Haplochromis squamipinnis Regan, 1921
|
publication ID |
https://doi.org/ 10.5852/ejt.2022.815.1749 |
|
publication LSID |
lsid:zoobank.org:pub:6AD0082E-7349-48DE-AFCA-1EE0BFBB3887 |
|
DOI |
https://doi.org/10.5281/zenodo.6502536 |
|
persistent identifier |
https://treatment.plazi.org/id/038F87A6-6144-DA10-FDF2-555A2741F894 |
|
treatment provided by |
Felipe |
|
scientific name |
Haplochromis squamipinnis Regan, 1921 |
| status |
|
Haplochromis squamipinnis Regan, 1921 View in CoL
Figs 1–2 View Fig View Fig , 38–40 View Fig View Fig View Fig ; Table 1 View Table 1
Haplochromis squamipinnis Regan, 1921: 636 View in CoL .
Haplochromis squamipinnis View in CoL – Trewavas 1933: 338 (redescription). — Greenwood 1973: 204, fig. 31 (redescription).
Harpagochromis squamipinnis View in CoL – Greenwood 1980: 13.
Differential diagnosis
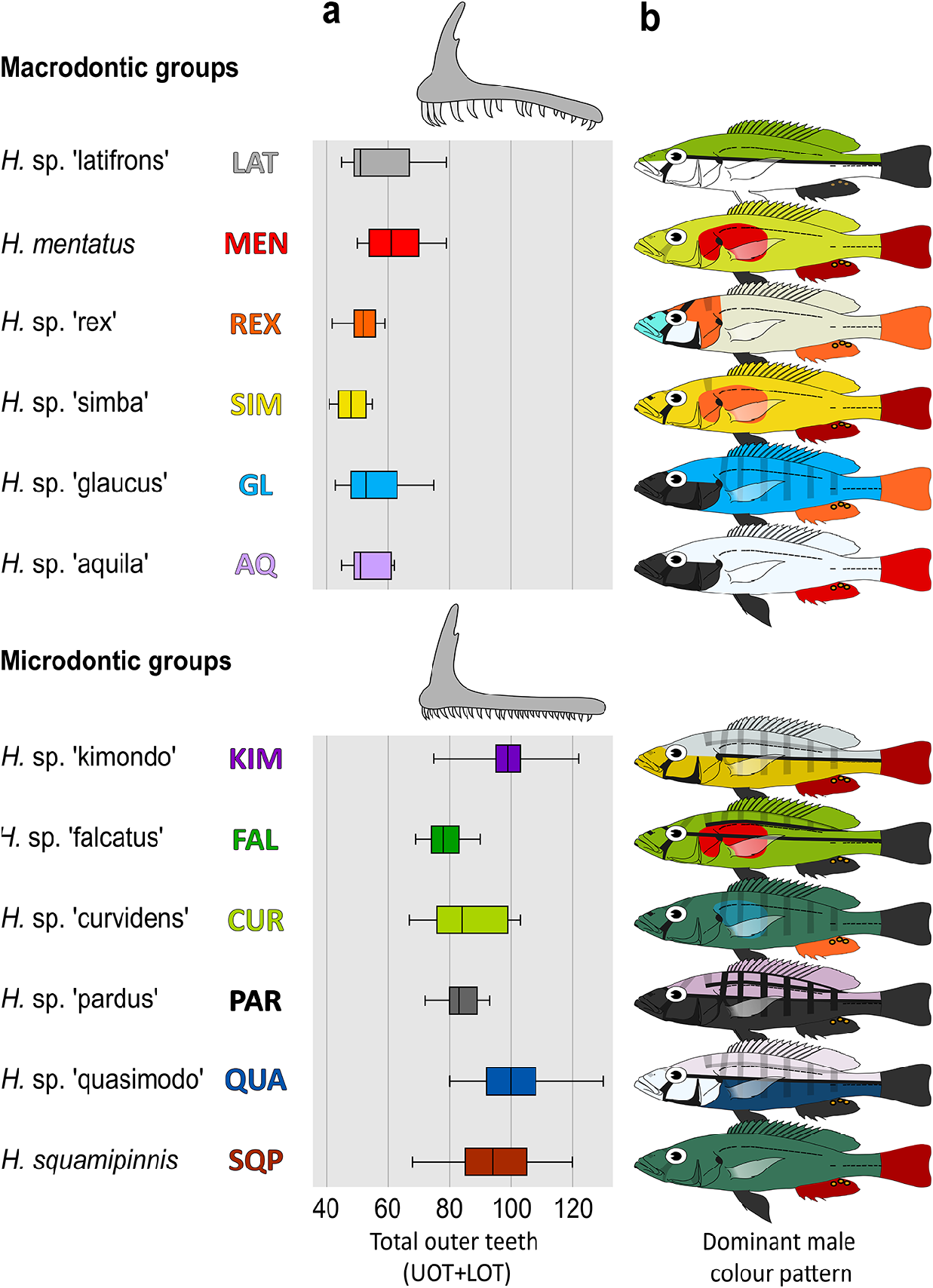
Species with a piscivorous morphology; body rather deep [BD 32.4–39.3 (mean 35.7) % SL]; oral jaws very long [LJL 47.8–58.6 (52.7) % HL], narrow [LJW 32.6–44.7 (37.2) % LJL], and steep (gape inclination 30–45°); outer oral teeth many and small [UOT 39–79 (median 58)]; dominant males slate blue.
Amongst piscivorous species from the Lake Edward system, H. squamipinnis differs from all by presence vs absence of minute scales on proximal part of dorsal fin (rarely few scales present in H. quasimodo sp. nov.).
It further differs from H. latifrons sp. nov. and H. mentatus by the combination of small vs large outer oral teeth, a larger number of outer upper jaw teeth [UOT 39–79 (58) vs 22–47 (27–36)], a steeper gape (30–45° vs 15–30°), and a deeper body [BD 32.4–39.3 (35.7) vs 27.2–32.3 (28.6–31.2) % SL]; from H. mentatus by dominant males uniformly slate blue vs yellow-green with a red anterior part of flank.
It further differs from H. rex sp. nov., H. simba sp. nov., H. glaucus sp. nov., and H. aquila sp. nov. by the combination of small vs large outer oral teeth, a larger number of outer upper jaw teeth [UOT 39–79 (58) vs 22–47 (27–36)], and dominant males uniformly slate blue vs cream-coloured with an orange operculum, yellow with an orange anterior part of flank, light blue with a dusky to black head, or light grey with a black head, respectively; further from H. rex sp. nov., H. simba sp. nov., and H. glaucus sp. nov. by a steeper gape (30–45° vs 15–30°); further from H. aquila sp. nov. by a smaller eye [ED 23.1–29.7 (26.6) vs 30.0–31.5 (30.6) % HL].
It further differs from H. kimondo sp. nov. by a concave to straight vs convex dorsal outline of head, a gentler snout inclination (30–40° vs 40–50°), and dominant males slate blue vs grey dorsally and yellow ventrally; further from H. falcatus sp. nov. by a shorter head [HL 35.1–36.9 (36.0) vs 36.6– 39.6 (38.2) % SL] and dominant males slate blue vs olive-green with an orange-red anterior part of flank; further from H. curvidens sp. nov. and H. pardus sp. nov. by a deeper cheek [ChD 24.9–36.0 (29.0) vs 20.8–24.9 (22.5–23.2) % HL]; further from H. pardus sp. nov. by a larger adult size (max. 211 vs 96 mm SL) and colour pattern of small specimens (<100 mm SL) light coloured vs speckled to uniformly black.
It differs from H. quasimodo sp. nov. by the combination of a broader interorbital area [IOW 48.6–55.6 (51.9) vs 40.5–48.7 (43.9) % HW], a longer lower jaw [LJL 47.8–58.6 (52.5) vs 44.2–49.6 (47.1) % HL], a steeper gape inclination (30–45° vs 20–35°), and dominant males slate blue vs light grey dorsally and blue-black ventrally.
Etymology
Specific name not explained in original description, from the Latin ‘ squamus ’ for ‘scale’, and ‘ pinnis ’ for ‘fin’; probably referring to minute scales on basal parts of dorsal and anal fins.
Material examined
Holotype DEMOCRATIC REPUBLIC OF THE CONGO (most likely) • 1 ♀, 136.9 mm SL; Lake Edward; 1907– 1908; H. Schubotz leg.; NHMUK 1914.4.8.32 .
Other material DEMOCRATIC REPUBLIC OF THE CONGO • 1 ♀, 75.9 mm SL; “Lac Edouard: Bugazia” [Lake Edward: Bugazia ]; 0°23′40.8″ S, 29°23′02.0″ E (inferred); 16 May 1935; IRSNB 12939 View Materials GoogleMaps • 1 ♂, 168.4 mm SL; “Lac Edouard: au large de la riv. Talia” [Lake Edward: offshore Talia River ]; 0°31′05″ S, 29°20′26″ E (inferred); 23 Apr. 1953; KEA exped. leg.; IRSNB 13475 View Materials GoogleMaps • 1 ♀, 167.7 mm SL; “Lac Edouard: au large de la riv. Kigera” [Lake Edward: offshore of the Kigera River ]; 0°29′42″ S, 29°38′14″ E (inferred); 25 May 1953; KEA exped. leg.; IRSNB 13477 View Materials GoogleMaps • 1 ♀, 210.5 mm SL; “Lac Edouard: 2–3 km à l’Ouest de Kiavinionge” [Lake Edward: 2–3 km west of Kiavinionge ]; 0°11′39″ S, 29°32′31″ E (inferred); 1 Jun. 1953; KEA exped. leg.; IRSNB 13482 View Materials GoogleMaps .
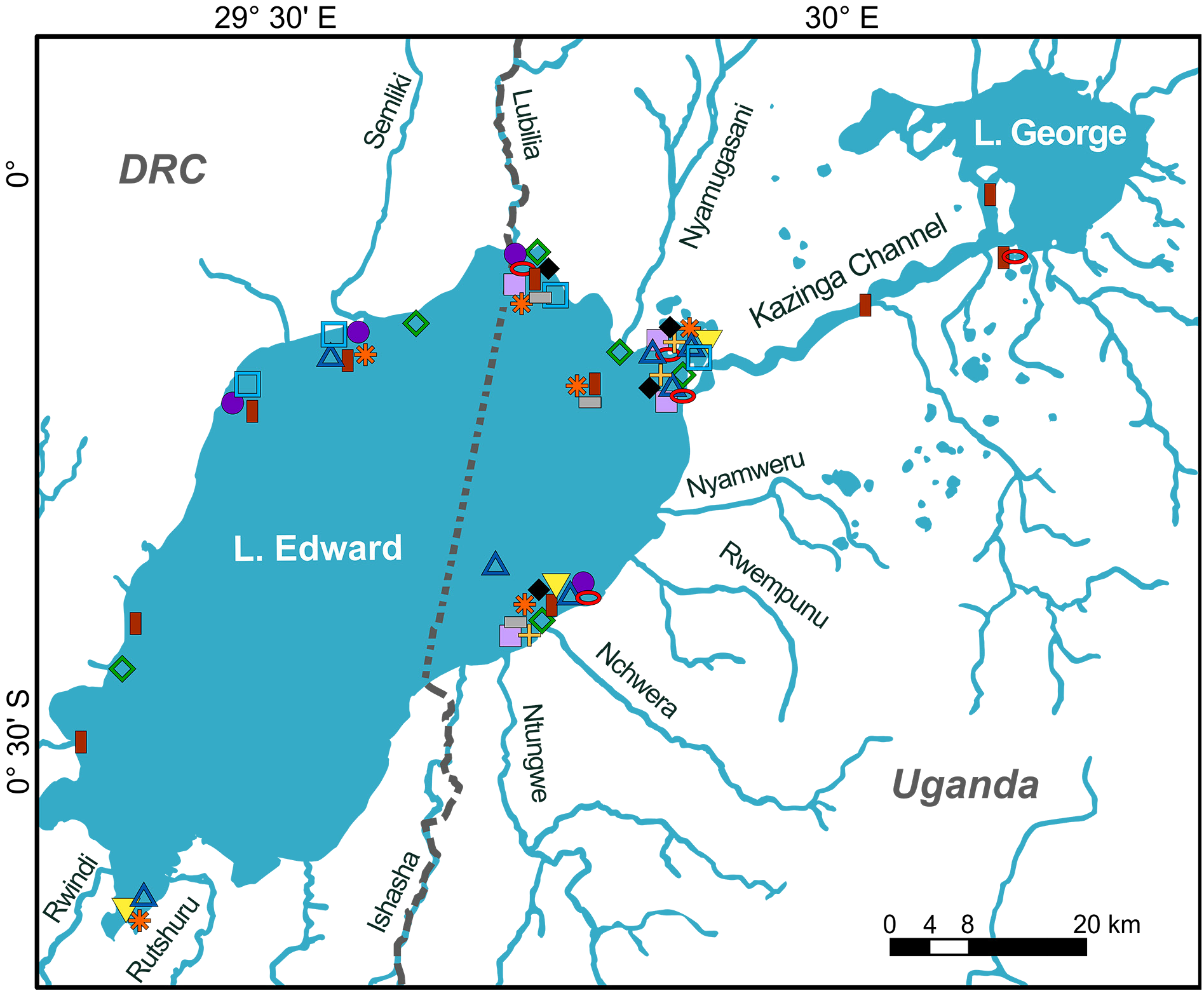
UGANDA – Lake Edward • 2 ♀♀, 110.9, 117.4 mm SL; 0°12′00.0″ S, 29°47′38.4″ E; 23 Oct. 2016; HIPE1 exped. leg.; deep catch, open water ± 20 m deep; RMCA 2016.035.P.0235, 0238 GoogleMaps • 2 ♂♂, 182.0, 211.4 mm SL; 0°24′16.0″ S, 29°46′24.8″ E; 9 Nov. 2016; HIPE1 exped. leg.; bought at Rwenshama landing site; RMCA 2016.035.P.0251, 0254 GoogleMaps • 1 ♂, 2 ♀♀, 148.5–177.3 mm SL; mouth of Kazinga Channel , hard substrate; 0°12′14.4″S, 29°52′37.2″ E; 23 Mar. 2017; HIPE2 exped. leg.; RMCA 2017.006.P.0375, 0377, 0379 GoogleMaps • 1 ♂, 105.4 mm SL; Rwenshama rocky shore; 0°24′05.7″ S, 29°46′35.1″ E; 25 Mar. 2017; HIPE2 exped. leg.; RMCA 2017.006.P.0385 GoogleMaps • 1 ♀, 113.2 mm SL; Kayanja , offshore; 0°05′31.2″ S, 29°45′30.3″ E; 21 Jan. 2018; HIPE3 exped. leg.; RMCA 2018.008.P.0368 GoogleMaps . – Kazinga Channel • 1 ♂, 111.3 mm SL; near Queen Elisabeth Bush Lodge ; 0°08′09.6″ S, 30°02′27.6″ E; 28 Oct. 2016; HIPE1 exped. leg.; RMCA 2016.035.P.0244 GoogleMaps . – Lake George • 1 ♂, 80.7 mm SL; Akika Island ; 0°01′26.7″ S, 30°09′38.2″ E; 28 Mar. 2017; HIPE2 exped. leg.; RMCA 2017.006.P.0387 GoogleMaps • 1 ♀ (90.9 mm SL); Akika Island ; 0°01′26.7″ S, 30°09′38.2″ E; 29 Mar. 2017; HIPE2 exped. leg.; RMCA 2017.006.P.0398 GoogleMaps • 1 ♂, 180.1 mm SL; Kashaka bay , north of inlet; 0°04′52.2″ S, 30°10′47.3″ E; 2 Feb. 2018; HIPE3 exped. leg.; RMCA 2018.008.P.0369 GoogleMaps • 2 ♀♀, 77.6, 107.4 mm SL; Kashaka bay , north of inlet; 0°04′52.2″ S, 30°10′47.3″ E; 2 Feb. 2018; HIPE3 exped. leg.; RMCA 2018.008.P.0371 to 0372 GoogleMaps .
Description
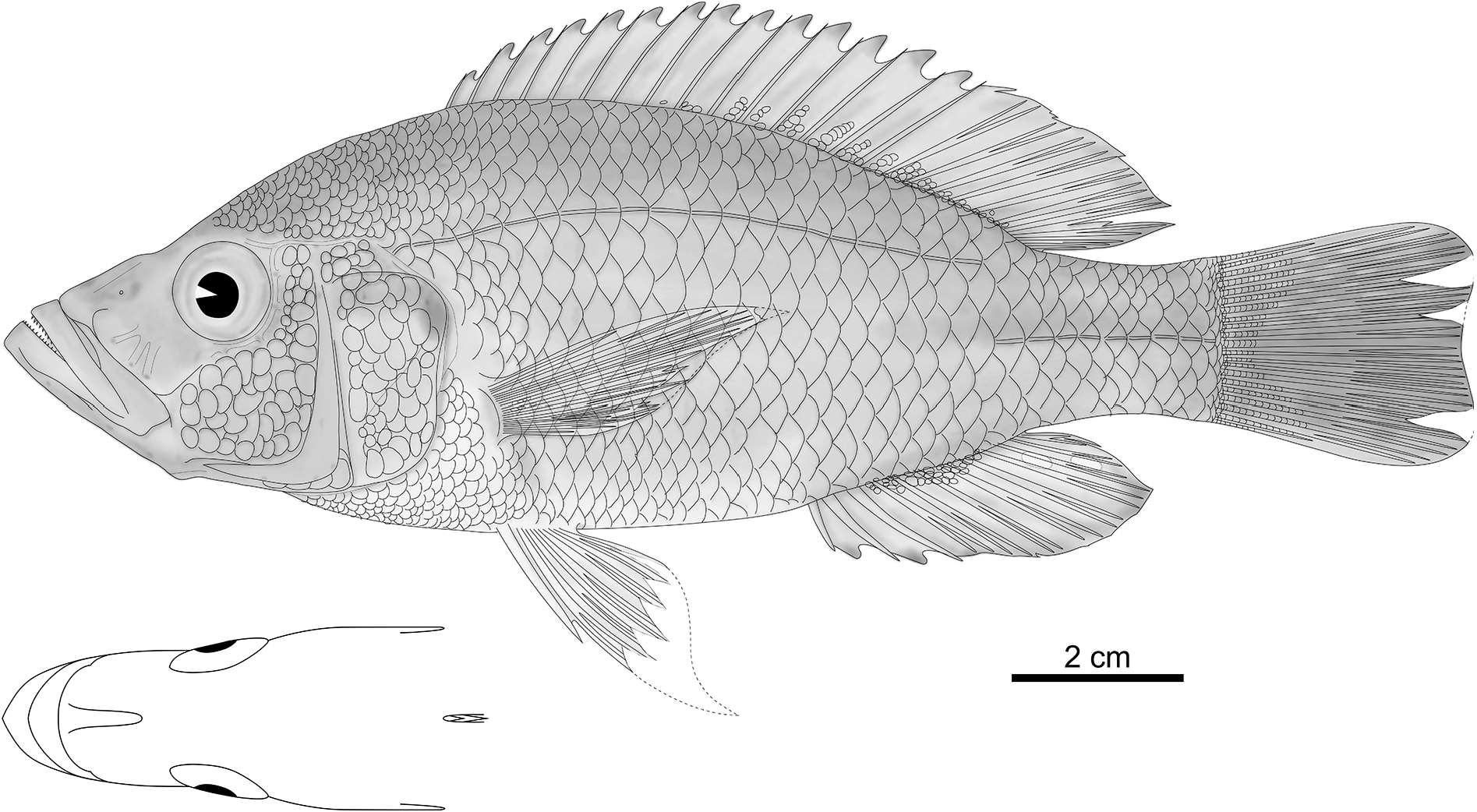
Based on 20 specimens (75.9–211.4 mm SL); body average in depth in comparison to generalised H. elegans (but deep for a piscivorous species; Table 1 View Table 1 ) and oval to rhomboid ( Fig. 38 View Fig ). Head long, narrow, and with a straight to concave dorsal outline; eye small; interorbital area narrow; cheek and lacrimal deep. Snout long, acute, and slopes very gently at 30–35°; premaxillary pedicel very long and prominent. Jaws isognathous to strongly prognathous, slim, very narrow, and rounded in dorsal view; upper jaw long and lower jaw very long; gape large and slopes steeply at 30–45°; maxilla extends to between verticals through anterior margins of orbit and pupil. Lower jaw shallow and with a straight ventral outline in lateral view, mental prominence weakly or strongly developed, and lower jaw side steep with an inclination of 35° to horizontal in anterior view. Upper jaw expanded slightly anteriorly and ventrally. Lips and oral mucosa thin. Neurocranium average in depth, ethmo-vomerine block horizontally inclined, preorbital region shallow (19–25% NL), orbital region average in depth (28–32% NL), and supraoccipital crest deep and pyramidical or weakly wedge-shaped ( Fig. 39b View Fig ).
Outer oral teeth numerous, unicuspid, and small. Necks stout, conical, and straight; crowns weakly recurved in lower jaw, recurved in upper jaw, and acutely pointed. Dental arcades rounded. Outer teeth closely and regularly set with neck-distances of ½–1 neck-width. In upper jaw, 1–3 posteriormost teeth sometimes slightly enlarged. Inner teeth small, weakly recurved, unicuspid in large specimens (> 120 mm SL), tri- to rarely unicuspid in upper jaw and uni- to weakly tricuspid in lower jaw of small specimens (<120 mm SL), and acutely pointed in acutely pointed in all specimens. Tooth bands very slender crescent-shaped with 1–3 rows of inner teeth, and narrow posteriorly until only outer row remains past ⅔ length of tooth band. Inner teeth closely and regularly set on 1–3/2 outer neck-widths from outer row; implantation recumbent; size uniform throughout tooth band.
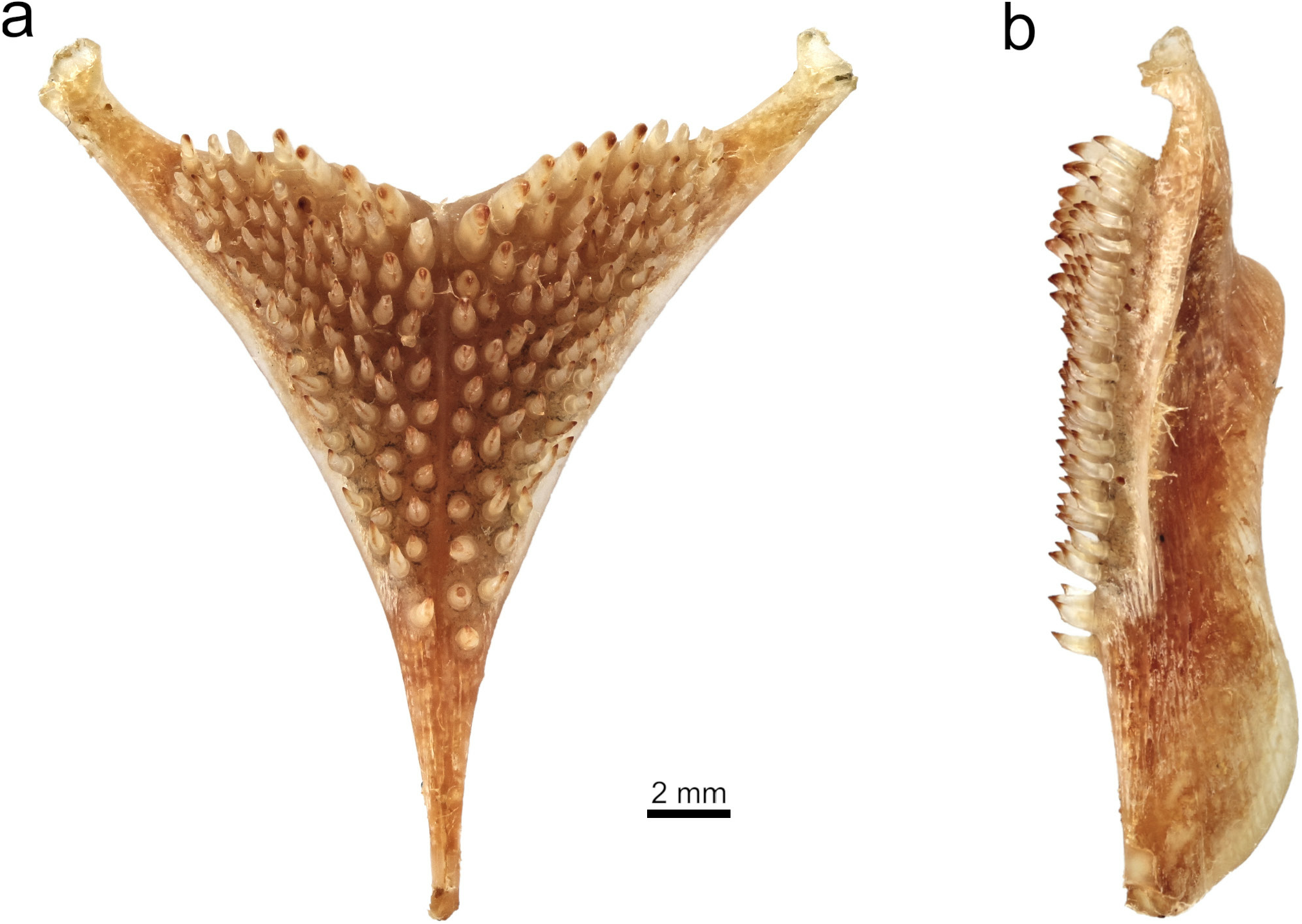
Lower pharyngeal bone long, narrow, slim, and shallow with a slightly deeper keel ( Fig. 40 View Fig ). Pharyngeal teeth relatively large and slender; major cusps acutely pointed; cusp gaps nearly straight; minor cusps and cusp protuberances small. Teeth in two median longitudinal rows equal in size and form to lateral teeth, 11 in each row. Posterior transverse row with 17–18 teeth, implanted erectly with a lateral inclination; major cusps nearly straight, bluntly pointed, and laterally compressed; minor cusps mostly present.
Chest scales small; transition to larger flank scales gradual. Basal parts of membranes of dorsal and anal fins covered by minute, ellipsoid scales; between some pairs of fin rays, up to two rows of 1–10 scales extend from body onto fin; scales very variable in distribution and density and mostly invisible to naked eye; no scales present anteriorly of fourth dorsal-fin spine ( Fig. 38 View Fig ). Minute scales on proximal half of caudal fin.
Caudal fin emarginate to weakly subtruncate; dorsal and anal fins reach to between verticals through caudal-fin base and two scales posterior to this vertical. Pectoral fin reaches to between first and third anal-fin spines; pelvic fin reaches to between first and third anal-fin spine in females, to third anal fin branched ray in males; first branched pelvic-fin ray elongated in all specimens.
Ceratobranchial gill rakers in outer row of first gill arch short, relatively slender, and simple; posteriormost rakers sometimes anvil-shaped or weakly bifid. Epibranchial gill rakers slender and simple.
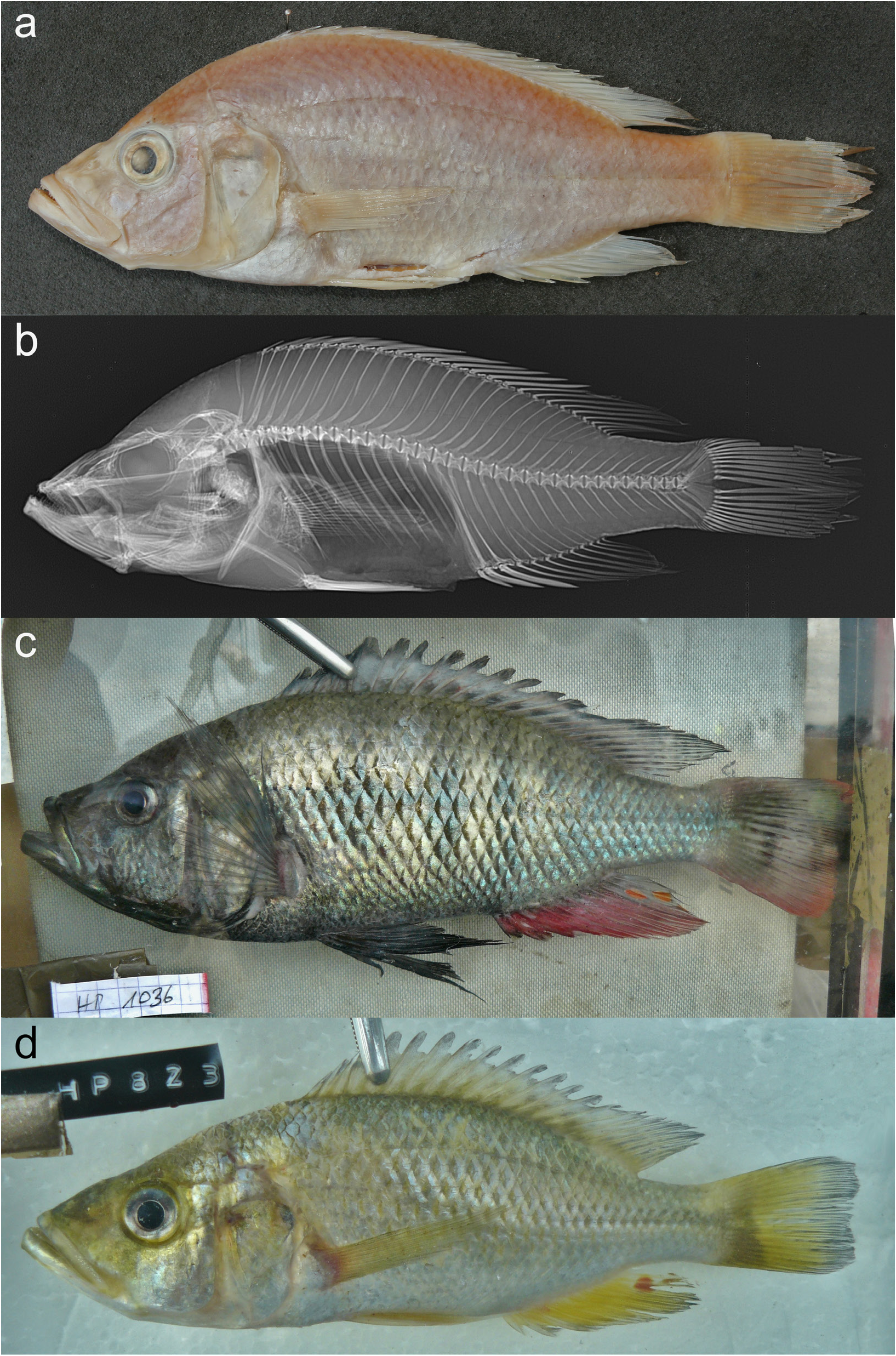
Colouration in life
Dominant males: body and head uniformly slate blue; belly and chest black; flank with very faint mid-lateral band and 5–7 vertical stripes in some specimens; snout dusky; lacrimal stripe and mental blotch present; eye with (dark) grey outer ring and silver to golden inner ring ( Fig. 39c View Fig ). Pectoral fin dusky; pelvic fin black; caudal fin crimson and with dusky base and maculated dorsal part. Dorsal fin dusky and with black lappets and, in posterior part, maculated crimson; anal fin crimson and with black lappets, a dusky base and posterior part, and 3–4 large orange egg-spots (i.e., twice distance between rays) with hyaline rings. Non-dominant males: body and head yellow-green; chest and belly white.
Females and juveniles: dorsum and dorsal part of head golden; belly, chest, operculum, and cheek white; transition gradual ( Fig. 39d View Fig ). Snout and lower jaw dusky; lacrimal stripe faint, mental blotch present; eye with (dark) grey outer ring and silver to golden inner ring. Flank with a very faint mid-lateral band and 5–7 vertical stripes in some specimens. Pectoral, pelvic, anal, and caudal fin yellowish; anal fin with hyaline base, dusky distal part, and 3–4 spots resembling egg-spots; caudal fin with dusky distal part and maculated dorsal part; dorsal fin dusky and with black lappets.
Preserved colouration
Dorsal part of body dusky brown, ventral part of body white, transition gradual; in dominant males, belly and chest black ( Fig. 39a View Fig ). Flank rarely with a very faint mid-lateral band and very faint 5–7 vertical stripes. Cheek light yellowish; snout dusky. Nostril, interorbital, lacrimal, and vertical opercular stripes faint; mental blotch present; lacrimal stripes present in dominant males. Pectoral fin dusky; pelvic fin yellowish with dusky distal parts in females, black in males; caudal fin dusky and with black distal margin. Dorsal fin dusky and with black lappets and posterodistal margin; anal fin dusky and with a black posterodistal margin, a white base in females, a dark base and 2–3 egg-spots in males.
Distribution and ecology
Endemic to the Lake Edward system; found in offshore benthic areas in mostly shallow and deep waters over muddy substrates. Introduced into Lake Kachira, Lake Victoria drainage, Uganda ( Schraml 2004). Piscivorous diet. Diet of Lake George specimens consists mainly of H. nigripinnis Regan, 1921 followed by H. angustifrons Boulenger, 1914 ( Moreau et al. 1993); insects and to a lesser degree small fishes and plant fragments contribute to diet in small specimens (<150 mm SL) ( Greenwood 1973; Moriarty et al. 1973). Sexual dimorphism in size, as observed by Greenwood (1973), seems absent; both sexes reach> 210 mm SL.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Haplochromis squamipinnis Regan, 1921
| Vranken, Nathan, Steenberge, Maarten Van, Heylen, Annelies, Decru, Eva & Snoeks, Jos 2022 |
Harpagochromis squamipinnis
| Greenwood P. H. 1980: 13 |
Haplochromis squamipinnis
| Greenwood P. H. 1973: 204 |
| Trewavas E. 1933: 338 |
Haplochromis squamipinnis
| Regan C. T. 1921: 636 |