Camelus bactrianus, Linnaeus, 1758
|
publication ID |
https://doi.org/ 10.5281/zenodo.5719719 |
|
DOI |
https://doi.org/10.5281/zenodo.5719743 |
|
persistent identifier |
https://treatment.plazi.org/id/03928E69-9A49-FFC0-D040-FC84F6FFF24B |
|
treatment provided by |
Conny |
|
scientific name |
Camelus bactrianus |
| status |
|
5 View On .
Bactrian Camel
Camelus bactrianus View in CoL
French: Chameau / German: Kamel / Spanish: Camello bactriano
Other common names: Two-Humped Camel, Double-Humped Camel, Asiatic Camel, Wild Bactrian Camel
Taxonomy. Camelus bactrianus Linnaeus, 1758 View in CoL ,
habitat in Africa. Restricted to “Bactria” ( Uzbekistan, Bokhara) by Thomas in 1911 based on domestic stock.
Bactria and Bactriana were the Greek and Latin names for the ancient Persian provincial capital Baxtriya in Central Asia. Aristotle (384-322 Bc) applied the term “Bactrian” to the domestic camel associated with this eastern region of the Achaemenid Persian Empire, which prospered from Bactrian Camel caravans that transported goods between the East and West over the famed Silk Road. The question of the relatedness and genetic distinctiveness of wild and domestic Bactrian Camelsis actively being pursued. Scientists argue variously that: 1) the wild Bactrian is the progenitor of today’s domestic Bactrian; 2) wild Bactrians are escapees and feral forms of domestics; 3) both derived from a common ancestor now extinct; or 4) both derived from separate ancestors now extinct. Preliminary work by Chinese geneticists first suggested the two Bactrians were sufficiently different to warrant subspecies or potentially even species separation. Recent mtDNA studies by Austrian scientists favor the final argument, estimating divergence between the two camelids at 700,000 years ago in the Pleistocene long before Bactrian domestication (4000-6000 years ago). Some classify the wild Bactrian as Camelus gobi or Camelus bactrianus gobi, while others apply the name Camelus ferus due to a ruling by the International Commission on Zoological Nomenclature because C. ferus was first applied to the wild species. In this account, the two animals are differentiated at the subspecies level (wild = C. b. ferus and domestic = C. b. bactrianus ). Genetic justification for recognizing the domestic form as a separate species is growing, and all workers agree that the critically endangered wild Bactrian is in desperate need of protective conservation measures. Two subspecies recognized here.
Subspecies and Distribution.
C. b. bactrianusLinnaeus, 1758 — aridlandsanddesertsofC & SAsiaasdomesticatedlivestock.
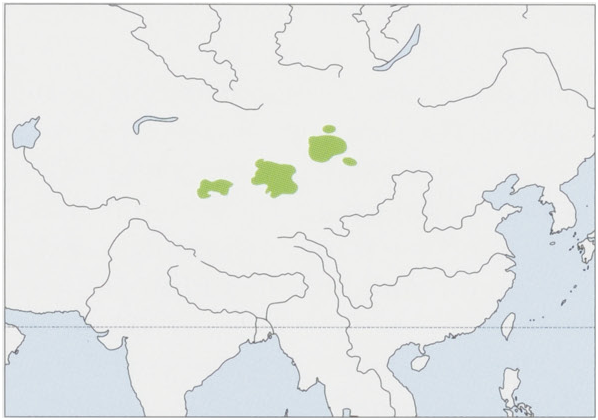
C. b. ferus Przewalski, 1878 — NW China (C & SE Xinjiang, Nei Mongol, NW Qinghai & NW Gansu) and Mongolia. View Figure
Descriptive notes. Head—body 320-350 cm, tail 51-64 cm, shoulder height 160-180 cm; weight 450-500 kg. Compared to Dromedary Camels ( C. dromedarius ), Bactrians have stouter and thicker bodies with relatively shorter legs, and two humps instead of one. Dromedaries are slimmer and taller. In winter the Bactrian’s long, woolly coat is sandy beige to dark brown, shedding in large clumps as temperatures warm in spring. They have a mane and a beard of long hair on the neck and throat, with hairs up to 25 cm long. Long eyelashes and sealable nostrils help to keep out dust in sandstorms. Two broad toes on each foot with undivided soles create a large, flat footpad that spreads widely as an adaptation for walking on sand. Their long face is somewhat triangular, with a split upper lip. Bactrians are well suited to cold and hot temperatures and are suspected to have physiological adaptations similar to those of Dromedaries. Their humps function the same way as Dromedaries’ humps, storing fat convertible to water and energy when there is a shortage of sustenance and enabling the animals to endure harsh desert conditions and periods of travel without water. As the fat depletes, the humps become floppy and flabby. Like Dromedaries, Bactrians rarely sweat, facilitating conservation of fluids. Dehydrated animals are able to drink 135 1 of water in 13 minutes. Although wild and domestic Bactrians have similar conformation and body structure, field observers report obvious differences in behavior, habits, and general appearance. Unlike the ponderous domestic Bactrians, wild ones are relatively lithe. Wild Bactrians are slimmer and smaller-bodied (laterally compressed), with slender limbs. They are sandy-gray-brown rather than predominantly dark brown, with shorter, sparser wool. There is no tuft of hair on top of the head, and the hair on the neck,tail, and knee joints is shorter. They do not have growths on the inner foreleg or callosities on the knees. The wild form has smaller ears and very narrow feet. Notably, their humps are lower, pointed, conical-shaped, and usually about half the size orless of the domestic Bactrian’s. They are timid and elusive.
Habitat. Bactrians are adapted for living in plains and hills where vegetation and water sources are sparse. They inhabit some of most inhospitable terrain in the world, centered in the extreme Gobi Desert of Central Asia. Gobi means “the rocky place” and it is primarily a rocky desert covered with small stones. There are low valleys and eroded hills, rocky-mountain massifs, “hamadas” (flat pavement-like stony plains), vast washed-out plains, high sand dunes, and rarely, poplarfringed oases. Summers are torrid, winters are cold. Annual precipitation is 50-200 mm. This is a region of extreme drought and scarce food and water. The Taklamankan Desert is sandy; Lop Nur Desert is both a rocky and sandy desert. Field studies in the Lop Nur Lake region of China revealed a Bactrian habitat preference for riparian communities and piedmont foothills; they were more often found in higher,hilly landscape than in the open. The Bactrians avoided gravel desert substrate and areas with hard, salt-crusted soil or moving sand dunes, preferring moderately hard ground. They stayed away from areas occupied by people. They used locations where the distance between foraging habitats and water sources did not exceed ¢.50-60 km and where there was relatively high coverage of halophytic vegetation, especially along lakeshores. In Mongolia preliminary results of satellite telemetry revealed a weak preference for habitat dominated by saxaul (Haloxylon ammodendrum) and no selection of areas with higher productivity or closer to permanent water sources. Climate change has altered Bactrian habitat by increasing the rate of desertification and reducing water resources and vegetation. Wild Bactrians have few native predators. Contrary to some accounts, Snow Leopards (Panthera uncia) pose no threat, and although Gray Wolf (Canis lupus) predation does occur,its impact is poorly understood.
Food and Feeding. Bactrians are opportunistic feeders capable of utilizing low-quality forage when more desirable herbage is unavailable. They are both grazers and browsers. Research on free-ranging domestic Bactrians in wild Bactrian habitat in the Gobi Desert provides the best information available on feeding habits. Those studies found that the camels’ main diet switched from senesced forbs in winter to a shrub in spring and to an increasing dependence on forbs in autumn. Although the shrub Haloxylon ammodendron was the dominant and highest biomass species in this desert community and a staple component of the camels’ diet each season, it was not preferred forage. Still, the shrub was essential when preferred forbs were not available in spring and summer. In autumn preferred annual forbs provided sufficient biomass. New plant growth during the wet season in summer and autumn was vitally important for replenishing the camels’ depleted fat reserves in preparation for winter. Domestic Bactrians are dependent on a year-long forage supply from the fragile and extremely arid environment they occupy. They show a preference for grazing on forbs, but are highly dependent upon browsing on shrubs. It has been reported that Bactrians can utilize salt water, with some researchers stating that salty water is necessary for their survival. When sufficient food is available in spring, summer, and autumn, Bactrians rarely have to drink, obtaining water requirements from plant moisture. In winter they have been observed to eat ice and snow.
Breeding. Breeding season is called the “musth” after the thick fluid exuded from a gland on the neck behind the head in adult males. A study of semi-captive wild Bactrians found that females reached puberty after three years and males at 5-7 years of age. Wild rutting males in Mongolia begin forming harems in late autumn (November) and mating in early winter, with most mating in January and February, but occasionally as late at March, or even May. Young males are driven out of the harems by rutting males and form bachelor herds; young females remain with their mothers. Males employ spitting (saliva and pseudorumen contents), kicking, biting, and screaming as offensive and defensive behaviors against each other during mating season. During the rut, wild males stretch their necks, shake their heads while roaring, and make loud noises with their teeth. Simultaneously they are foaming/frothing from the mouth and shaking their lips to throw foam on their head and chests. Occasionally they widen their hindlegs to urinate on their tails, using the tail to spread urine on their hips, hump, and head. The concentrated brown secretion from the 1-5 cm x 5 cm gland behind the head is rubbed on the forehump, giving it a darker color. The penis is 4 cm wide x 13-5 cm long. There is no use of dung piles by either sex. Estrous females are usually receptive in January or February for 4-7 days, at which time they follow males, knocking mating males off females and occasionally urinating. To mate, females lift theirtails after lying down in a sternal recumbancy position. Males typically mate with females 2-3 times in the morning or evening for 3-5 minutes each time. After mating, the genitals of females swell and become pink. Females are semen-induced ovulators. Ovulation occurs 30-48 hours after mating. Gestation of semi-captives in Mongolia was 390-430 days (13-14 months) depending upon the age of the female and the number of previous calves. Females give birth to a single offspring while standing, but only every 2-3 years. Spring birth season is from March to April. Females giving birth isolate themselves from other animals and people, remaining alone for about two weeks. Calves are able to stumble-walk within 15-30 minutes. They weigh 32-34 kg at birth and nurse every 1-2 hours. Body growth levels off at seven years. Domestic Bactrians produce ¢.760 I of milk during 16 months of lactation. Calves are commonly weaned at 10-11 months but may nurse for up to 18 months. In captive Bactrians there is liberal and indiscriminate allonursing (adult females nursing non-offspring); this has been observed about one-third of the time, and was not correlated with the age of the mother or relatedness of the calf. Spring to autumn calf mortality is high, at 50%. Poor calf survival is often the result of drought, sandstorms, and freezing temperatures. Aerial surveys in the early 2000s counted only 3-9 young/ 100 adults,significantly lower than surveys in 1980 and 1998. The remaining Bactrian population cannot sustain itself with such a low calf-survival rate. Wild and domestic Bactrian Camels readily interbreed, producing fertile-hybrid offspring that can be differentiated by genetic testing based upon fixed mitochondrial sequence polymorphism. They can live as long as 35 years.
Activity patterns. Activity budgets are an indication of an animal’s welfare since its primary activities are centered on energy acquisition, conservation, or expenditure. Bactrian Camels are diurnal feeders and nocturnal sleepers. In winter they spend less time foraging and more time resting than in spring and summer. There is high foraging activity in spring, triggered by readily available shrubs with minimal walking required. Daily patterns of activity are structured to expend the least amount of energy to obtain food, even when the food is not preferred forage.
Movements, Home range and Social organization. Bactrians often move 50-100 km seeking permanent surface water and patches of foraging habitat. Some populations are suspected to be migratory, especially those in the Chinese Lop Nur Lake region where the animals move between lakeside riparian communities in winter and cooler foothills in the summer. Researchers tracked seven wild camels in the Mongolian Gobi for one year, using satellite telemetry. One adult female moved a minimum of 4527 km in a home range of 17,232 km. She spent 75% of her time within 8699 km?. Ground surveys in Mongolia report that the Bactrian population was composed of 82% adults, 12% juveniles (non-reproductive young), and 6% calves (depending upon the time of year). Groups sighted in China numbered 4-40 individuals and in Mongolia from one animal to dozens, with researchers frequently encountering groups of more than 50 animals. From 1982 to 1989, when a total of 2370 wild Bactrians and 675 groups were sighted, average herd size was six (5-3—-6-5) with 6-20 animals/group the most common. Other than sporadic and irregular sightings in the vast Gobi Desert, where low densities of Bactrian Camels occur, we know almost nothing of their year-round social organization. However, it would not be surprising to find that the Bactrian social system is similar to that observed in wild/feral Dromedaries of Australia. Gray Wolves have been cited as a contributing factor to the Bactrian’s decline in both China and Mongolia, but the evidence is weak because it is primarily based upon camel remains in scats, which could have been from scavenging.
Status and Conservation. The wild subspecies is classified as Critically Endangered on The IUCN Red List and Endangered by the US Fish and Wildlife Service. It is considered by some to be on the verge of extinction. Protected nationally in China and Mongolia. The Bactrian’s original distribution and natural habitat was the entire Asian Trans-Altai Gobi Desert, stretching from the great bend of the Yellow River in north-east China throughout Mongolia to central Kazakhstan in the west, at elevations 1500-2000 m above sea level in an area that encompassed nearly 100,000 km *. Today they are greatly reduced to a few small and fragmented populations in south-western Mongolia and nearby areas of north-western China in a total area ¢. 28,000 km *. Four populations of wild Bactrians survive: the Taklamakan Desert; Altun Mountains and Archik Valley; and Gashun Desert populations in China; and the Outer Gobi Desert population of Mongolia and China, which migrates between the two countries. Overall numbers greatly reduced in the past century because of hunting for their meat and hides, habitat loss due to grazing, and competition for water with domestic livestock (domestic camels, sheep, goats, horses, and cattle), interbreeding with domestic camels, mining activities, and poor reproduction and survival of young. Because of harsh climatic conditions, lack of appropriate aircraft for aerial surveys, and the remote and vast region where the remaining Bactrians live, there is uncertainty as to the total numbers remaining. The famous Russian explorer Nikolai Przhevalsky, who is given credit for discovering the wild Bactrian Camel of Asia during his travels to Mongolia and China, noted in 1876: “According to our informants wild camels are numerous in north-western Tsaidam, where the country is barren, the soil being clay, overgrown with budarbana, and so destitute of water that they have to go several miles to drink, and in winter are obligated to satisfy their thirst with snow. The herds are small, averaging five to ten in each, never more than twenty. Their appearanceis slightly different from the domesticated breed: their humps are smaller, the muzzle more pointed, and the color of the hair gray.” It has been reported that wild Bactrians experienced drastic reduction in numbers and range over the past 10-20 years, but the most recent and reliable data suggests that populations in Mongolia have been relatively stable if not increasing. Actual numbers are unclear and little is known. Estimates of total population size from incomplete ground surveys are as low as 730-950 individuals with projections from aerial surveys in Mongolia of 4335 individuals. Attempts to assist with Bactrian conservation efforts in Mongolia through a captive breeding program have been unsuccessful. In China, the population is suspected to be decreasing in the Taklamakan Desert, because of oil development, but not in the Lop Nur Wild Camel National Nature Reserve. The wild Bactrian Camel may well be one of the most endangered large mammals on Earth, and our knowledge ofits basic biology and ecology is dismally poor. Research is needed on the comparative genetics of wild and domestic forms, reproductive physiology, population structure and dynamics, and habitat requirements. Bactrian camels need immediate conservation attention, including standardization of methodology for accurate population surveys in both China and Mongolia; development of a comprehensive conservation program; increasing support among local people, including control of illegal hunting; habitat improvement; prevention of hybridization with domestic camels that threatens the gene pool of the Mongolian wild camel population, particularly in the Great Gobi “A” Strictly Protected Area and its associated Buffer Zone; and an increase in the number and size of protected areas. The Lop Nur Wild Camel National Nature Reserve in China, a former nuclear test site, is especially critical because it is believed to contain the most genetically pure individuals of the species.
Bibliography. Adiya et al. (2004), Al-Ani (2004), Bannikov (1976), Burger & Charruau (2011), China Statistical Yearbook (2008), Gauthier-Pilters & Dagg (1981), Gentry et al. (2004), Grubb (2005), Guoying (2001), Han Jie et al. (2002), Hare (1996, 1997, 1998, 2008), Indra et al. (2002, 2003), Ji Rimutu et al. (2009), Menglia et al. (2006), Mijiddorj (2002a, 2002b), Mix et al. (2002), Peters & von den Driesch (1997b), Potts (2004), Reading, Blumeret al. (2005), Reading, Enkhbileg & Galbataar (2002), Reading, Mix, Blumer et al. (2002), Reading, Mix, Lhagvasuren & Blumer (1999), Schaller (1998), Silbermayr et al. (2010) Tilson (1986), Tserenbaljid (2002), Tulgat (2002), Tulgat & Schaller (1992), Weidong et al. (2002), Wang Zhenghuan et al. (2002).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.