Apteronotus magdalenensis (Miles, 1945)
|
publication ID |
https://doi.org/10.1590/S1679-62252011000300005 |
|
persistent identifier |
https://treatment.plazi.org/id/039087C2-FFBF-FFD5-FC4B-71540CBED173 |
|
treatment provided by |
Carolina |
|
scientific name |
Apteronotus magdalenensis (Miles, 1945) |
| status |
|
Apteronotus magdalenensis (Miles, 1945) View in CoL Figs. 1-2 View Fig View Fig
Ubidia magdalenensis Miles, 1945: 461-463 , figs. 10-12 [original description, illustration].-Triques, 1993: 119 [phylogenetic relationships].-Mago-Leccia, 1994: 37, fig. 56 [listing of genus; key to apteronotid genera; illustration; diagnosis].-Campos-da-Paz, 1995: 30, 33, 34 [phylogenetic relationships].-Campos-da-Paz, 2000: 526 [taxonomy]. -Mojica & Castellanos, 2002: 187, 188 [endangered category; illustration].-Triques, 2005: 142 [phylogenetic relationships].
Apteronotus magdalenensis .-Albert & Campos-da-Paz, 1998: 431 [new combination; phylogenetic relationships]. -Albert, 2001: 76 [phylogenetic relationships].-Albert, 2003: 499 [checklist].-Maldonado-Ocampo & Albert, 2003: 153 [checklist].-de Santana et al., 2004: 2-9 [comparison to Apteronotus eschmeyeri ].-de Santana & Maldonado- Ocampo, 2005: fig. 5b [illustration; key to species of Apteronotus in Colombia].-Maldonado-Ocampo et al., 2005: 180, 294 [distribution; illustration].-Mojica et al., 2006: 34 [checklist]. -Villa-Navarro et al., 2006: 17 [checklist].- Maldonado-Ocampo et al., 2008: 213 [checklist].-Agudelo- Zamora et al., 2009 [distribution extension].-Albert & Crampton, 2009: 91 [snout elongation].
Diagnosis. Apteronotus magdalenensis belongs to Apteronotus sensu stricto and is distinguished from all other species by one autapomorphy: mouth rictus not reaching posterior naris.
Additional characters that helps to differentiate the species but not exclusive to A. magdalenensis among other species of Apteronotus sensu stricto are: sphenoid region of neurocranium more than one-third total head length in mature specimens ( vs. sphenoid region of neurocranium less than one-third total head length in mature specimens, except A. cuchillo Schultz, 1949 ); body coloration blotchy ( vs. even brown to black, except A. cuchillo and A. eschmeyeri de Santana, Maldonado-Ocampo, Severi & Mendes, 2004 ); large body size, attaining a total length greater than 300 mm ( vs. attaining a total length smaller than 300 mm, except A. cuchillo and A. eschmeyeri ); total number of anal-fin rays 180-213 [ vs. 133-148 in A. camposdapazi de Santana & Lehmann, 2006 ; 146-154 in A. caudimaculosus de Santana, 2003 , 138- 155 in A. cuchillejo (Schultz, 1949) ; 160-175 in A. eschmeyeri ; 145-165 in A. galvisi de Santana, Maldonado-Ocampo & Crampton, 2007 ; 167 in A. jurubidae (Fowler, 1944) ; 145-156 in A. magoi de Santana, Castillo & Taphorn, 2006 ; 130-165 in A. milesi de Santana & Maldonado-Ocampo, 2005 ; 162-180 in A. mariae (Eigenmann & Fisher, 1914) ; 153-162 in A. rostratus (Meek & Hildebrand, 1913) ; 171-179 in A. spurrellii (Regan, 1914) ]; the presence of scales on the middorsal region of body [ vs. absence in A. ellisi (Alonso de Arámburu, 1957) ]; and the presence of an ossified lateral ethmoid ( vs. unossified in A. cuchillejo , A. rostratus , and A. spurrellii ).
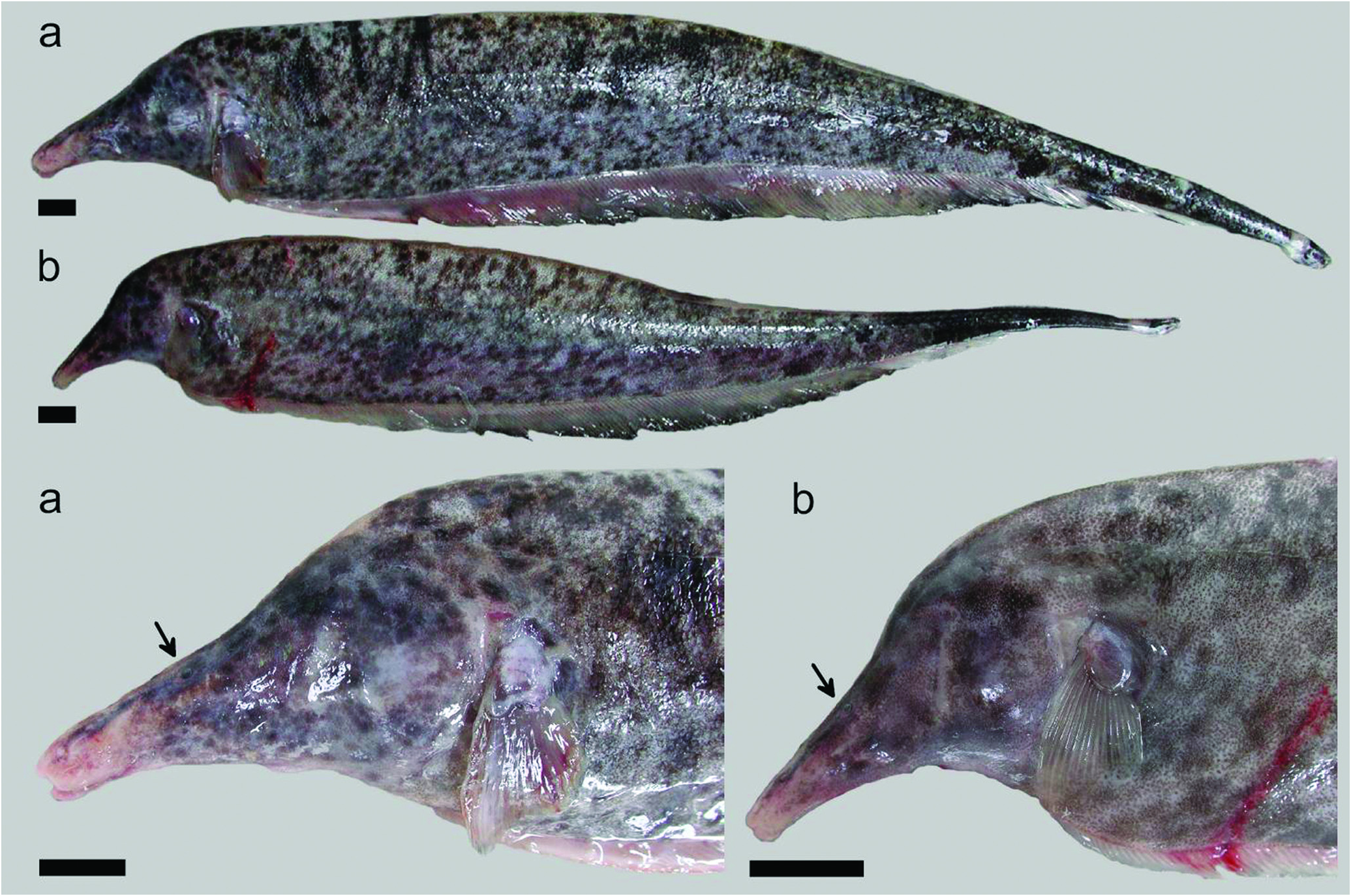
Description. Figs. 1-2 View Fig View Fig illustrate body shape and pigmentation. Table 1 presents morphometric and meristic data for Apteronotus magdalenensis . Maximum observed body size 449 mm TL (males) and 363 mm TL (females). No sexual dimorphism of cranial morphology (based on Principal Component Analysis of multiple morphometric landmarks in 13 males and 15 females). Body elongate. Dorsal profile straight. Maximum body depth at abdominal cavity or, slightly posterior. Lateral line extending to base of caudal fin, but absent on it. First perforated scale above pectoral-fin origin. Head laterally elongate. Maximum head depth at occiput. Postorbital region distinct longer than preorbital portion of head. Eye very small 3.2-6.3 in HL (n=34), laterally located and covered by membrane. Mouth short 14.5-27.4 in HL (n=32) and terminal. Mouth rictus not surpassing a vertical trough posterior naris. Upper jaw longer than lower jaw. Anus and urogenital papilla adjacent, ventral, at vertical posterior to eye. Dorsal mid-sagittal dorsal thong origin on posterior half of body and inserted into a narrow middorsal groove. Scales present on middorsal region of body. Pectoral fin somewhat elongated, broad and pointed distally, with ii,16-ii,19 rays (n=34). Anal fin with 180-213 (n=34) total anal-fin rays. 13 -14 precaudal vertebrae (n=14).
Color in live specimens. (See Fig. 2 View Fig ). Base color light pinkish or light brown, with darker brown or black spots of small size and irregular shape forming a more or less dense marbled pattern with posterior increasing spot size. Towards caudal third, coloration becomes darker and pattern more dense to form a dark caudal end. Two thin creamy white bands, one in caudal peduncle and other in tip of caudal fin. Base of anal fin pinkish, with dark distal border in mid and posterior sections. Mottling intensified on head.Anterior portion of snout largely free of mottling.
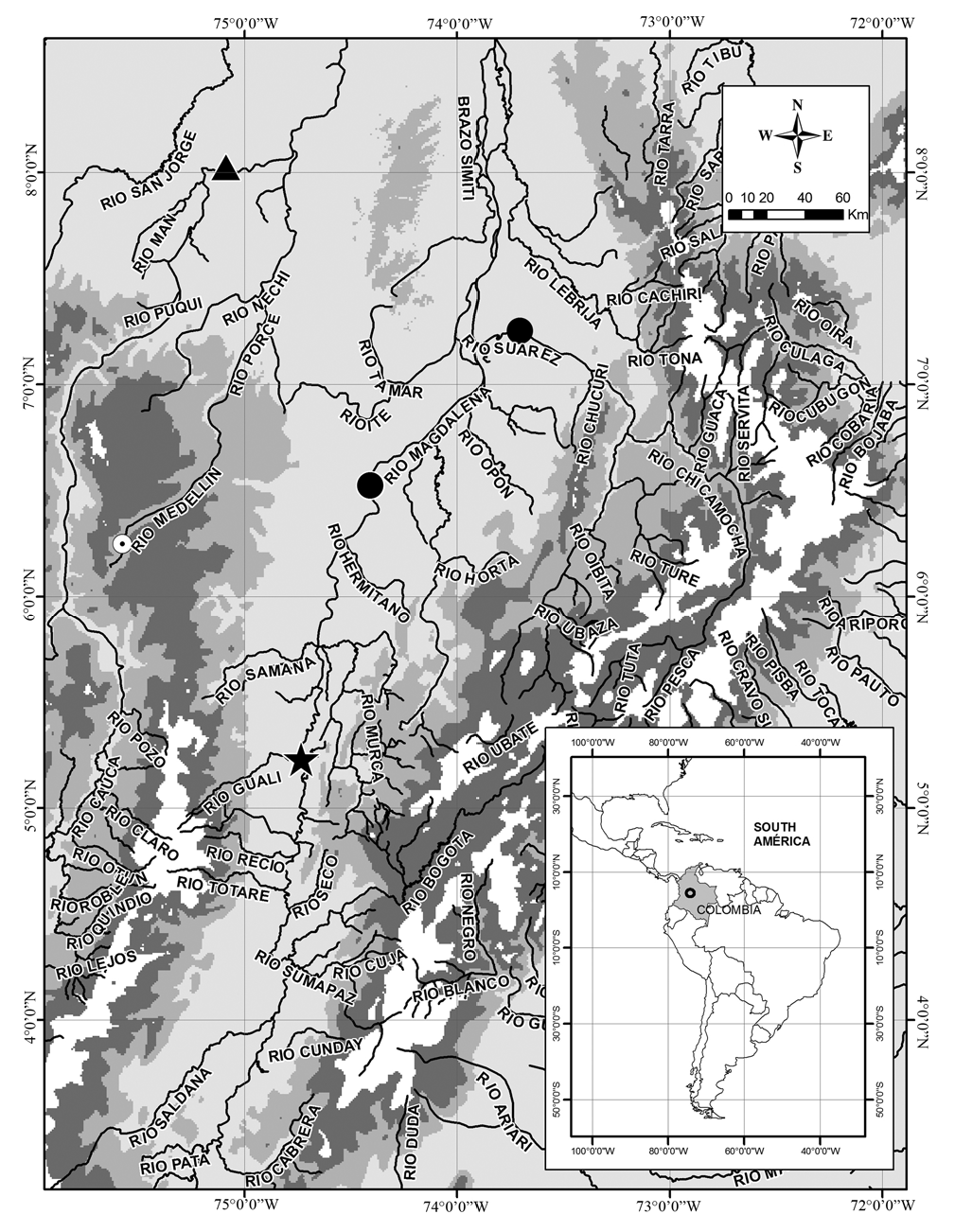
Distribution. Apteronotus magdalenesis is endemic to the río Magdalena-Cauca basin in the trans-Andean region of
Colombia. Until May 2008, it was only known from the type locality in the Torrents of Honda, río Magdalena. However, recent collections performed at the lower stretches of the río Magdalena and río Cauca, captured four additional individuals, two in the main channel of the río Magdalena in the municipality of Puerto Berrio ( 186 km downriver from the type locality), one in the main channel of the río Sogamoso close to its confluence with the río Magdalena almost 210 km downriver from the type locality ( Agudelo-Zamora et al., 2009), and one in the main channel of the lower río Cauca in the municipality of Caucasia (approximately 168 km from the confluence between the río Cauca and río Magdalena). These records extend the species distribution range in the río Magdalena-Cauca basin ( Fig. 3 View Fig ) .
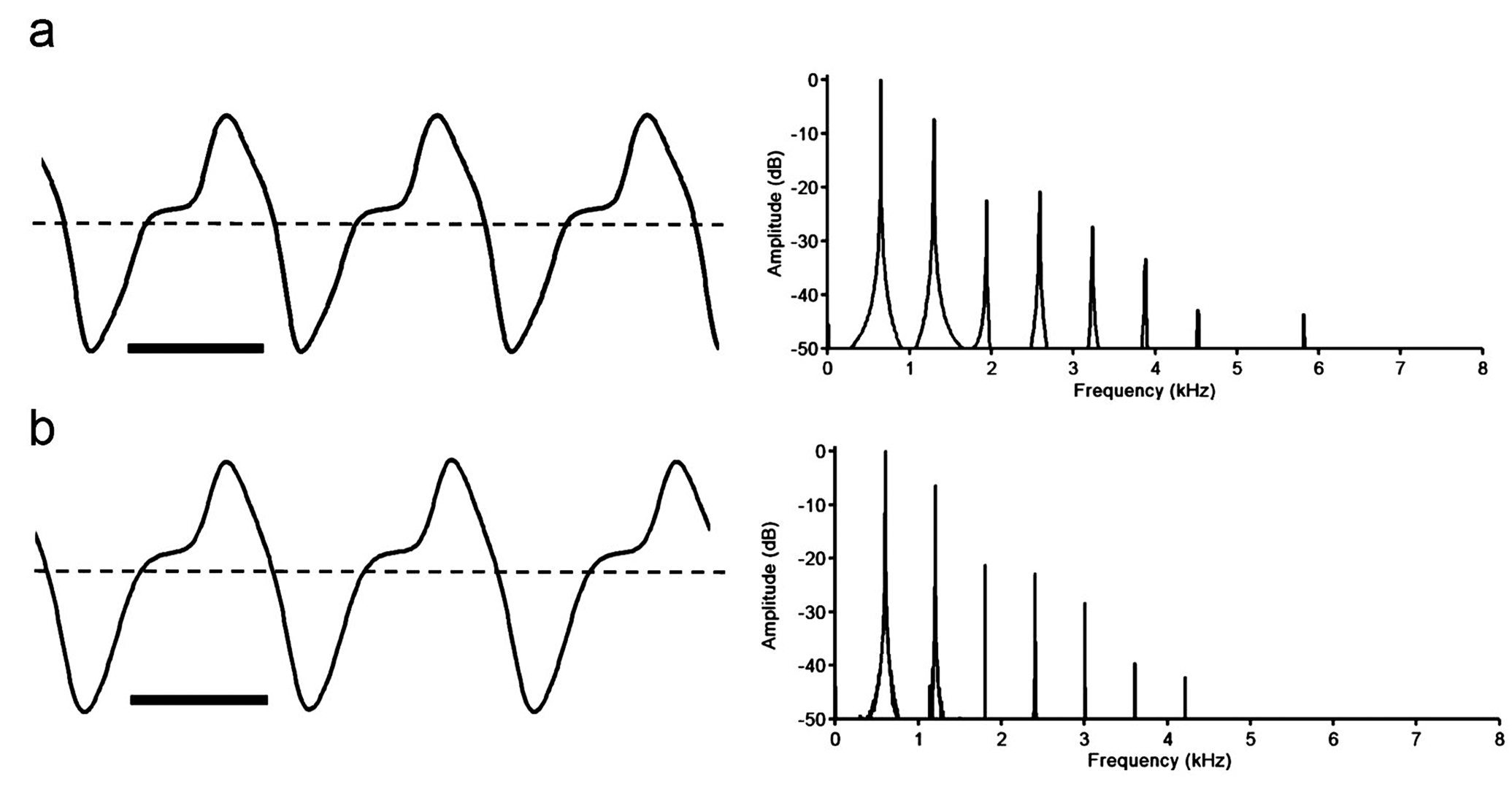
Electric organ discharges (EOD). Apteronotus magdalenensis generates a continuous wave (tone) – type EOD with a stable fundamental frequency (cycle rate) of 534- 996 Hz at 27.0°C +/- 0.2°C (mean 709, SD 129, n = 26). The EOD waveform contains two phases of alternating polarity, corresponding to a type-C category of apteronotid EOD (following the classification of Crampton & Albert, 2006), where there is a noticeable inflection in the ascending voltage component of the waveform, above the baseline ( i.e. 0 volts). In Fig. 4 View Fig we present typical EODs from fully mature male and female specimens. We found no sexual differences in EOD discharge rate. Considering all mature specimens at Nikolsky stages 2-4, we noted no significant sex difference in the EOD fundamental frequency (males - 697 Hz, n = 9, females - 671 Hz, n = 8, df 15 p = 0.61). Considering all mature specimens at Nikolsky stages 3-4, we also noted no significant sex difference in the EOD fundamental frequency (males – 691 Hz, n = 7, females – 634 Hz, n = 8, df 13, p = 0.26). Likewise, considering only fully mature specimens, at Nikolsky stage 4, we still noted no significant difference in the EOD fundamental frequency (males – 691 Hz, n = 7, females – 618 Hz, n = 4, df 9, p = 0.24). We also noted no obvious sex differences in EOD waveform shape. The Peak Power Frequency ( PPF) of the power spectral density ( Fig. 4 View Fig ) corresponds invariably to the fundamental frequency (n = 26). No spontaneous amplitude modulations (chirps) or other EOD modulations were observed. However, we suspect that in the absence of differences in EOD fundamental frequency and waveform shape between sexually mature males and females, must may be encoded by modulations of the EOD during courtship (see Turner et al., 2007) .
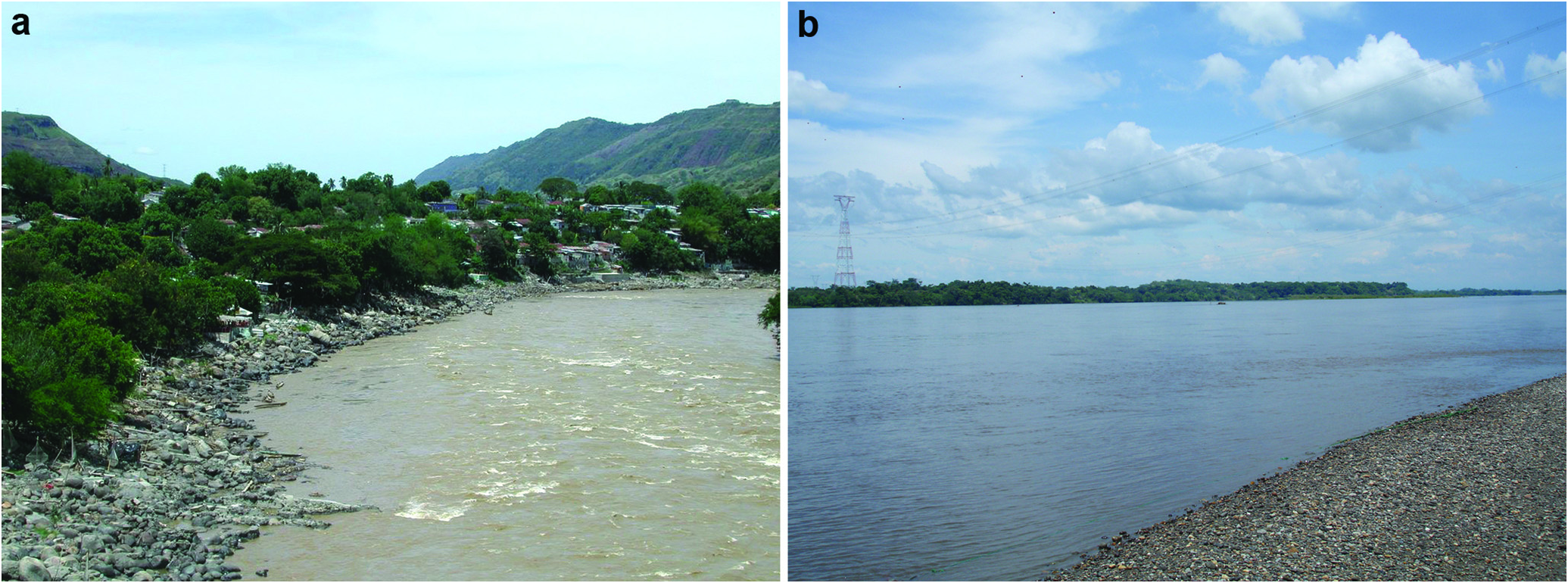
Ecological notes. The type locality, the Torrents of Honda in the río Magdalena ( Fig 5a View Fig ), is located at approximately 250 m above sea level. The main channel of the river, which reaches about 10 m deep, is characterized by a rocky substrate comprising small stones to very large (> 2 m diameter) boulders, with some gravel, sand, and mud beaches. There current is fast (> 1.5 ms - 1 in mid channel) and the water is turbid due to a high sediment load of suspended silt ( Fig. 5a View Fig ). In March 9- 12 2006 we recorded the following water quality parameters: dissolved oxygen, 5.0- 5.2 mg -1; electrical conductivity, 140- 150 Scm -1; pH 6.2-6.4; transparency with Secchi disk 0.28-0.4 m; temperature, 25.6-27.1 °C, with an average of 26.5 °C. We noted fluctuations in the water level of some 1-2 m over a period of less than 48 h, corresponding to rain in upstream areas of the basin. The coolest temperatures corresponded to pulses of elevated water level. The locality records downstream of Honda, in the municipality of Puerto Berrio and Puerto Wilches (see Materials Examined) (Agudelo- Zamora et al., 2009), present similar conditions - white waters with heavy sediment load characteristics of the río Magdalena but with a slower current ( Fig. 5b View Fig ).
At the type locality, other Gymnotiformes that occur syntopically with A. magdalenensis are: Apteronotus eschmeyeri , A. mariae , Eigenmannia cf. virescens , Eigenmannia humboldtii (Steindachner, 1878) and Sternopygus aequilabiatus (Humboldt, 1805) . We found males and females in reproductive condition in both the rainy and dry seasons – suggesting an extended breeding period. As has been reported for the majority of Gymnotiformes species, A. magdalenensis feeds on the larval stages of aquatic insects.
Remarks. The range of the total lengths (TL) of the type series is 280-335 mm, however Miles (1945) reported that Apteronotus magdalenensis reach lengths approximating one meter (referring to A. magdalenensis , he wrote: “It has been found only at Honda, its extreme scarcity accounting for its absence from previous collections, although it attains a considerable size (about 1 meter), the type being somewhat smaller 280 mm ”). We also noted that the total length of all collected individuals does not exceed 449 mm TL (n = 33), with a minimum size of maturity of 301 mm TL in males (n = 13), and 324 mm in females (n = 15). Moreover, the maximum size for the species observed by local fishermen exceeds no more than ca 500 mm TL. This leaves little doubt that Miles’ (1945) comments refer instead to the syntopic species Sternopygus aequilabiatus , which apparently does reach lengths of a little more than one meter TL, according to local fishermen; we captured S. aequilabiatus at Honda to a maximum of 842 mm TL.
The anal and pectoral fins rays counts presented by Miles (1945) are respectively about 175 and ii,15, however the inspection of the two available paratypes shows higher values: 192-195 anal-fin rays and ii,16-ii,17 pectoral-fin rays. The differences in type series values between Miles description and the presented here, at least for anal-fin rays, could be result of the difficulty of counting the posterior fin rays at the end of the anal fin, without the aid of a dissecting microscope with transmitted backlight. Counting these rays from x-rays images permitted the accurate counts we present here.
In the description by Miles (1945), the single illustration of A. magdalenensis indicate that the snout tip cross below a horizontal imaginary line through the origin of the anal-fin base. However, this condition is highly variable in the additional specimens collected at the type locality (for example the specimens in Fig. 2 View Fig do not exhibit this condition).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.