Microcambeva bendego, Medeiros & Moreira & Pinna & Lima, 2020
|
publication ID |
https://doi.org/10.11646/zootaxa.4895.1.6 |
|
publication LSID |
lsid:zoobank.org:pub:988D35CE-5652-485F-A1F4-01E55D3F20C9 |
|
DOI |
https://doi.org/10.5281/zenodo.4323864 |
|
persistent identifier |
https://treatment.plazi.org/id/03925B12-ED6B-FFB6-FF5F-FF7EFDBDFE95 |
|
treatment provided by |
Plazi |
|
scientific name |
Microcambeva bendego |
| status |
sp. nov. |
Microcambeva bendego new species
( Fig. 1 View FIGURE 1 , Table 1 View TABLE 1 )
Holotype. MNRJ 52042 View Materials , 28.1 mm SL; Brazil: Rio de Janeiro State, Guapimirim Municipality; rio Guapiaçu near Cachoeiras de Macacu, rio Guapi-Macacu basin, 22°35’33”S 42°53’20”W, P. A. Buckup, D. F. Moraes-Jr and V. Brito, 31 Aug 2016. GoogleMaps
Paratypes. MNRJ 48616 View Materials , 1, 26.9 mm SL, collected with holotype GoogleMaps . MZUSP 125789 View Materials , 1, 27.8 mm SL, collected with holotype GoogleMaps .
Diagnosis. Microcambeva bendego is distinguished from all congeners by the long finger-like projections, as long as orbital diameter ( vs. projections absent in M. filamentosa and smaller than orbital diameter in the remaining congeners), and the more numerous opercular odontodes (19 vs. 6–7 in M. barbata , 9–12 in M. mucuriensis , 9–14 in M. ribeirae , 11–12 in M. draco and M. filamentosa , and 13–15 in M. jucuensis ). It is also distinguished from all congeners, except M. ribeirae , by the unmodified first pectoral-fin ray ( vs. first pectoral-fin ray filamentous). It differs from all species of the genus, except M. ribeirae and M. filamentosa , by the absence of ossification in the anterior cartilage of the autopalatine ( vs. presence), and by the supraorbital pores (s6) fused into a single median pore, positioned on the middle of head ( vs. paired s6 pore). The new species differs further from M. filamentosa by having 8 interopercular odontodes ( vs. 6) and 33 vertebrae ( vs. 36), and from M. ribeirae by the rictal barbels reaching the anterior portion of the interopercular patch of odontodes ( vs. reaching middle of orbit), and the three first rays of the dorsal-fin unbranched ( vs. two).
Description. Morphometric data of holotype and two paratypes presented in Table 1 View TABLE 1 . Body elongated, cylindrical immediately posterior to head to pelvic-fin origin, gradually compressed towards caudal peduncle ( Fig. 1a View FIGURE 1 ). Dorsal profile gently convex from snout to dorsal-fin origin, and straight along caudal peduncle. Ventral profile convex from jaw to pelvic-fin insertion, straight from that point to terminus of caudal peduncle.
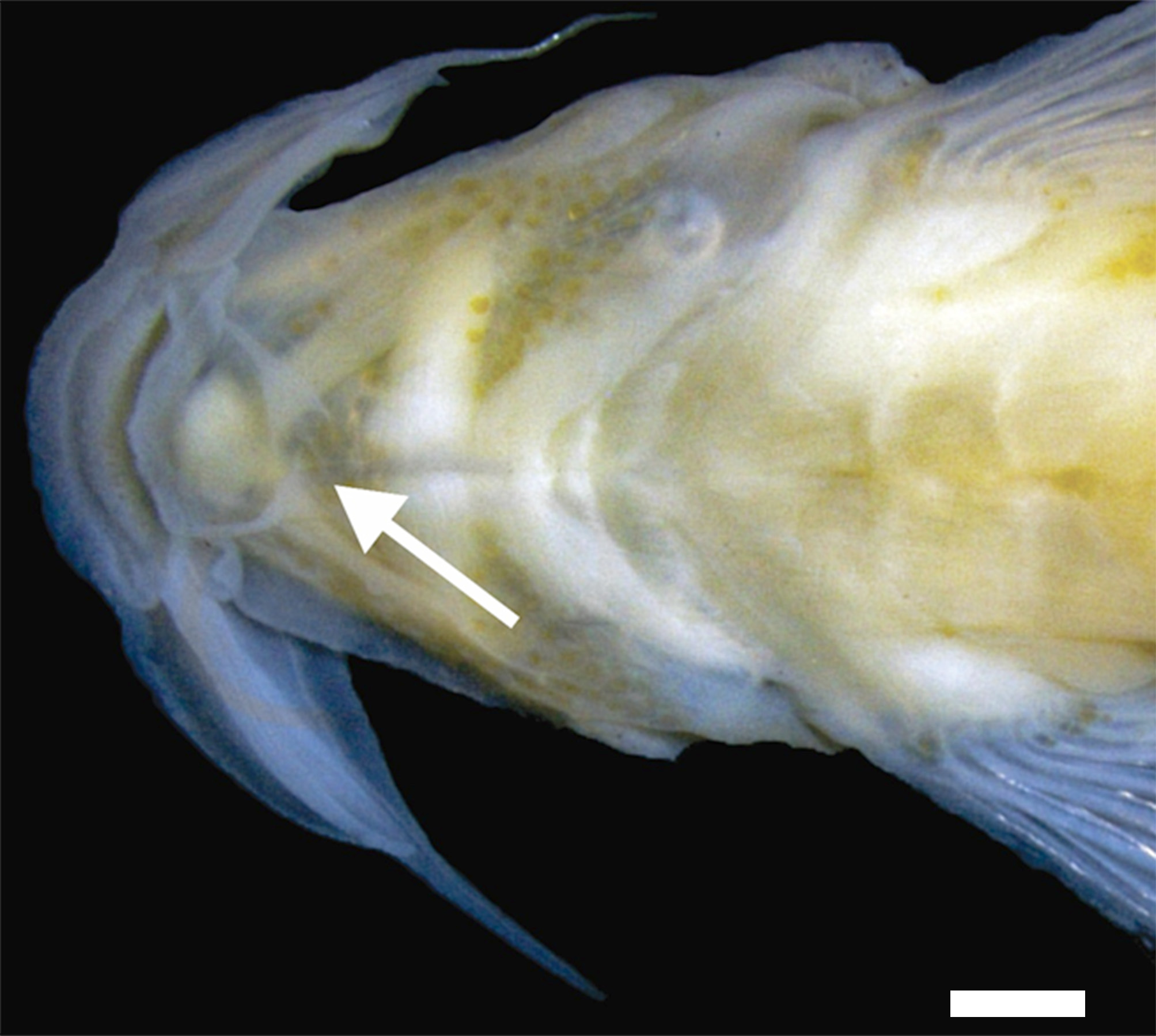
Head triangular in dorsal view, moderately depressed, longer than wide ( Fig. 1b View FIGURE 1 ). Mouth subterminal. Snout rectangular, with slight lateral compression after origin of maxillary barbel. Nostrils circular, posterior ones larger than anterior ones. Anterior nostril surrounded by skin continuous with nasal barbel, posterior nostril with small half-moon skin fold, approximately as large as nasal opening. Posterior nostril closer to anterior nostril than to margin of eye, anterior nostril closer to upper lip than posterior nostril. Barbels tapering distally. Nasal barbel originating on median portion of anterior nostril, reaching posterior portion of posterior nostril. Maxillary barbel reaching posterior half of interopercular patch of odontodes. Rictal barbel reaching anterior half of interopercular patch of odontodes. Pair of finger-like projections approximately as long as eye diameter, inserted anterior to branchial isthmus ( Fig. 2 View FIGURE 2 ). Eyes round, positioned dorsally at middle distance between snout and posterior portion of opercular patch of odontodes.
Pectoral fin subtriangular, with seven rays (i+6). First unbranched ray not filamentous, approximately 60% as long as first branched ray, last branched ray shortest. Tips of rays extending beyond interradial membrane. Adipose organ round, translucent, dorsal to pectoral fin. Dorsal fin semicircular, nine rays (iii+6), origin at vertical through 14 th vertebrae. Pelvic fins subtriangular, with five rays (i+4) extending beyond interradial membrane; pelvic-fin origin at vertical through 10 th vertebrae, its tip not reaching urogenital papilla. Urogenital papilla conic. Anal fin with seven rays (iii+4) and semicircular distal margin, originating at vertical through 19 th vertebrae. Caudal fin truncated, with 13 rays (i+11+i), six in dorsal plate (i+5) and seven in ventral plate (6+i). Six procurrent caudal-fin rays dorsally and ventrally. Vertebrae 33, 27 caudal and six precaudal. Five pleural ribs. Six branchiostegal rays.
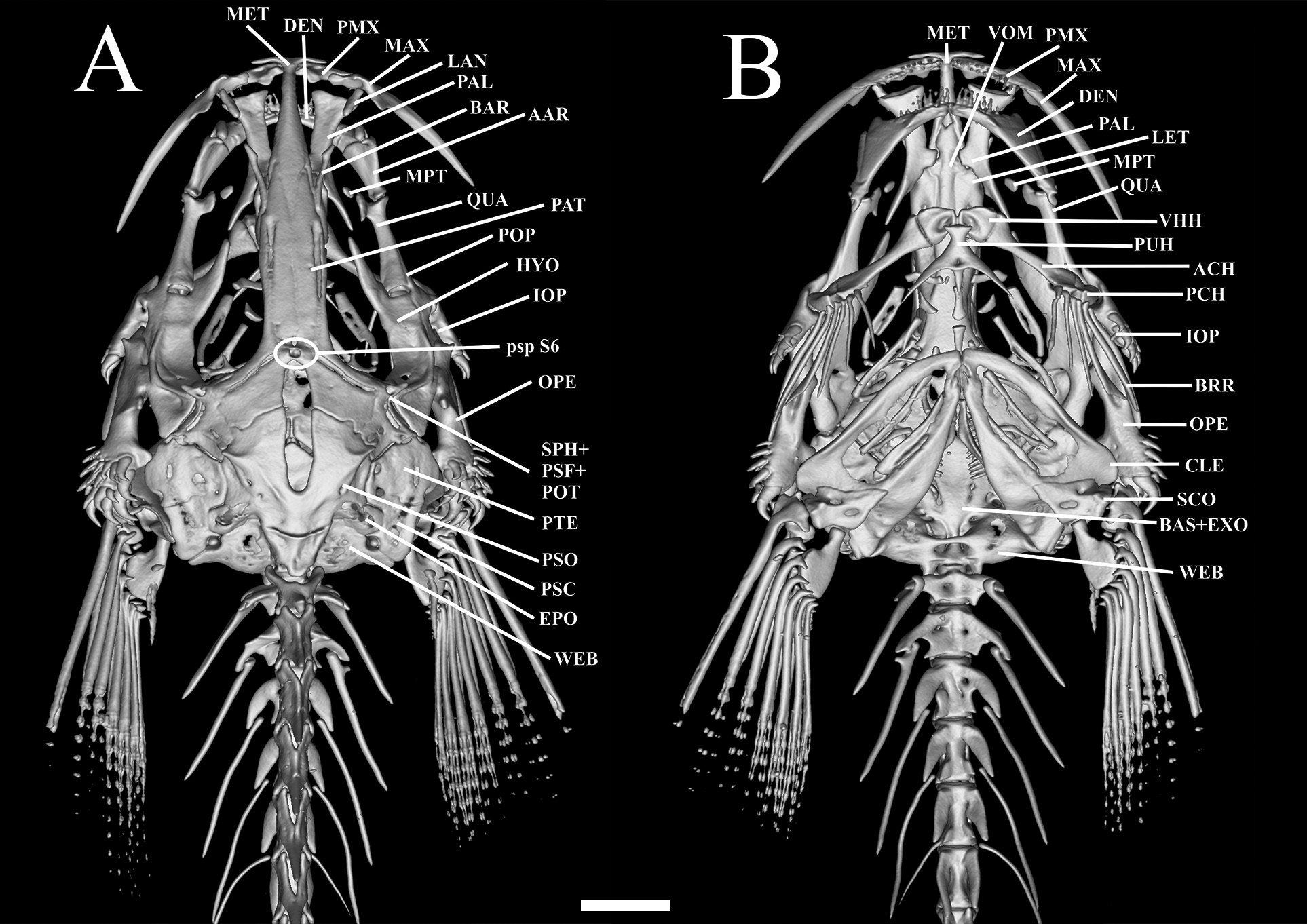
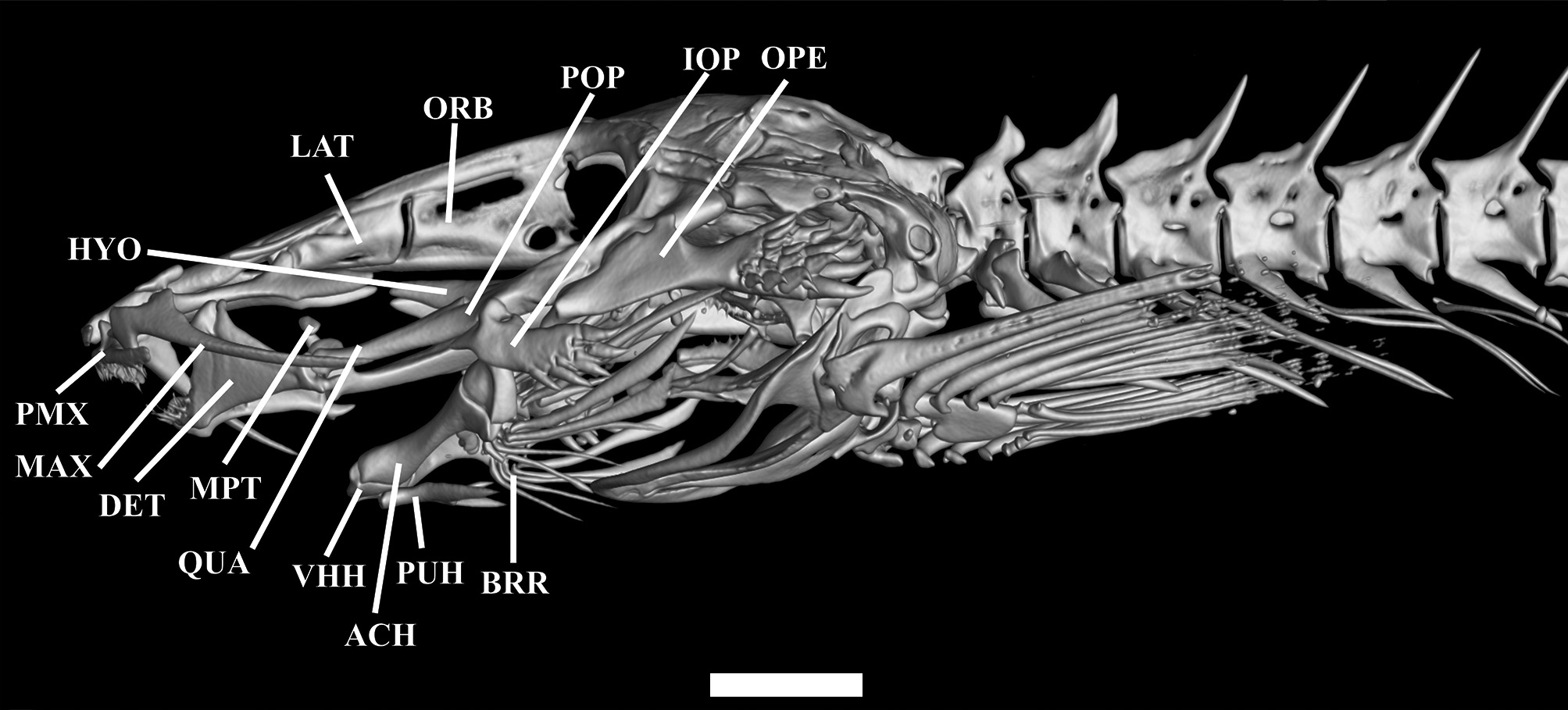
Mesethmoid long, cornua short, straight and pointed; slight depression at anterior portion of the mesethmoid; main body of bone compressed at posterior portion, and pointed. Lateral margin straight along mid-length ( Fig.3 View FIGURE 3 ). Frontals slender; progressively more spaced from each other posteriorly. Two pores of supraorbital canal present, s3 pore opening at anterior portion of frontal, and median s6 pore at center of neurocranium, near anterior edge of cranial fontanel. Cranial fontanel extending for approximately 75% of neurocranium length, with rounded anterior and posterior ends. Sphenotic+ pterosphenoid+ prootic trapezoid, prominent, with elongated and pointed lateral process at middle portion, bearing part of laterosensory canal, opening as single pore i11. Pterotic square-like, without lateral process, bearing preopercle pore i11. Epioccipital rectangular. Posttemporo-supracleithrum rectangular, with small lateral process near its proximal portion. Vomer long, arrow-shaped, with lateral constriction at anterior portion and forked anteriorly; thin lateral projections anterior to constriction. Lateral ethmoid rectangular, without lateral projections. Basioccipital fused with exoccipital anteriorly and with Weberian complex posteriorly.
Premaxilla with two tooth rows. Eight conical teeth in labial row and 12 in lingual; premaxillary dorsal protuberance present and expanded near palatine cartilage ( Fig. 3 View FIGURE 3 ). Maxilla long, 50% larger than premaxilla, its distal tip pointed and triangular expansion on its ventrolateral portion. Autopalatine long with moderately concave lateral margin, and straight mesial margin. Posterior process of autopalatine long and pointed. Lacrimal-antorbital elongated and cylindrical approximately 75% of barbular length. Barbular bone long and cylindrical; lacrimal-antorbital and barbular disposed in line, separated by gap approximately equivalent to length of lacrimal-antorbital.
Metapterygoid small and elliptical. Quadrate elongated and concave, with small dorsally-directed process forming distal synchondrosis with metapterygoid ( Fig. 4 View FIGURE 4 ). Quadrate-hyomandibula fenestra lacking defined shape. Hyomandibula narrow, long, with pointed anterior process with slightly ventrally-curved anteriorly. Ventroposterior margin of hyomandibula with lateral condyle articulating with opercle. Preopercle narrow, pointed anteriorly and round posteriorly, its anteromedial region concave with lateral extension articulating with interopercle, its central portion with pointed process. Opercle narrow, with 19 conical, posteriorly-directed odontodes arranged obliquely in five irregular rows on posterior region. Opercle with pronounced dorsomedial concavity and small pointed process anteriorly. Opercle articulating dorsally with hyomandibula via small condyle on anterior medial process. Interopercle narrow with eight conical, posteriorly-oriented odontodes arranged in two oblique irregular rows on posterior region. Interopercle with deep concavity in dorsomedial region, with small pointed process on anterior portion. Odontodes progressively larger and more strongly curved posteriorly, on both opercle and interopercle. Dentary straight, with two rows of small conical teeth, seven teeth in inner row and nine in outer one. Prominent coronoid process and associated Meckel’s cartilage.
Parurohyal with two long wings tapering gradually from base to tip, with slight concavity between them. Posterior median process straight and pointed, shorter than lateral wings. Hypobranchial foramen small and circular. Anterior portion of parurohyal with two small condyles articulating with ventral hypohyals ( Fig. 3B View FIGURE 3 ). Ventral hypohyal triangular, with gentle concavity on dorsal portion and slightly depression on ventral portion, accommodating parurohyal condyles. Anterior ceratohyal cylindrical, with constriction at middle; its anterior margin nearly straight, its posterior margin concave dorsally and convex ventrally with both ends lined with cartilage. Posterior ceratohyal roughly triangular, with irregular margins. Six branchiostegal rays, cylindrical and pointed, three on posterior ceratohyal, one on interceratohyal cartilage and two on posterior ceratohyal.
Basibranchial 1 absent. Basibranchial 2–3 rod-like with anterior and posterior tips cartilaginous; basibranchial- 4 fully cartilaginous, circular and flattened, with slight constriction at middle ( Fig. 3B View FIGURE 3 ). Hypobranchial 1, rod-like, anterior and posterior ends with globose cartilages; hypobranchial 2–3 conical, with constriction at middle. Ceratobranchial 1–4 cylindrical, with irregular dorsal margins. Ceratobranchial 1–2 with small laminar expansion at ventromedial region; ceratobranchial 3 with small round process at ventromedial region; ceratobranchial 4 with pointed process near anterodorsal margin. Five conical pharyngeal teeth irregularly arranged on ceratobranchial-5. Epibranchial 1 Y-shaped, with pronounced concavity at distal portion. Epibranchial 2 rod-like, with two lateral expansions, a mid-dorsal one directed anteriorly, and an anteroventral one directed posteriorly. Epibranchial 3 laminar with expansion distally at anteroventral portion; epibranchial 4 rectangular. Pharyngobranchials 1–2 absent; pharyngobranchial 3 rod-like, with thin roundish cartilages at tips; pharyngobranchial 4 entirely cartilaginous, inserted at anterior portion of upper pharyngeal tooth plate. Tooth plate large, trapezoid in shape, with 12 conical teeth distributed in single row on ventral surface.
Cleithrum triangular and flat; scapulacoracoid ossified, roughly triangular. Basipterygium square, excluding anterior processes; internal and external processes long and slender with pointed tips; external process larger than internal; posterior process rudimentary; pelvic splint pointed and small. Hypural complex composed of two plates ( Fig. 5 View FIGURE 5 ). Lower plate rectangular, supposedly corresponding to fused parhypural plus hypurals 1–2, and upper plate corresponding to hypurals 3–5. Uroneural long and equal in size to adjacent neural spine, in contact with, but not fused to, upper hypural plate. Two procurrent rays associated with uroneural. Neural spine long and pointed, reaching approximately half of upper hypural plate. Hemal spine larger than neural one, not contacting lower hypural plate, and reaching to distal portion of lower hypural plate; two procurrent rays associated with hemal spine. Ventral procurrent rays larger than dorsal ones ( Fig. 5 View FIGURE 5 ).
Color in alcohol. Dorsum, lateral and ventrum of body, almost yellowish pale. Four rows of large dark spots, two along sides, one middorsal and one midventral. Lower lateral row midlateral, with nine unevenly-spaced spots extending along longitudinal skeletogenous septum. First spot dorsoposteriorly to base of pectoral fin. Last spot of midlateral series largest, covering hypural plate and central portion of base of caudal fin. Upper lateral row extending along dorsal part of flanks, with three well-defined, bilaterally-aligned anterior spots (third one at transverse line through origin of dorsal fin), plus three or four poorly-defined and bilaterly misaligned ones. Middorsal series with three spots evenly spaced between occiput and origin of dorsal fin (with third one right at fin origin), plus fourth one at middle of dorsal-fin base. Anterior two middorsal spots intercalated with those of upper lateral row. Isolated final middorsal spot on terminus of edge of caudal peduncle. Midventral row with three spots, aligned respectively with distal portion of pelvic fins, middle of anal-fin base and middle of caudal peduncle. Faint files of dark chromato-phores along limits of myotomes, more pronouncedly on epaxial series. Head with large dark field extending over middle of posterior part of neurocranium, from slightly anteriorly to eyes to limit of epaxial musculature, forming two dark arms at that limit. Cephalic color pattern poorly-defined. Eyes and iris black. Narrow dark field extending laterally between anterior and posterior nostrils. Remainder of dorsal surface of head with light uniform covering of dark chromatophores. Nostrils outlined in abrupt white, in stark contrast with remainder of dorsal surface of head. Dark concentration on opercular patch of odontodes. Ventral part of head entirely white. Maxillary and nasal barbels with dark chromatophores concentrated at base. Rictal barbel white. Dark concentrations on proximal third of pectoral fin. Remaining fins hyaline. Pale-gray vertical bar on caudal-fin base.
Etymology. The specific epithet is derived from the second-largest meteorite discovered in Brazil, the Bendegó. Found in 1794 in Northeastern Brazil, it was transported to the Museu Nacional in 1888, where it became part of its exhibit ever since. In 2018, a fire of huge proportions destroyed part of the Museu Nacional, Universidade Federal do Rio de Janeiro (MNRJ) including some of its bicentennial collections. Even though part of the building collapsed, the Bendegó remained intact at the main entrance of the museum, where it was seen by the crowd that gathered the day after the fire, becoming a symbol of the resistance of the institution. This is not only an homage to the MNRJ, its employees and students, but also an allusion to the resilience of the species herein described in Atlantic Forest basin severely impacted by anthropic actions. A noun in apposition.
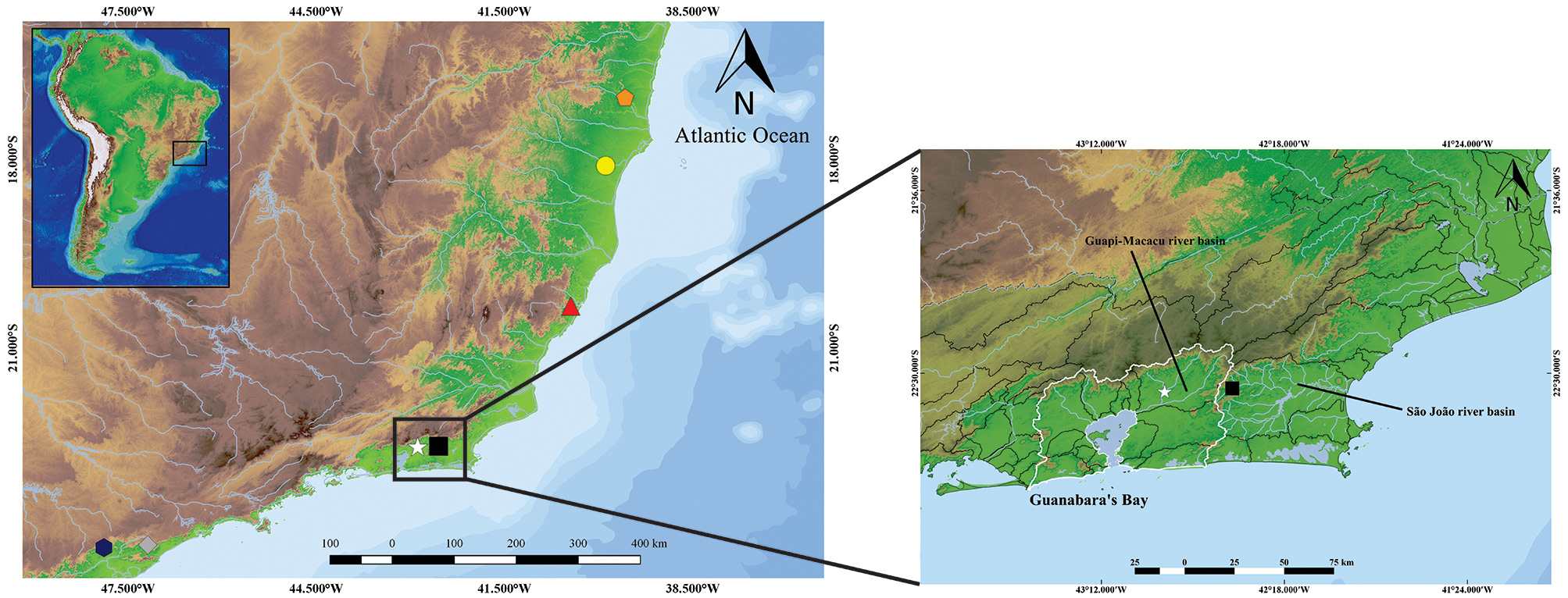
Distribution. Known only from its type locality, in the middle section of the rio Guapiaçu, in the Guapi-Macacu system, a basin that drains directly into the northeastern portion of Guanabara Bay ( Fig. 6 View FIGURE 6 ).
Habitat and ecological notes. Microcambeva bendego was found in the middle course of rio Guapiaçu, in a stream approximately 10 m wide and 1 m deep, with sandy bottom and riparian vegetation composed mostly of grass-like Cyperaceae . The new species was collected along with Hypostomus affinis (Steindachner 1877) , Parotocinclus maculicauda (Steindachner 1877) and Rineloricaria sp., plus two introduced aquarium species, the three-spot gourami Trichopodus trichopterus (Pallas 1770) and the jewel tetra Hyphessobrycon eques (Steindachner 1882) . A recent visit to the type locality did not secure any Microcambeva specimens. According to local fishermen, the African catfish Clarias gariepinus (Burchell 1822) is also found in the stream.
Discussion. Microcambeva bendego has all four putative synapomorphies so far proposed for the genus, viz., a reduction in the quadrate process, a rectangular ventral hypural plate; and a reduced number of opercular and interopercular odontodes ( Costa & Bockmann 1994; Costa et al. 2020a; 2020b), although polarity of those characters are debatable. The new species has the largest number of opercular odontodes (19) in the genus ( 6–7 in M. barbata , 9–14 in M. mucuriensis , M. ribeirae , M. draco and M. filamentosa , and 13–15 in M. jucuensis ) ( Costa & Bockmann 1994; Costa et al. 2004; 2019a; 2020b).
An additional putative synapomorphy of the genus is the presence of a distinct pair of finger-like projections, similar in position to a mentonian barbel. This barbel of unknown function occurs in all species of the genus, except M. filamentosa ( Costa et al. 2020b) , and at least five unrelated trichomycterid species, Pseudostegophilus maculatus (Steindachner 1879) , Malacoglanis gelatinosus Myers & Weitzman 1966 , Stenolicmus sarmientoi de Pinna & Starnes 1990 , Ammoglanis pulex de Pinna & Winemiller 2000 , and A. obliquus Henschel, Bragança, Rangel-Pereira & Costa 2019 ( de Pinna & Winemiller, 2000; Henschel et al. 2020). The structure in M. bendego is the largest so far recorded for the genus, in stark contrast to the minute barbels seen in M. draco , M. jucuensis , and M. mucuriensis and the medium-sized ones in M. barbata and M. ribeirae .
Microcambeva ribeirae , M. filamentosa and M. bendego share three character-states not observed in other congeners: median fusion of the third pore (s6) of the supraorbital canal, absence of the anterior autopalatine ossification, and a deep concavity between the base of the ascending process of opercle and the patch of odontodes. Among Microcambeva , the new species additionally shares exclusively with M. ribeirae two distinct features: a non-filamentous first pectoral-fin ray and conspicuous series of dark spots along the body. In other congeners, the first pectoral-fin ray is modified as a filament, as the long filament in M. filamentosa (70–80% pectoral-fin length without filament), medium sized in M. barbata (40%) and M. draco (50%), and short in M. jucuensis and M. mucuriensis (5% to 10%). In the sarcoglanidines Sarcoglanis simplex Myers & Weitzman 1966 and Malacoglanis gelatinosus , the trichomycterine Scleronema Eigenmann 1917 , and in the stegophiline Ochmacanthus batrachostoma ( Miranda Ribeiro 1912) a small reduction in the size of the first pectoral filament is observed ( Myers & Weitzman 1966; Neto & de Pinna 2016; Ferrer & Malabarba 2020); however this reduction is not comparable to that observed in M. ribeirae and M. bendego .
Several psammophilic species display a reduction in integumentary pigmentation, as observed in the sarcoglanidines S. sarmientoi , Stauroglanis gouldingi de Pinna 1989 , Microcambeva and Ammoglanis Costa 1994 species. Species of Scleronema , have a yellowish background and patterns composed of rounded brown blotches ( de Pinna, 1989; de Pinna & Winemiller 2000; Ferrer & Malabarba 2020). A unique pattern of color of the body, composed of a yellowish background with larger dark spaced spots is observed in Stenolicmus ix Wosiacki, Coutinho & Montang 2011, M. ribeirae and M. bendego , however in the Amazon species, the spots are distributed irregularly along the middle of the body, while in Atlantic forest species, the spots form a series along the middle of the body. These shared characteristics suggest that M. bendego is more closely related to M. ribeirae from the Ribeira de Iguape ecoregion than to species in its own Fluminense ecoregion.
The apparent sister-group relationship between M. bendego and M. ribeirae is surprising given that the new species is geographically closer to M. barbata , with the two type localities separated by mere 35 km in straight line. Geomorphological data, however, lend support to this biogeographic pattern. The Guapi-Macacu and S„o Jo„o drainages are separated by the Serra do Sembê, which extends from the Serra dos Órg„os Mountains to the Cabo Frio Magmatic Lineament (CFML) ( Riccomini et al. 2005). These high and ancient rocky escarpments act as a geographical barrier, which has influenced the vicariant patterns and the high diversity of freshwater fish species in the Fluminense ecoregion ( Lima et al. 2017). This is reflected in some taxa with different species on each side of this divide, such as the Atlantirivulus Costa 2008 rivulids, and Parotocinclus Eigenmann & Eigenmann 1889 loricari-ids, and Listrura and Homodiaetus Eigenmann & Ward 1907 trichomycterids ( Koch 2002; Villa-Verde et al. 2012; Roxo et al. 2017; Lima et al. 2017). In addition, Santos et al. (2009) and Pereira et al. (2013) also demonstrated that Hoplias malabaricus (Bloch 1794) lineages’ from Guanabara Bay drainages are more closely related to those from the distant Paranaguá Bay, in Paraná State, than to the rio S„o Jo„o basin, corroborating the effectiveness of this barrier for the freshwater aquatic biota ( Riccomini et al. 2005; Lima et al. 2017). Thus, the apparently geographically counter-intuitive relationships of M. bendego actually fits a broader biogeographical pattern.
It is not ideal to describe a species based on few specimens, because obviously there is not enough material to address such basic questions as ontogenetic or intraspecific variation ( de Pinna et al. 2020). Still, some important recent discoveries on trichomycterid diversity were based on few individuals, e.g: M. barbata (n=4), M. draco (n=2), Listrura boticario de Pinna & Wosiacki 2002 (n=1), L. depinnai Villa-Verde, Ferrer & Malabarba 2014 (n=2), and Trichogenes beagle de Pinna, Reis & Britski 2020 (n=3) ( Costa & Bockmann, 1994; de Pinna & Wosiacki 2002; Mattos & Lima 2010; Villa-Verde et al. 2014; de Pinna et al. 2020). The realities of this kind of research sometimes make it imperative to release new taxonomic knowledge on elements of biodiversity even with limited material. Species must be made available for phylogenetic, biogeographic, ecological and conservation strategies ( Silva et al. 2019; de Pinna et al. 2020), especially for those occurring in highly impacted areas, such as the Guanabara Bay area in the present case.
Conservation status. The Guanabara Bay is composed of approximately 40 rivers and streams, and the region has been sampled by many naturalists since the 19 th century. The type locality of the new species is near large urban conglomerates and rural properties concentrating approximately 70% of the metropolitan region of Rio de Janeiro State, including a major industrial center ( Bizerril & Primo 2001). The rio Guapi-Macacu basin has an area of ca. 1.600 km ², making it the largest drainage flowing into the Guanabara bay. Inserted in the inner portion of the basin, with its headwaters in the Serra dos Órg„os mountain range, it is considered as a biodiversity pocket of the Guanabara Bay region. It has been heavily impacted by unrestrained urban occupation, intensified in the mid-1930, deforestation of riparian forests, fires, diversion of river courses and water pollution ( Bizerril & Primo 2001).
The intense degradation of the Guanabara’s Bay aquatic environments is evidenced by its numerous threatened freshwater fish species, such as the ‘endangered’ Brycon insignis Steindachner 1877 , Hyphessobrycon flammeus Myers 1924 , and Spintherobolus broccae Myers 1925 , and the ‘critically endangered’ Characidium grajahuense Travassos 1944 , Listrura nematopteryx de Pinna 1988 , Kryptolebias brasiliensis (Valenciennes 1821) , Leptolebias marmoratus (Ladiges 1934) , Leptopanchax splendens (Myers 1942) , and L. opalescens (Myers, 1942) (ICMBio, 2018). While species previously considered extinct such as L. splendens , were later rediscovered in remnant natural areas of the Guanabara Bay region ( Costa et al. 2019b), others like Leptopanchax sanguineus Costa 2019 , may already be extinct (Costa 2019). Considering that Microcambeva bendego was only collected in a single locality consisting in a highly degraded habitat of well-sampled drainage, that recent efforts failed in the obtaining additional specimens, and its area is occupation of less than 20 km 2, the B2ab(iii) critically endangered category is recommended as an obvious preventive measure (IUCN 2012).
The coastal basins of Atlantic Forest harbor many endemic and restricted-range freshwater fish species. However, the habitat loss along five centuries of colonization has left its mark, with several of those species currently threatened ( Abilhoa et al. 2011). To some of these ‘endangered’ species, the creation or expansion of conservation units encompassing small hydrographic basins and associated riparian zones in southeastern Brazil are important measures ( Menezes et al. 2007). In the estuarine portion of rio Guapi-Macacu basin, there are two conservation units, the Guanabara Ecological Station (GES) between the municipalities of Itaboraí, Guapimirim, S„o Gonçalo and Magé, covering an area of 1,420km ², and the Guapi-Mirim Environmental Protection Area (GMEPA), covering an area of 138.25 km ². Outside the protected areas this region is severely impacted, with the riparian zone deforested, diversion of river courses to agriculture, and the introduction of exotic species ( Bizerril & Primo 2001). Although the type locality of M. bendego is close to the buffer zone of the GES inside in GMEPA, its geographic distribution is limited to the middle section of the rio Guapiaçu. A measure that will contribute to the conservation, not only of the new species, but also of other aquatic species, is the expansion of the GES area to the middle portions of the rio Guapi-Macacu river basin to protect most freshwater habitats and their associated biota ( Azevedo-Santos et al. 2019).
Comparative material. Rio de Janeiro: Microcambeva barbata: MZUSP 43678, 24.7 mm SL, holotype, rio S„o Jo„o. MZUSP 43679, 1, 18.7 mm SL, paratype. DZSJRP-Pisces 13861, 25.1 mm SL, affluent rio S„o Jo„o. MNRJ 49371, 22.7 mm SL, rio Aldeia Velha. MNRJ 47108, 19.6 mm SL, rio Aldeia Velha. MNRJ 37572, 26.3 mm SL, rio S„o Jo„o. São Paulo: Microcambeva ribeirae: MZUSP 84301, 47.8 mm SL, holotype, rio S„o Lourencinho. MZUSP 79953, 7, 25.9–36.6 mm SL, paratypes, rio Espraiado. MZUSP 74699, 10, 35.0– 47.5 mm SL, paratypes, rio Faú. MNRJ14304, 3, 29.5–32.4 mm SL, paratypes, tributary of rio S„o Lourencinho. MNRJ 37165, 35.1 mm SL, rio Jacupiranga. Paraná: Microcambeva ribeirae: UFRGS 24759, 37.7 mm SL, tributary of rio Guaraqueçaba. Bahia: Microcambeva draco: MCP 17796, 2, 24.6– 22.6 mm, holotype, rio Jucuruçu. Microcambeva jucuensis: MZUSP 91641, 11, 19.5–24.6 mm SL, rio Formate.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |