriptions of four new species from Brazil, Ecuador and French Guiana. Zootaxa, 4849 (2), 187 - 206. https: // doi. org / 10.11646 / zootaxa. 4869.2.2
Yasunaga, T. (2000) The mirid subfamily Cylapinae (Heteroptera: Miridae) or fungal inhabiting plant bugs in Japan. Tijdschrift voor Entomologie, 143, 183 - 209. https: // doi. org / 10.1163 / 22119434 - 99900044
Yasunaga, T., Miyamoto, S. (2006) Second report on the Japanese cylapinae plant bugs (Heteroptera, Miridae, Cylapinae), with description of five new species. In: Rabitch W (Ed.), Hug the Bug - For Love of True Bugs. Festschrift zum 70. Geburtstag von Ernst Heiss. Denisia, 19, 721 - 735.
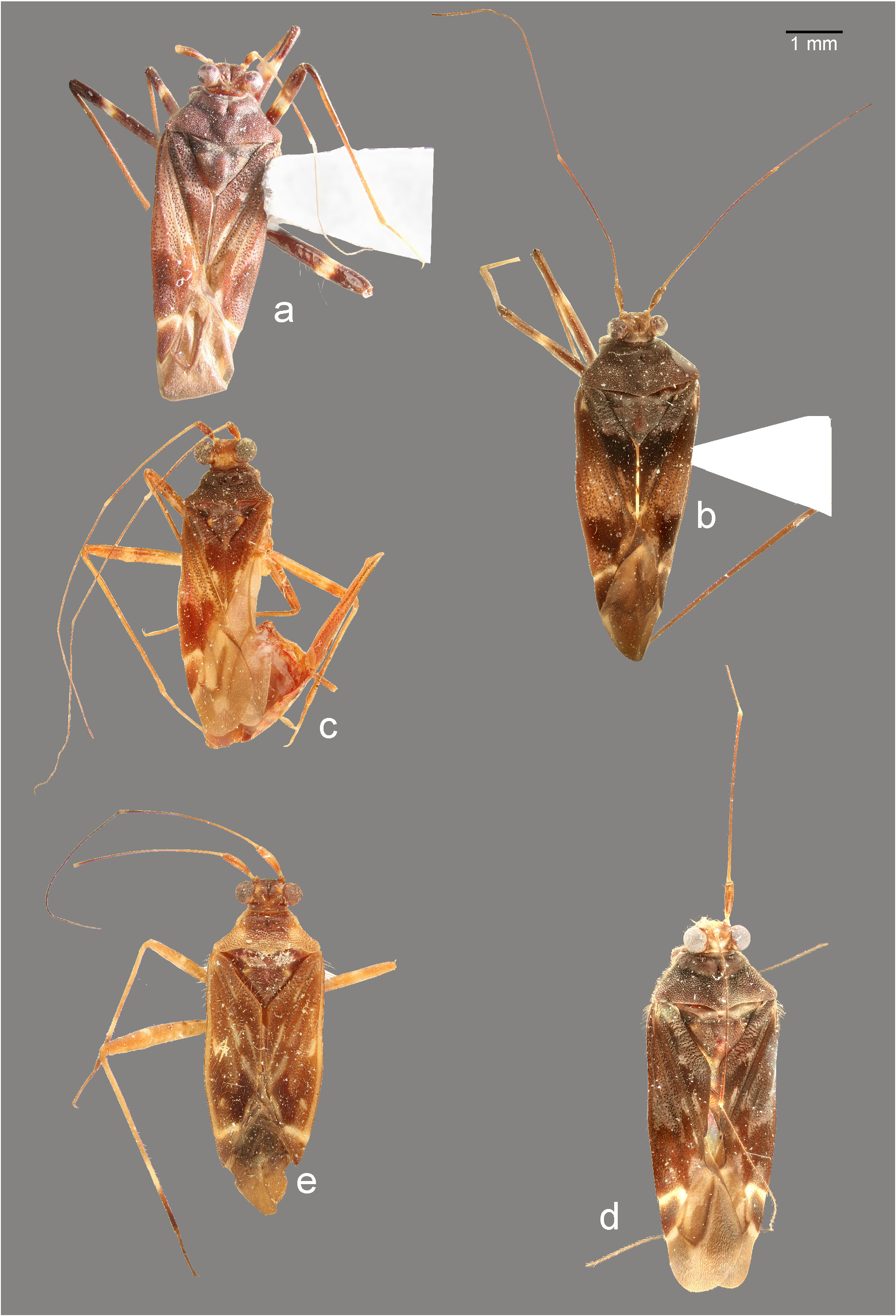
FIGURE 3. Dorsal habitus photographs. a. Cylapinus minusculus (♂, Ecuador); b. Cylapinus yasunagai (holotype); c. Cylapoides bicolor (holotype); d. Cylapoides unicolor (♂, Ecuador).
FIGURE 4. Dorsal habitus photographs. a. Peltidocylapus calyciformis (paratype, ♂); b. Peltidocylapus caudatus (holotype); c. Peltidocylapus cerbereus (lectotype); d. Peltidocylapus ecuadorensis (holotype); e. Peltidocylapus festinabundus (♀, Peru).
FIGURE 6. Dorsal habitus photographs. a. Peltidocylapus rugosus (lectotype); b. Peltidocylapus scutellaris (♂, Costa Rica); c. Peltidocylapus simplex (holotype); d. Peltidocylapus spinosus (holotype).
FIGURE 7. Lateral view. a. Cylapinus minusculus (♀); b. Cylapinus yasunagai (paratype); c. Cylapoides unicolor (♀); d. Peltidocylapus calyciformis (paratype); e. Peltidocylapus cerbereus (lectotype).
FIGURE 8. Head and pronotum, anterior (a–j, l, m) and lateral (k) views. a. Cylapinus yasunagai (paratype); b. Cylapoides unicolor (♀); c. Cylapus ruficeps Bergroth (♂); d. Cylapus tenuicornis (♂); e. Peltidocylapus calyciformis (paratype); f. Peltidocylapus caudatus (paratype); g. Peltidocylapus rugosus (holotype); h. Peltidocylapus simplex (paratype); i. Cylapomorpha sp. (♂); j, k. Vanniusoides clypeatus (paratype); l. Fulvius pallens (♂); m. Rhinocylapus vittatus (♀).
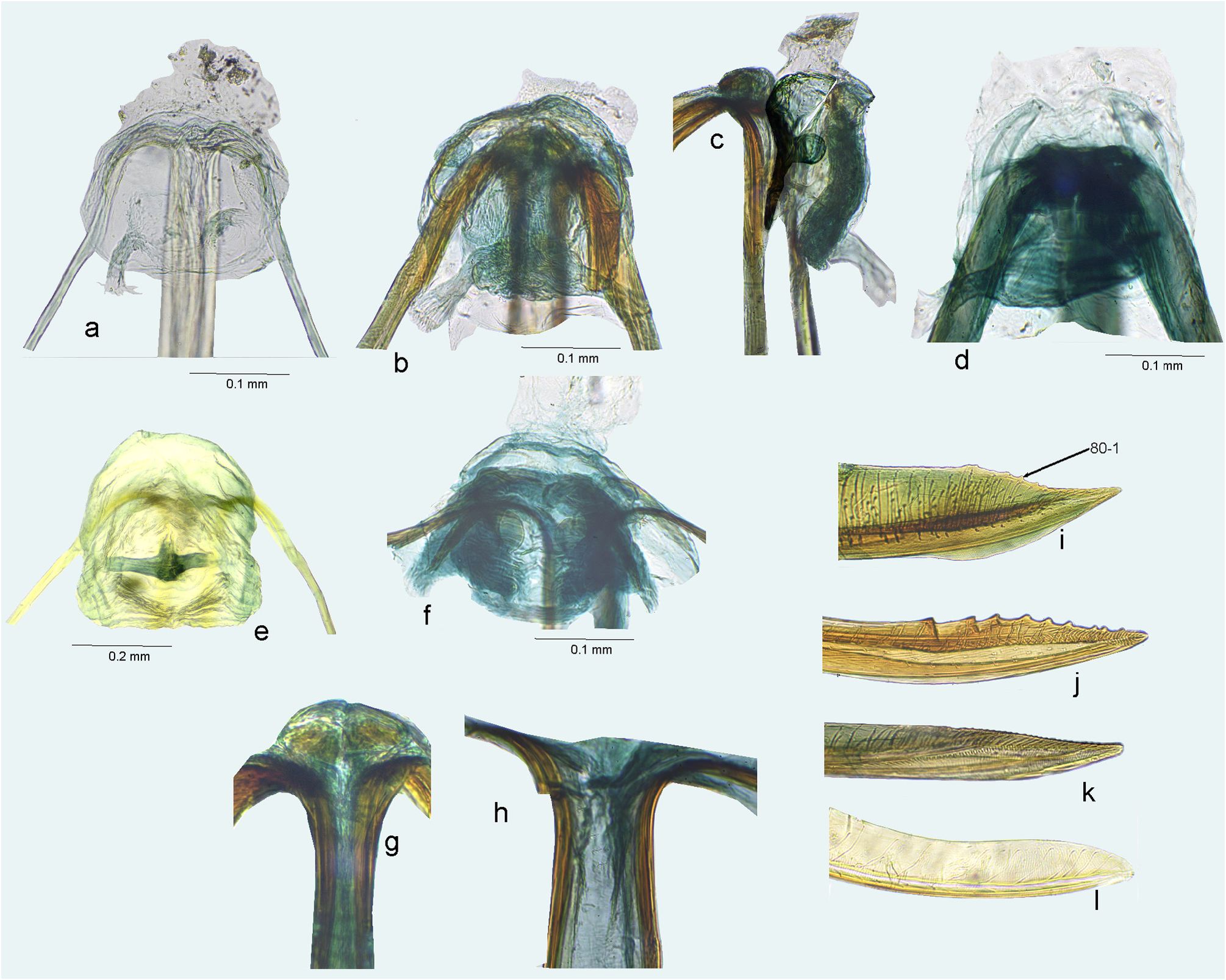
FIGURE 9. Scanning electron micrographs. a–f. Head and pronotum (left lateral view); g–i. Head (anterior view); j, k. Labium; l. Body (left lateral view). a. Valdasus flavinotum; b, h. Corcovadocola sp.; c, i. Vannius podager; d, k. Bothriomiris dissimulans; e. Fulvius pallens; f. Rhinomiris sp.; g. Valdasus schoechnerri; j. Cylapus tenuicornis Say; l. Cylapocoris sp..
FIGURE 10. Scanning electron micrographs. a. Pronotum (left lateral view); b–h. Thoracic pleura; i–m. Metatarsus. n, o. Pretarsal claw. a. Valdasus schoechnerri; b. Amapacylapus amapariensis; c, i. Cylapus tenuicornis; d. Peltidocylapus scutellaris; e, j. Valdasus henryi; f. Bothriomiris dissimulans; g. Cylapocoris sp.; h. Psallops sp.; k, o. Vannius sp.; l. Fulvius sp.; m. Cylapocoris sp.; n. Cylapinus minusculus.
FIGURE 11. Scanning electron micrographs. a, e, g, m. Dorsal habitus; b, n. Lateral habitus; h, l. Scutellum; c, f, i. Thoracic pleura; d, j, o. Tarsus. k. Pretarsal claw. a–d. Cylapinus minusculus; e, f. Cylapoides unicolor; g–j. Peltidocylapus caudatus; k. Peltidocylapus calyciformis; l. Peltidocylapus ecuadorensis; m–o. Peltidocylapus scutellaris.
FIGURE 12. Male genitalia. a, d, g, j, p. Endosoma (dorsal view); b, e, h, m, s. Left paramere (right lateral view). c, f, i, o, u. Right paramere (left lateral view); n, t. Left paramere (dorsal view). k, q. Transparent portion of distal sclerotized portion of ductus seminis inside endosoma; l, r. Endosoma (ventral view). a–c. Cylapinus minusculus; d–f. Cylapinus yasunagai; g–i. Cylapoides unicolor; j–o. Peltidocylapus calyciformis; p–u. Peltidocylapus caudatus. ap = apical process; bp = basal process; dss = distal part of ductus seminis inside endosoma; pb = paramere body; sl = sensory lobe. Scale bars 0.2 mm.
FIGURE 13. Male genitalia. a, g, m. Endosoma (dorsal view); d, j, p. Left paramere (right lateral view). e, k, q. Left paramere (dorsal view). f, l, r. Right paramere (left lateral view). b, h, o. Endosoma (ventral view); c, i, n. Endosoma (lateral view). a–f. Peltidocylapus ecuadorensis; g–l. Peltidocylapus pallidus; m–r. Peltidocylapus parallelus. dss = distal part of ductus seminis inside endosoma. Scale bars 0.2 mm.
FIGURE 14. Male genitalia. a, f, l, r. Endosoma (dorsal view); b, h, n. Endosoma (ventral view); g, m. Endosoma (right lateral view); c, i, o, s. Left paramere (right lateral view). d, j, p, t. Left paramere (dorsal view). e, k, q, u. Right paramere (left lateral view). a–e. Peltidocylapus rugosus; f–k. Peltidocylapus scutellaris; l–k. Peltidocylapus simplex; r–u. Peltidocylapus spinosus. dss = distal part of ductus seminis inside endosoma. Scale bars 0.2 mm.
FIGURE 15. Female genitalia. a, b, f, g, h. Bursa copulatrix (dorsal view); c, d. Ventral labiate plate; e. Sclerotized rings of dorsal labiate plate. a. Amapacylapus amapariensis; b, c. Amapacylapus unicolor; d–f. Cylapinus minusculus; g. Cylapoides unicolor; h. Cylapomorpha sp. dlp = dorsal labiate plate; odl = lateral oviduct; sd = seminal depository; sg = spermathecal gland; sr = sclerotized ring; vlp = ventral labiate plate.
FIGURE 16. Female genitalia. a, d, g. Bursa copulatrix (dorsal view); b, e, h. Bursa copulatrix (dorsal view, ventral labiate plate dissected). c, f, i. Ventral labiate plate and seminal depository (ventral view, with remainder of bursa copulatrix dissected). j. First gonapophyses and bursa copulatrix (ventral view); k. Bursa copulatrix (left lateral view). a–c. Cylapus amazonicus; d–f. Cylapus antennatus; g–k. Cylapus citus. dlp = dorsal labiate plate; odl = lateral oviduct; sd = seminal depository; sg = spermathecal gland; sr = sclerotized ring; vlp = ventral labiate plate; vstb = vestibulum.
FIGURE 17. Female genitalia. a, c, g, j, Bursa copulatrix (dorsal view); b. Bursa copulatrix (ventral view); d, k. Bursa copulatrix (dorsal view, with ventral labiate plate and seminal depository dissected); e, i, m. Ventral labiate plate and seminal depository (ventral view, with remainder of bursa copulatrix dissected); f, h, l. Bursa copulatrix (ventral view, with ventral labiate plate and seminal depository dissected). a, b. Cylapus marginicollis; c–f. Cylapus ruficeps; g–i. Cylapus tenuicornis; j–m. Cylapus tucuruiensis. dlp = dorsal labiate plate; odl = lateral oviduct; sd = seminal depository; sg = spermathecal gland; sr = sclerotized ring; vlp = ventral labiate plate.
FIGURE 18. Female genitalia. a–c. Bursa copulatrix (dorsal view); d. Ventral labiate plate (ventral view, with remainder of bursa copulatrix dissected). a. Peltidocylapus calyciformis; b–d. Peltidocylapus ecuadorensis. dlp = dorsal labiate plate; odl = lateral oviduct; sd = seminal depository; sg = spermathecal gland; sr = sclerotized ring; vlp = ventral labiate plate.
FIGURE 19. Female genitalia. a, b, d, e. Bursa copulatrix (dorsal view); c. Ventral labiate plate (ventral view, with remainder of bursa copulatrix dissected); f, g. Bursa copulatrix, posterior wall. a–c. Peltidocylapus parallelus; d, e, f. Peltidocylapus simplex; g. Cylapus tenuicornis. dlp = dorsal labiate plate; odl = lateral oviduct; sd = seminal depository; sg = spermathecal gland; sr = sclerotized ring; vlp = ventral labiate plate.
FIGURE 20. Female genitalia. Vestibular area. a. Amapacylapus amapariensis; b. Cylapinus minusculus; c. Cylapoides unicol- or; d. Cylapus antennatus; e. Cylapus citus; f. Cylapus tenuicornis; g. Peltidocylapus parallelus; h. Valdasus flavinotum.
FIGURE 21. Female genitalia, ovipositor. a–i. Apex of first gonapophysis; j–q. Apex of second gonapophysis. a, j. Amapacylapus amapariensis; b. Cylapinus minusculus; c, k. Cylapoides unicolor; d, l. Cylapomorpha sp.; e, m. Cylapus amazonicus; f, n. Cylapus citus; g, o. Cylapus ruficeps; h, p. Peltidocylapus ecuadorensis; i, q. Peltidocylapus parallelus.
FIGURE 22. Female genitalia. a, b, d–f. Bursa copulatrix (dorsal view); c. Bursa copulatrix (lateral view); g, h. Base of first gonapophyses; i–l. Apex of second gonapophysis. a. Psallops sp.; b, c, g, j. Afrovannius schmitzi; d. Vannius sp.; e, k. Cylapocoris sp.; f, h, l. Fulvius pallens; i. Leprocapsus scutellaris.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
1 (by plazi, 2021-12-06 08:45:40)
2 (by ExternalLinkService, 2021-12-06 08:57:06)
3 (by carolina, 2021-12-15 18:31:45)
4 (by ExternalLinkService, 2021-12-15 18:50:25)
5 (by ExternalLinkService, 2021-12-15 19:12:56)
6 (by ExternalLinkService, 2021-12-15 19:46:04)
7 (by plazi, 2023-11-08 10:55:03)