Megophrys (Panophrys) frigida, Tapley & Cutajar & Nguyen & Portway & Mahony & Nguyen & Harding & Luong & Rowley, 2021
|
publication ID |
https://doi.org/ 10.1080/00222933.2020.1856952 |
|
publication LSID |
lsid:zoobank.org:pub:EBB06109-3FCE-4E57-96E8-00558EE13369 |
|
persistent identifier |
https://treatment.plazi.org/id/69340DD7-B21E-4DD0-836F-6741878013BB |
|
taxon LSID |
lsid:zoobank.org:act:69340DD7-B21E-4DD0-836F-6741878013BB |
|
treatment provided by |
Carolina |
|
scientific name |
Megophrys (Panophrys) frigida |
| status |
sp. nov. |
Megophrys (Panophrys) frigida sp. nov.
( Figures 4–7 View Figure 4 View Figure 5 View Figure 6 View Figure 7 ; Tables 1, 2)
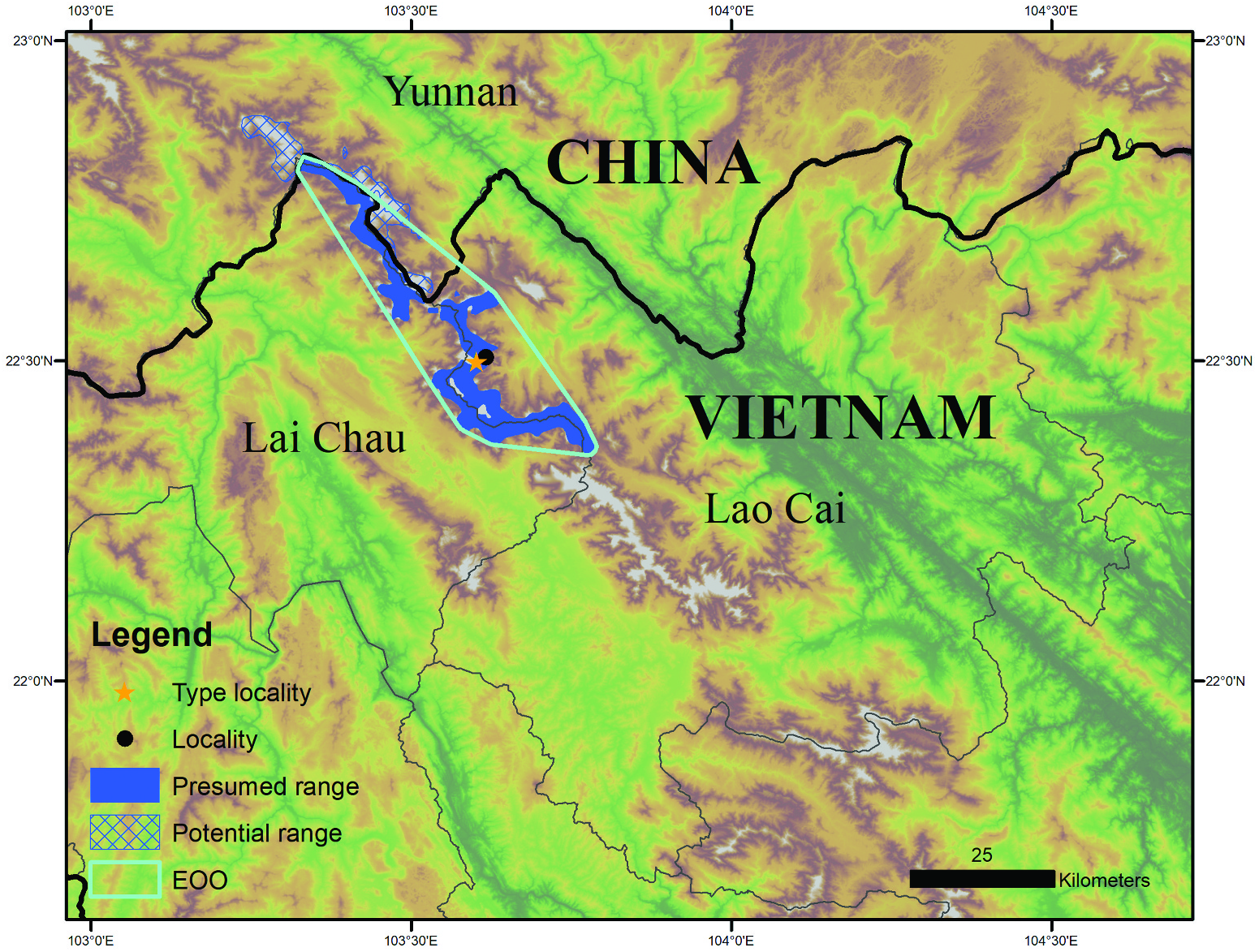
Holotype. Adult male ( VNMN 010948 View Materials ; field tag BX009: Figures 5 View Figure 5 (a–e), 6(a,b,i,k), 7(a)) found beside a 1.5 m wide mountain stream in disturbed upper montane forest on Mount Ky Quan San, Bat Xat District, Lao Cai Province, Vietnam (22.499°N, 103.602°E, 2668 m asl; Figures 1 View Figure 1 , 8 View Figure 8 (a,b)), collected at 22:00 h on 9 September 2017 by Nguyen Thanh Chung, Luan Thanh Nguyen, Luke Harding, Timothy Cutajar, Jodi J. L. Rowley and Benjamin Tapley. GoogleMaps
Paratypes. Adult male ( AMS R186131 ; field tag BX011: Figures 5 View Figure 5 (f–h), 6(e–f,j)) same collection locality, date and collectors as holotype, collected at 21:00 h . Adult male ( AMS R186132 ; field tag BX014: Figure 6 View Figure 6 (g,h,l)) found resting on soil substrate in dense vegetation beside a swampy stream headwater in disturbed broadleaf forest, Mount Ky Quan San, Bat Xat District, Lao Cai Province, Vietnam (22.508°N, 103.615°E, 2118 m asl; Figure 1 View Figure 1 ), collected at 21:30 h on 10 September 2017, same collectors as holotype GoogleMaps .
Referred specimen. Adult male ( HLNP 20170910 00006; field tag BX010 ) found perched on a leaf, same collection locality, date and collectors as holotype, collected at 21:00 h . This specimen is not included in the type series due to it being deposited in a local collection. Its taxonomic identity is not in question.
elevations above 2000 m above sea level in the Hoang Lien Range: M. fansipanensis , M. hoanglienensis , M. jingdongensis and M. rubrimera . Parameter values are
given as means (and ranges). Bold text indicates non-overlapping call parameters between compared species.
Etymology. Specific epithet ‘ frigida ’ is a femine adjective; the Latin word ‘ frigida ’ meaning cold, in reference to the relatively cold temperature at the type locality of the species, Mount Ky Quan San. At some high-elevation sites in the Hoang Lien range, the climate is
almost temperate; temperatures range from −3 to +20°C. In the coldest months there is frequent ground frost ( Nguyen and Harder 1996).
Suggested vernacular name. Mount Ky Quan San horned frog (English), Cóc sừng Ky Quan San (Vietnamese).
Diagnosis. Based on the type series and the referred specimen, which are all adult males (N = 4), Megophrys frigida sp. nov. differs from its congeners by a combination of the following characters: (1) small adult male size SVL 30.3–31.8 mm; (2) small blunt tubercle present on outer edge of upper eyelids; (3) dorsolateral ridges present; (4) toes lacking distinct interdigital webbing; (5) subarticular tubercles absent on fingers and toes; (6) palmar tubercles absent; (7) inner metatarsal tubercle present on feet; (8) tympanum clearly defined; (9) presence of vomerine ridges, vomerine and maxillary teeth; (10) nuptial pads covered with black microspinules; (11) advertisement call with a dominant frequency of 3.6 (3.5–3.7) kHz.
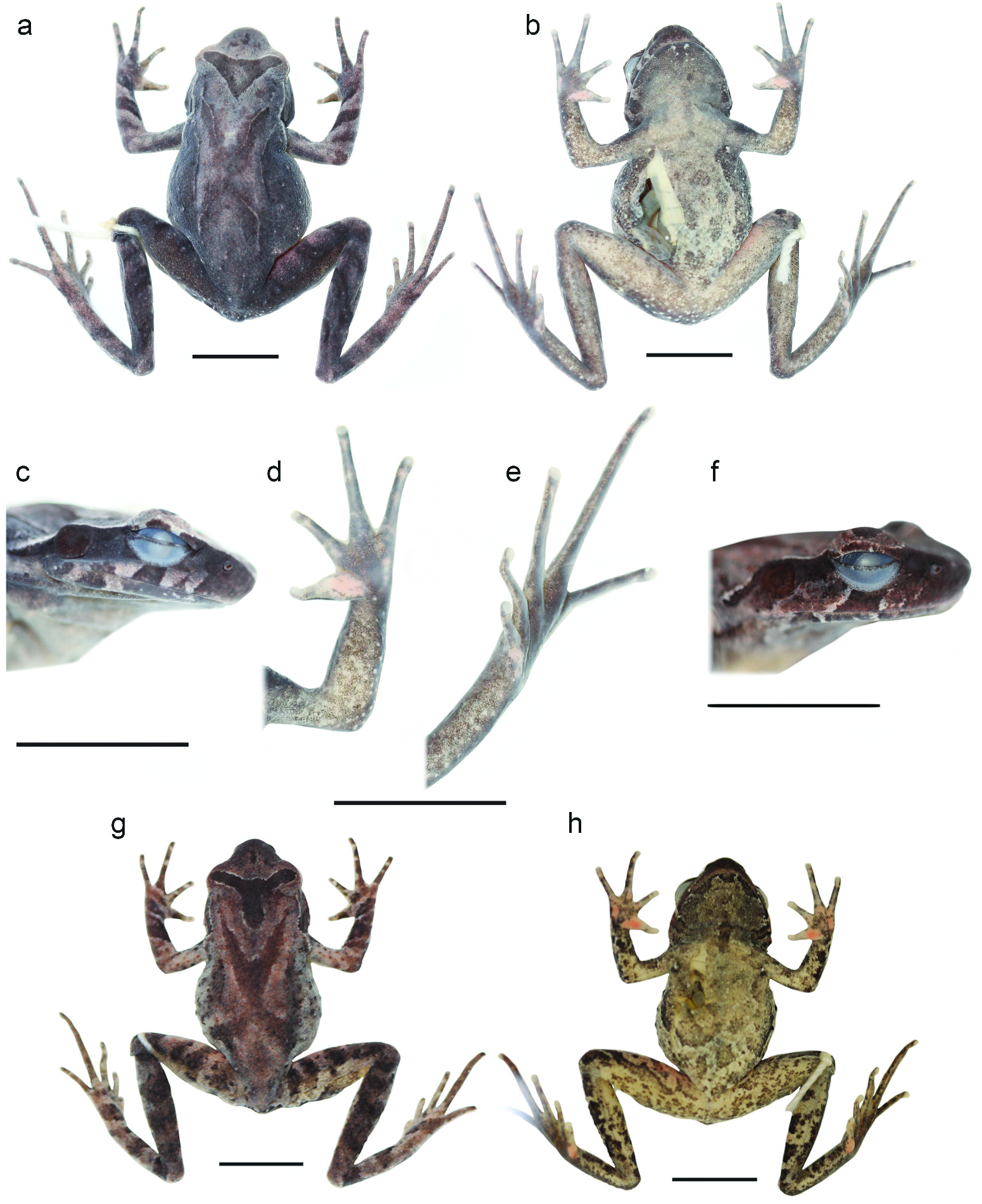
Description of holotype. Figures 5 View Figure 5 (a–e), 6(a,b,i,k) and 7(a): Sexually mature male. Head small, greater in length than in width; snout rounded in dorsal view, obtusely protruding in lateral view, rostral appendage absent; loreal region vertical and concave; canthus rostralis angular; eye length 24% longer than maximum diameter of tympanum and subequal to snout length; eye to tympanum distance shorter than maximum tympanum diameter; tympanum oval, orientated vertically. Pupil in life oval, vertically orientated when dilated; nostril round and orientated laterally, closer to snout tip than to eye; eyelid width subequal to narrowest point between upper eyelids, and greater than internarial distance; vomerine ridges present, obliquely orientated and barely separated from choanae anteriorly with small vomerine teeth; maxillary teeth present. Tongue large and not clearly notched posteriorly. Fore limbs long and thin, forearms not significantly enlarged relative to upper arms, forearm length shorter than hand length; fingers long and narrow without lateral fringes, finger length formula FIL <FIIL <FIVL <FIIIL; interdigital webbing absent, subarticular, supernumerary and palmar tubercles absent; thenar tubercle weakly defined; finger tips slightly expanded relative to adjacent digit width and flattened to oval pads; terminal grooves absent. Hind limbs relatively long and thin; foot length subequal to thigh length, both shorter than shank length; toe tips slightly dilated relative to adjacent digit width and flattened to oval pads, terminal grooves absent; webbing and lateral fringes on toes absent; outer metatarsal, subarticular and supernumerary tubercles absent; inner metatarsal tubercle very weakly defined.
Skin of dorsal surfaces of body, limbs, and dorsal and lateral surfaces of head weakly granular; gular region, chest, abdomen and ventral surfaces of limbs smooth; tympanum surface with small raised granular bumps lacking black-tipped asperities; tympanum border slightly raised; small blunt tubercle present on outer edge of upper eyelids; very small, black-tipped asperities present on posterior half of upper eyelid; no asperities circummarginally on lower jaw; white tubercles, some with black asperities present on angle of jaw; supratympanic ridges with black asperities, supratympanic ridge narrows as it passes above tympanum, terminating above axilla; tubercles above fore limb insertion with black-tipped asperities; flanks with small scattered tubercles lacking black-tipped asperities; thin dorsolateral ridge on each side, extending from behind supratympanic fold to approximately two-thirds of distance to groin, supratympanic fold with asperities along apex, these are black tipped on anterior half and without black tips on posterior half; a weak, ‘V’-shaped parietoscapular ridge present, its two sides extending posteriorly from above tympanum and meeting medially beyond level of axilla; a second, inverted ‘V’- shaped sacral ridge present on mid-dorsum which does not join laterally with dorsolateral ridges; ‘V’-shaped ridges joined at their apices by a weakly defined medial ridge; crest of parietoscapular and sacral ridge covered in asperities, which are black tipped on anterior half of parietoscapular ridge and without black tips on posterior half; small tubercles on dorsal surface of body lack black tips, except between parietoscapular ridge and supratympanic fold; small tubercles without black tips arranged into distinct transverse rows on dorsal surface of thighs, shanks and forearms. Large distinct tubercles present on dorsolateral surfaces of the body; slightly smaller tubercles present on dorsal and posterior surfaces of forearms, shanks and region surrounding cloaca, some tubercles surrounding cloaca with black-tipped asperities, small tubercles present on dorsal and ventral surfaces of thighs; ventral surfaces of fore limbs and shanks smooth; pectoral glands distinct, small, slightly raised, positioned level with axilla; femoral glands small, slightly raised, one positioned closer to knee than cloaca on posterior surface of each thigh.
Colour of holotype in life. Figures 6 View Figure 6 (a,b,i,k) and 7(a): Dorsally orange-brown; darker brown lines follow opposing ‘V’-shaped parietoscapular-sacral ridges; darker brown triangular marking between eyes with a lighter central blotch; lateral surfaces of snout, anterior to orbits dark brown, tip of snout light brown; a vertical dark brown bar present below eyes; supratympanic ridges longitudinally bicoloured, cream above and dark brown below; tympanic region dark brown; inguinal region olive green; tubercles on flanks whitish-blue in colour, some bordered with irregular brown blotches; tubercles around cloaca, posterior surfaces of forearms, shanks and thighs white; gular and pectoral region dark brown with grey flecks; broad dark brown longitudinal stripes extend ventrolaterally on abdomen, darkest anteriorly and fading on posterior third of abdomen; centre of abdomen mottled with different hues of grey and orange-brown; three dark brown blotches on anterior lateral surface of forearms; dorsal surface of fingers with dark brown blotches; anterior lateral surfaces of thighs dark orange, ventral surfaces of thighs and forearms grey-brown, speckled with darker brown; white tubercles on ventral surface of thighs; white pectoral and femoral glands; ventral surface of hands dark brown; thenar and hyperthenar region of hands dark orange, giving illusion of tubercles; ventral surfaces of feet dark brown; inner metatarsal tubercle dark orange; iris metallic orange-brown with black reticulations throughout.
Colour of holotype in preservative. ( Figure 5 View Figure 5 (a–e)): Majority of dorsal and lateral surfaces of head, body, fore limbs and hind limbs grey-brown; darker brown triangular marking with light central blotch between eyes; darker brown ‘X’-shaped marking over opposing ‘V’-shaped parietoscapular-sacral ridges; dorsolateral ridges, supratympanic ridges and flank tubercles brownish-cream; snout and lateral canthus rostralis dark brown; wide vertical dark brown bar below eyes and dark brown blotch covering tympanum and extending to posterior edge of eyes; three dark brown blotches on anterior lateral surface of forearms; dorsal surface of fingers with dark brown blotches; inguinal region darker brown than dorsal surface; gular region, chest and anterior part of abdomen primarily creamy-grey, with brown speckling in gular region. Abdomen light grey, distinctly blotched with dark brown; ventral surfaces of thighs and shanks with pale brown mottling; ventral surfaces of feet grey-brown; inner metatarsal tubercle dark orange; fore limbs ventrally mottled and blotched with light and dark brown; ventral surface of hands grey; thenar and hyperthenar region of hands light pink, giving illusion of tubercles; pectoral and femoral glands white.
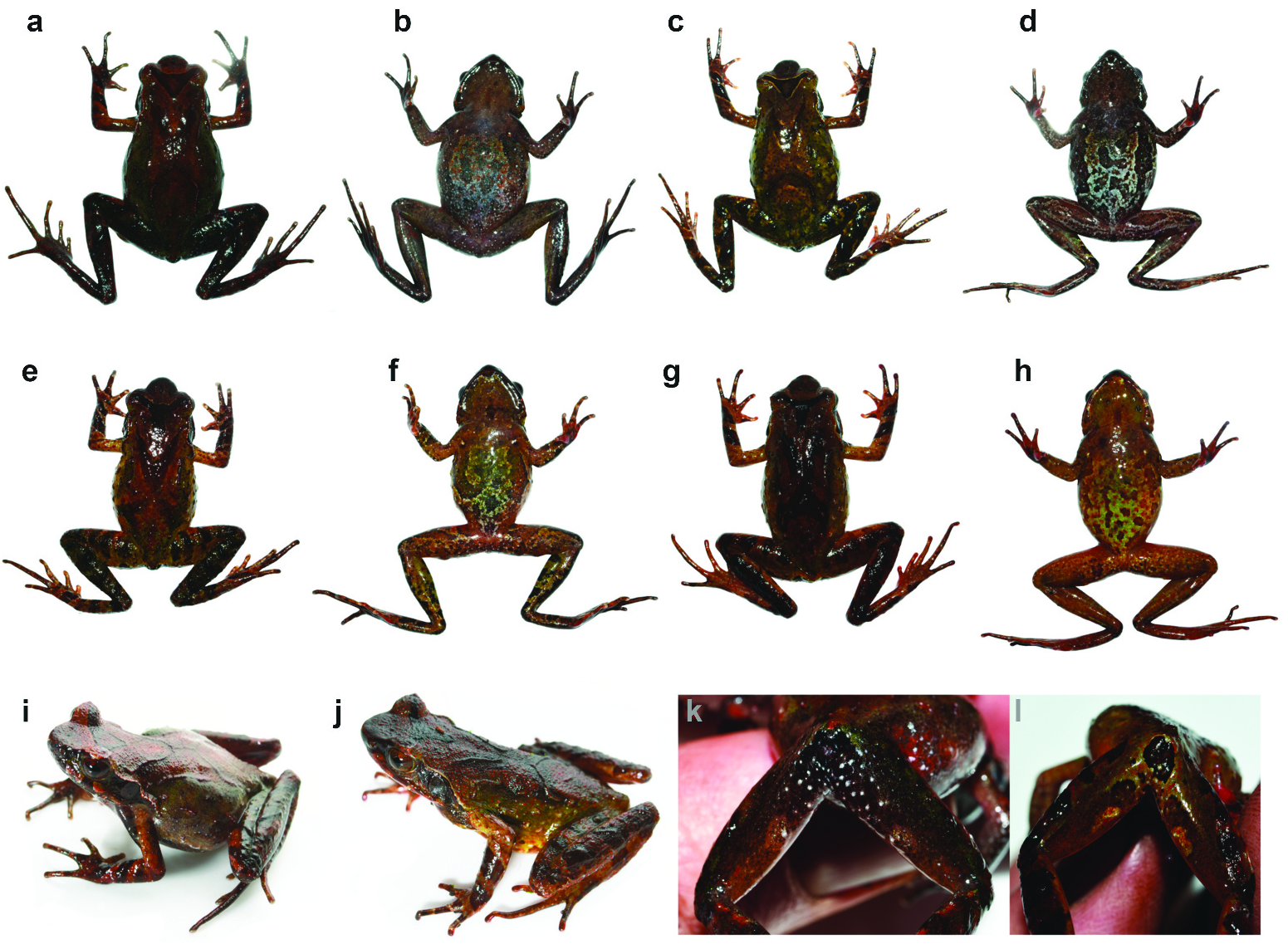
Variation. Morphometric measurements of type series are shown in Table 1. Paratypes and referred specimen generally agree with holotype morphologically, but with the following exceptions: head width greater than head length in HLNP 20170910 00006, AMS R186131 and AMS R186132 (vs head length greater than head width in holotype); EL/ SL 79–82% in paratypes and referred specimen (vs EL/SL 95% in holotype); FL>TL in AMS R186132 but TL> FL in AMS R186131 ; finger length formula for AMS R186131 and AMS R186132 agrees with holotype ( FIL < FIIL < FIVL < FIIIL), finger length formula of HLNP 20170910 00006 differs from holotype ( FIL < FIVL < FIIL < FIIIL); tongue of AMS R186131 appears notched posteriorly, a notch absent in VNMN 010948 View Materials , HLNP 20170910 00006 and AMS R186132 , this apparent absence of notching may be an artefact of fixation . Colouration and markings in life are highly variable (see Figures 6 View Figure 6 and 7 View Figure 7 ), one unvouchered specimen had a brick red dorsal surface ( Figure 7 View Figure 7 (b)); colouration of palmar surfaces of hands in life largely in agreement with holotype, though eminences over metacarpal regions observed in HLNP 20170910 00006, AMS R186131 and AMS R186132 were dark orange . Only holotype possessed ‘V’-shaped parietoscapular and inverted ‘V’- shaped sacral ridges; HLNP 20170910 00006 ( Figure 6 View Figure 6 (c)) had one ‘V’-shaped parietoscapular ridge and an opposing ‘U’-shaped sacral ridge with apexes not connected by a middorsal ridge, and AMS R186131 and AMS R186132 had ‘V’-shaped parietoscapular ridge and opposing ‘U’-shaped sacral ridges with apexes not connected by a medial ridge . Distribution of asperities in AMS R186131 agreed with holotype, although asperities were far fewer and more dispersed on all surfaces than on holotype and none of these appeared to be black tipped; coverage of asperities in AMS R186132 and HLNP 20170910 00006 also agreed with holotype on all surfaces, but those on upper eyelids, supratympanic ridges, dorsolateral ridges, sacral ridge, parietoscapular ridge and dorsolateral to axilla lacked black tips; on AMS R186132 and HLNP 20170910 00006, black-tipped asperities were only present on dorsal surfaces of body and these were concentrated on posterior dorsum .
Secondary sexual characters. All specimens collected were male and had slightly raised nuptial pads covered with black microspinules; oval nuptial pads covering most dorsal surfaces of FI and FII at their base; fleshy projection posterior to cloaca absent, a secondary sexual character of some male Megophrys , e.g. M. angka ( Wu et al. 2019) , M. caudoprocta Shen, 1994 , M. koui ( Kou 1985) and M. pachyproctus Huang and Fei (1981) .
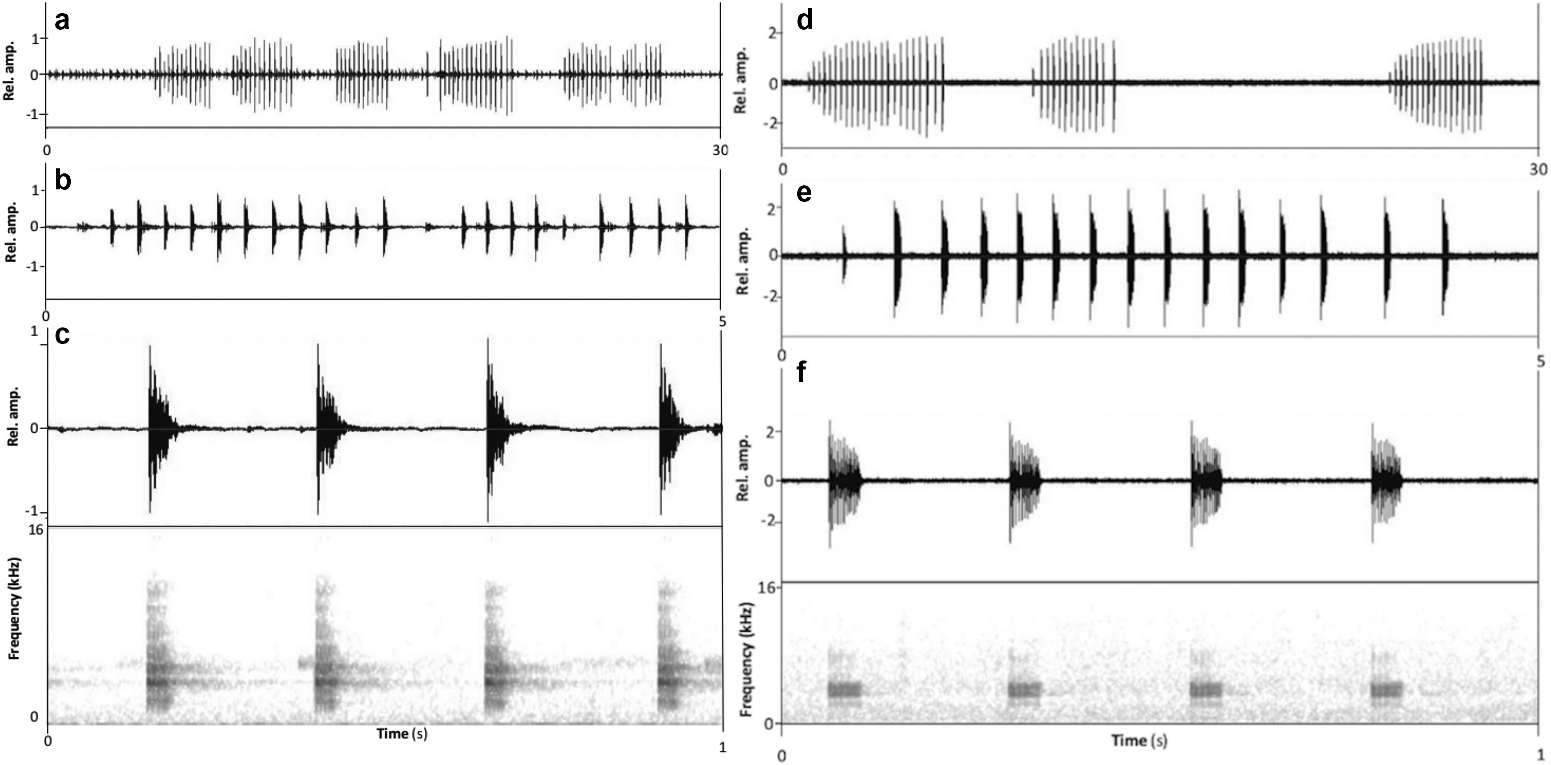
Advertisement call. Call descriptions are based on the calls of the holotype (VNMN 010948); five call groups and 20 calls in total were analysed ( Table 2; Figure 9 View Figure 9 (a–c)). Advertisement calls were recorded at 16.0°C ambient temperature. Refer to Table 2 for call characters. Call amplitude was relatively consistent within each call group, but the amplitude of pulses within each call dropped sharply after the first pulse ( Figure 9 View Figure 9 (a–c)). Weak harmonics were visible above the dominant frequency (at approximately 7.4 kHz) and below the dominant frequency at approximately 2.3 kHz.
Natural history. All specimens of Megophrys frigida sp. nov. were associated with disturbed secondary broadleaf forest and upper montane forest with a relatively open canopy. All individuals were encountered at night and observed in riparian habitat along 1–2 m wide streams with clear water and rocky stream beds. Males were calling from stream-side vegetation in September 2017. Tadpoles and females were not observed. Megophrys frigida sp. nov. is syntopic with M. jingdongensis , M. hoanglienensis and M. rubrimera at 2118 m asl ( Tapley et al. 2018b). At 2668 m, Megophrys frigida sp. nov. was the only Megophrys species encountered.
Distribution and conservation status. This species is currently only known from the recently designated Bat Xat Nature Reserve on Mount Ky Quan San at elevations between 2118 m asl and 2668 m ( Figure 1 View Figure 1 ). Despite an intensive survey effort at different times of the year (March, April, June, September and December), the species has not been encountered farther south on Mount Fansipan. The high-elevation sites where Megophrys frigida sp. nov. occurs face the immediate threat of habitat degradation; the forest in which this species occurs is being negatively impacted by fuelwood collection for the tourism industry and by the grazing of livestock ( Figures 8 View Figure 8 (b,c)), particularly the site at 2668 m asl. There was no evidence of excessive water extraction or water pollution. The fungal pathogen, Batrachochytium dendrobatidis, was not detected from 15 samples collected from 15 different anuran amphibians on Mount Ky Quan in September 2017; including six samples from Megophrys frigida sp. nov. ( Tapley et al. 2020a). If Megophrys frigida sp. nov. is restricted to a narrow, high-elevation band, it is likely that this species may be vulnerable to future climate change. The species’ EOO is currently predicted to be 832 km 2. We recommend that Megophrys frigida sp. nov. is listed as Endangered in accordance with the IUCN Red List of Threatened Species categories and criteria B1ab (iii) (see IUCN 2012).
Comparisons. Megophrys frigida sp. nov. can be distinguished from all species in the subgenus Panophrys found in mainland Southeast Asia, north of the Isthmus of Kra and nearby provinces of China (Yunnan, Guangxi and Guizhou) on the basis of morphology, and from all species in the subgenus for which comparable data is available on the basis of molecular and acoustic data . Comparisons with each subgenus are discussed separately below. The following comparisons are based on four adult males of Megophrys frigida sp. nov.
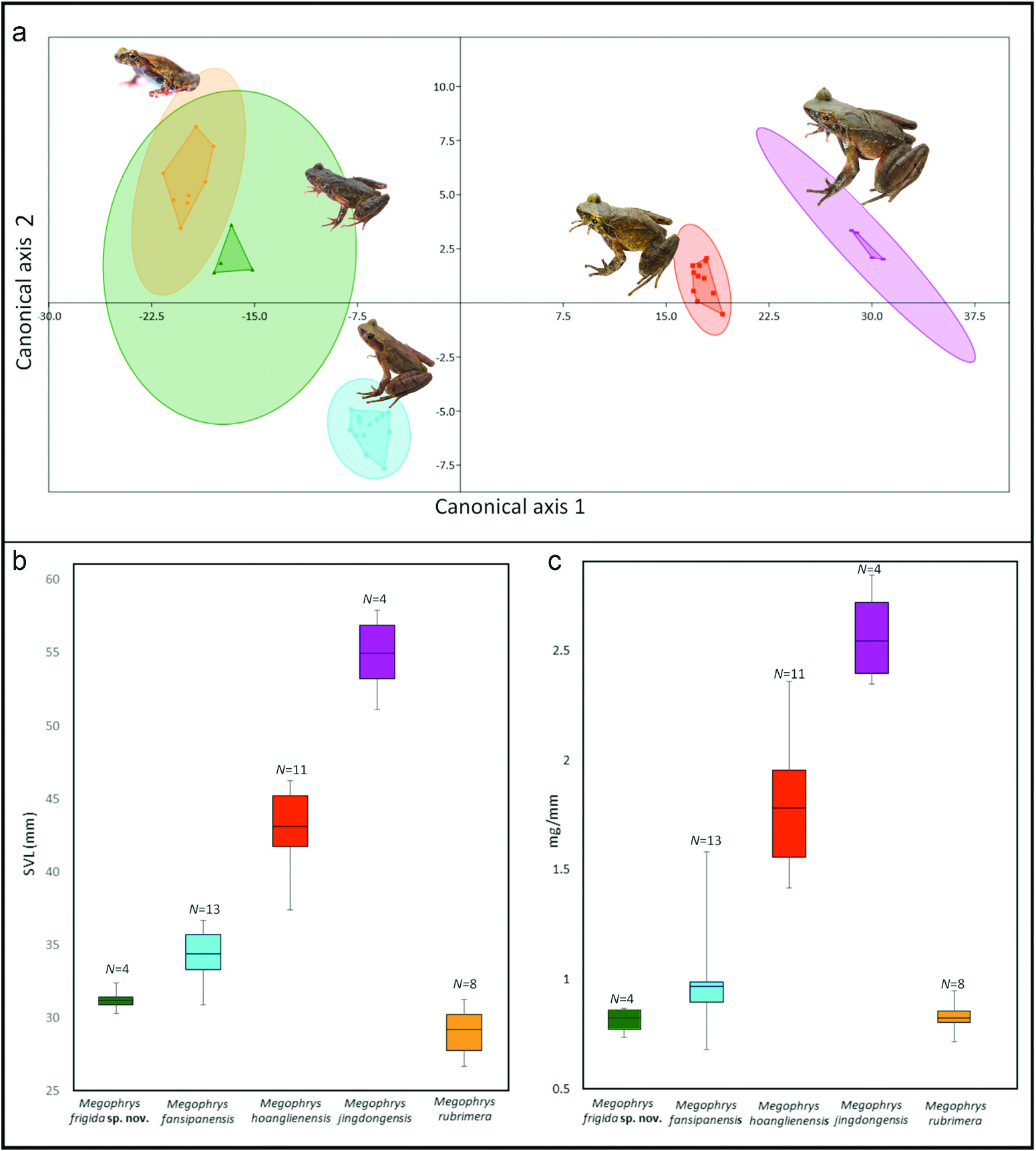
Subgenus Panophrys . Phylogenetic analysis places Megophrys frigida sp. nov. in the subgenus Panophrys . Megophrys frigida sp. nov. differs from M. angka by vomerine teeth and vomerine ridges present (vs ‘indistinct’ vomerine ridges and vomerine teeth absent in M. angka ; Wu et al. 2019) and subarticular tubercles absent (vs present on FI and FII in M. angka ; Wu et al. 2019); from M. binchuanensis by subarticular tubercles absent (vs present in M. binchuanensis ; Ye and Fei 1995) and dermal fringe on toes absent (vs present in M. binchuanensis ; Ye and Fei 1995); from M. binlingensis by having a smaller adult male size, SVL 30.3–31.8 mm (vs 45.1–51.0 mm, N = 3, in M. binlingensis ; Fei et al. 2009) and vomerine teeth present (vs absent in M. binlingensis ; Fei et al. 2009); from M. boettgeri by vomerine ridges and vomerine teeth present (vs absent in M. boettgeri ; Boulenger 1899; material examined), dorsolateral ridges present (vs absent in M. boettgeri N = 7; material examined) and male advertisement call (see Bioacoustic comparison); from M. brachykolos by having a smaller adult male size, SVL 30.3–31.8 mm (vs 34.1–40.5 mm, N = 14, in brachykolos ; Inger and Romer 1961; material examined), subarticular tubercles on fingers absent (vs present on base of all fingers in M. brachykolos ; Inger and Romer 1961; material examined) and vomerine ridges and vomerine teeth present (vs absent in M. brachykolos ; Inger and Romer 1961; material examined); from M. caobangensis by having a smaller adult male size, SVL 30.3–31.8 mm (vs 34.9–38.9 mm, N = 11, in M. caobangensis ; Nguyen et al. 2020), vomerine teeth present (vs absent in M. caobangensis ) and male TYD/EL 74–81% (vs 40– 47%, N = 11, in M. caobangensis ; Nguyen et al. 2020); from M. chishuiensis by having a smaller adult male size, SVL 30.3–31.8 mm (vs 43.4–44.1 mm, N = 3, in M. chishuiensis ; Xu et al. 2020), vomerine teeth present (vs absent in M. chishuiensis ; Xu et al. 2020) and male advertisement call (see Bioacoustic comparison); from M. daweimontis by having a smaller adult male size, SVL 30.3–31.8 mm (vs 34.0–37.0 mm, N = 18, in M. daweimontis ; Rao and Yang 1997). The ranges in measurements of characters of Megophrys frigida sp. nov. largely overlap with those of M. fansipanensis , but Megophrys frigida sp. nov. differs from M. fansipanensis ( Figure 4 View Figure 4 (a–c)) by an overlapping but typically smaller mean adult male size, SVL 31.1 (30.3–31.8) mm (vs 34.9 [30.9–44.3] mm, N = 13, in M. fansipanensis ; material examined; Figure 4 View Figure 4 (b)), a typically overlapping but smaller mean weight/SVL, 0.81 (0.74– 0.87) mg/mm (vs 1.01 [0.68–1.58] mg/mm, N = 13, in M. fansipanensis ; Tapley et al. 2018a; Figure 4 View Figure 4 (c)), typically relatively shorter thigh length, mean TL/SVL 50.7 (46.2–54.0)% (vs mean 53.0 [47.2–59.8]%, N = 13, in M. fansipanensis ; material examined), and advertisement call (see Bioacoustic comparison); from M. hoanglienensis ( Figure 4 View Figure 4 (a–c)) by having a smaller adult male body size, SVL 30.3–31.8 mm (vs 41.1–47.6 mm, N = 11, in M. hoanglienensis ; material examined; Figure 4 View Figure 4 (b)), smaller weight/SVL, 0.81 (0.74–0.87) mg/mm (vs 1.79 [1.42– 2.36] mg/mm, N = 11, in M. hoanglienensis ; Tapley et al. 2018a; Figure 4 View Figure 4 (c)), and advertisement call (see Bioacoustic comparison); from M. jiangi by having a smaller adult male size, SVL 30.3–31.8 mm (vs 34.4–39.2 mm, N = 9, in M. jiangi ; Liu et al. 2020), vomerine teeth present (vs absent in M. jiangi ; Liu et al. 2020) and male advertisement call (see Bioacoustic comparison); from M. jingdongensis by having a smaller adult male size, SVL 30.3–31.8 mm (vs 51.1–59.7 mm, N = 4, in M. jingdongensis ; material examined), wide dermal fringes on toes and interdigital webbing between toes absent (vs dermal fringes on toes present and toes being at least one-quarter webbed in M. jingdongensis ; material examined), and male advertisement call (see Bioacoustic comparison); from M. leishanensis by vomerine teeth present (vs absent in M. leishanensis ; Li et al. 2019 [“2018”]) and advertisement call (see Bioacoustic comparison); from M. liboensis by having a smaller adult male size, SVL 30.3– 31.8 mm (vs 60.5–67.7 mm, N = 5, in M. liboensis ; Zhang et al. 2017), and by lateral fringes on toes absent (vs present in M. liboensis ; Zhang et al. 2017); from M. minor by vomerine teeth present (vs absent in M. minor ; Stejneger 1926), adult male TYD/EL 74–81% (vs 47–48%, N = 2, in M. minor ; material examined) and male advertisement call (see Bioacoustic comparison); from M. mirabilis by having a smaller adult male size, SVL 30.3–31.8 mm (vs 55.8–61.4 mm, N = 2, in M. mirabilis ; Lyu et al. 2020), vomerine teeth present (vs absent in M. mirabilis ; Lyu et al. 2020), dermal fringes on fingers absent (vs present in M. mirabilis ; Lyu et al. 2020); and dermal fringes on toes absent (vs present in M. mirabilis ; Lyu et al. 2020); from M. omeimontis by having a smaller adult male size, SVL 30.3–31.8 mm (vs 51.6–60.9 mm, N = 5, in M. omeimontis ; material examined) and dermal fringes on toes absent (vs present in M. omeimontis ; material examined); from M. palpebralespinosa by dermal fringes on toes absent (vs present in M. palpebralespinosa ; Bourret 1937; Orlov et al. 2015; material examined), interdigital webbing between toes absent (vs present in M. palpebralespinosa ; Bourret 1937; Orlov et al. 2015; material examined) and a small blunt tubercle present on outer edge of upper eyelids (vs moderately large palpebral horn like structure on upper eyelids present in M. palpebralespinosa ; Bourret 1937; Orlov et al. 2015; material examined); from M. qianbeiensis by having a smaller adult male size, SVL 30.3–31.8 mm (vs 49.3–58.2 mm, N = 6, in M. qianbeiensis ; Su et al. 2020), lateral fringes on toes absent (vs present in M. qianbeiensis ; Su et al. 2020), interdigital webbing between toes absent (vs present in M. qianbeiensis ; Su et al. 2020), large keratinised spines on nuptial pads of sexually mature males absent (vs present in in M. qianbeiensis ; Su et al. 2020) and male advertisement call (see Bioacoustics comparison); from M. rubrimera by lateral fringes on toes absent (vs present in M. rubrimera ; Tapley et al. 2017; material examined), absence of a red-orange groin, inner thighs and outer surface of shanks in life (vs presence in M. rubrimera ; Tapley et al. 2017) and male advertisement call (see Bioacoustics comparison); from M. shimentaina by dermal fringes on fingers absent (vs present in M. shimentaina ; Lyu et al. 2020), dermal fringes on toes absent (vs present in M. shimentaina ; Lyu et al. 2020) and male advertisement call (see Bioacoustics comparison); from M. shuichengensis by interdigital webbing between toes absent (vs present, distinctly webbed in M. shuichengensis ; Tian et al. 2000) and dermal fringes on toes absent (vs present in M. shuichengensis ; Tian et al. 2000); from M. shunhuangensis by vomerine teeth present (vs absent in M. shunhuangensis ; Wang et al. 2019b), male TYD/EL 74–81% (vs 47–70%, N = 10, in M. shunhuangensis ; Wang et al. 2019b) and advertisement call (see Bioacoustic comparison); from M. spinata by interdigital webbing between toes absent (vs present, distinctly webbed in M. spinata ; Hu et al. 1973) and large keratinised spines on nuptial pads of sexually mature males absent (vs present in M. spinata ; Hu et al. 1973); and from M. wuliangshanensis by having a larger tympanum on males, mean male TYD/EL 78% (vs mean male TYD/EL 49%, N = 10, in M. wuliangshanensis ; Ye and Fei 1995), and vomerine ridges and vomerine teeth present (vs absent in M. wuliangshanensis ; Fei et al. 2009, 2012).
Subgenus Xenophrys Günther, 1864 . Megophrys frigida sp. nov. differs from M. auralensis by having a smaller adult male size, SVL 30.3–31.8 mm (vs 60.1 − 76.9 mm, N = 20, in M. auralensis ; Ohler et al. 2002; Neang et al. 2013); from M. damrei by having a smaller adult male size, SVL 30.3–31.8 mm (vs 47.7–57.1 mm, N = 7, in M. damrei ; Mahony 2011; Neang et al. 2013; material examined); from M. glandulosa by having a smaller adult male size, SVL 30.3–31.8 mm (vs 76.7–81.6 mm, N = 10, in M. glandulosa ; Fei et al. 2009; material examined), dermal fringes on toes absent (vs present in M. glandulosa ; Huang et al. 1998; Mahony et al. 2018; material examined), distinct interdigital webbing between toes absent (vs present as basal webbing in M. glandulosa ; Mahony et al. 2018; material examined) and a light-coloured upper lip stripe absent (vs present in M. glandulosa ; Fei et al. 1990; Mahony et al. 2018; material examined); from M. lekaguli by having a smaller adult male size, SVL 30.3–31.8 mm (vs 55.6–68.1 mm, N = 8, in M. lekaguli ; Stuart et al. 2006; material examined); from M. major by having a smaller adult male size, SVL 30.3–31.8 mm (vs 72.4–87.5 mm, N = 10, in M. major ; Mahony et al. 2018; material examined), distinct interdigital toe webbing absent (vs present as distinct basal webbing in M. major ; Mahony et al. 2018; material examined) and a light-coloured upper lip stripe absent (vs present in M. major ; Boulenger 1908; Mahony et al. 2018; material examined); from M. maosonensis by having a smaller adult male size, SVL 30.3– 31.8 mm (vs 58.0–76.0 mm, N = 6, in M. maosonensis ; Bourret 1937), distinct interdigital toe webbing absent (vs present, toes up to one-quarter webbed in M. maosonensis ; Bourret 1937) and a light-coloured upper lip stripe absent (vs present in M. maosonensis ; Bourret 1937); from M. pachyproctus by a larger TYD/EL 74–81% (vs 29%, N = 3, in M. pachyproctus ; Huang and Fei 1981) and a protruding projection posterior to cloaca on male specimens absent (vs present in M. pachyproctus Huang et al. 1981 ); from M. parva by a larger TYD/EL 74–81% (vs 40–55%, N = 4, in M. parva ; material examined); and from M. takensis by having a smaller adult male size, SVL 30.3–31.8 mm (vs 47.3–53.0 mm, N = 3, in M. takensis ; Mahony 2011; material examined).
Subgenus Atympanophrys Tian and Hu, 1983 . Megophrys frigida sp. nov. can be distinguished from M. gigantica by having a distinct tympanum (vs obscured in M. gigantica ; Liu et al. 1960), vomerine teeth present (vs absent in M. gigantica ; Liu et al. 1960), lateral fringes on toes absent (vs present in M. gigantica ; Liu et al. 1960) and smaller adult male size, SVL 30.3–31.8 mm (vs 80.5–107.0 mm in M. gigantica ; Fei et al. 2012); and from M. shapingensis by having a distinct tympanum (vs obscured in M. shapingensis ; Liu 1950) and vomerine teeth present (vs absent in M. shapingensis ; Liu 1950).
Subgenus Brachytarsophrys Tian and Hu, 1983 . Megophrys frigida sp. nov. can be distinguished from M. carinense , M. feae , M. intermedia and M. platyparietus by transverse
ridge at base of head absent (vs present in M. carinense , M. feae , M. intermedia and M. platyparietus ), and by having a smaller adult male size, SVL 30.3–31.8 mm (vs> 70.7 mm; material examined; Fei and Ye 2001; Li et al. 2020).
Subgenus Ophryophryne Boulenger, 1903 . Megophrys frigida sp. nov. can be distinguished from M. elfina by brown nuptial pads in life (vs bright orange; Poyarkov et al. 2017); from M. gerti by vomerine ridges present (vs absent in M. gerti ; Poyarkov et al. 2017; material examined); from M. hansi by having a smaller adult male size, SVL 30.3–31.8 mm (vs 33.4–44.4 mm, N = 12, in M. hansi ; Poyarkov et al. 2017; material examined); from M. koui by protruding fleshy projection above cloaca in sexually mature males absent (vs present in M. koui ; Poyarkov et al. 2017); from M. poilani by having a smaller adult male size, SVL 30.3–31.8 mm (vs 32.6–38.1 mm, N = 14, in M. poilani ; Poyarkov et al. 2017); from M. synoria by having a smaller adult male size, SVL 30.3–31.8 mm (vs 38.2–53.7 mm, N = 14, in M. synoria ; Poyarkov et al. 2017; material examined), and microspinules on nuptial pads of sexually mature males (vs microgranules on nuptial pads of sexually mature male M. synoria ; material examined); and from M. microstoma and all other species in the subgenus Ophryophryne due to maxillary teeth present (vs absent; Poyarkov et al. 2017; material examined).
Species not yet assigned to a subgenus. Megophrys frigida sp. nov. differs from M. feii by having a larger adult male body size, SVL 30.3–31.8 mm (vs 24.3–25.1 mm, N = 4, in M. feii ; Yang et al. 2018), vomerine ridges and vomerine teeth present (vs absent in M. feii ; Yang et al. 2018), lateral fringes on toes absent (vs present in M. feii ; Yang et al. 2018), nuptial pads on breeding males present (vs absent in M. feii ; Yang et al. 2018), and a protruding projection posterior to cloaca absent (vs present in both sexes of M. feii ; Yang et al. 2018).
Bioacoustic comparison. The male advertisement call (N = 20 calls) of Megophrys frigida sp. nov. recorded at 16.0°C differs from those of its 10 congeners (subgenus Panophrys ) found in mainland Southeast Asia, north of the Isthmus of Kra and nearby provinces of China (Yunnan, Guangxi and Guizhou) for which calls have been described. The male advertisement call of Megophrys frigida sp. nov. differs from that of M. boettgeri (data from Wang et al. 2014, N = 76 calls recorded at 15.0–18.0°C) by having an average call duration of 46.5 (43.0–50.0) ms (vs 54 ms [range not provided] in M. boettgeri ), and weak harmonics at both 2.3 and 7.4 kHz (vs relatively clear harmonics at approximately 5.0 kHz in M. boettgeri ); from M. chishuiensis (data from Xu et al. 2020, N = 7 calls recorded at 24.5°C) by having an average call duration of 47 (43–50) ms (vs 70–120 ms in M. chishuiensis ) and an average dominant frequency of 3.6 (3.5–3.7) kHz (vs 5.9 [5.7–6.1] kHz in M. chishuiensis ); from M. fansipanensis (data from Tapley et al. 2018a, N = 60 calls recorded at 15.3–18.3°C) by having an average call repetition rate of 3.5 (3.5–3.6) calls/s (vs 3.9 [3.8–4.0] calls/s in M. fansipanensis ), and a consistent call amplitude within each call group and amplitude of pulses within each call dropping sharply after the first pulse (vs more variable amplitude within each call group and amplitude of pulses within each call declining gradually after the first pulse in M. fansipanensis ; Figure 9 View Figure 9 (d)); from M. hoanglienensis (data from Tapley et al. 2018a, N = 20 calls recorded at 18.5°C) by having an average call duration of 47 (43–50) ms (vs 103 [96–108] ms in M. hoanglienensis ), an average of 10.8 (10.0–11.0) pulses per call (vs 18.7 [12.0–22.0] in M. hoanglienensis ), and an average call repetition rate of 3.5 (3.5– 3.6) calls/s (vs 2.6 calls/s in M. hoanglienensis ); from M. jiangi (data from text in Liu et al. 2020, N = 90 calls recorded at 19.5°C) by having an average call duration of 47 (43–50) ms (vs 170–370 ms in M. jiangi ) and an average dominant frequency of 3.6 (3.5–3.7) kHz (vs 5.8 [5.7–6.0] kHz in M. jiangi ); from M. jingdongensis (data from Cutajar et al. 2020, N = 17 calls recorded at 18.5°C) by having an average call duration of 46.5 (43.0–50.0) ms (vs 132.7 [117.0–147.0] ms in M. jingdongensis ), an average dominant frequency of 3.6 (3.5–3.7) kHz (vs 2.5 [2.4–2.6] kHz in M. jingdongensis ), and an average call repetition rate of 3.5 (3.5–3.6) calls/s (vs 3.9 calls/s in M. jingdongensis ); from M. leishanensis (data from Li et al. 2019 [“2018”], N = 36 calls recorded at 18.9°C) by having an average of 10.8 (10.0–11.0) pulses/call (vs 13.0 [12.0–14.0] pulses/call, N = 5, in M. leishanensis ), and an average call repetition rate of 3.5 (3.5–3.6) calls/s (vs 2.6 [1.2–3.2] calls/s in M. leishanensis ); from M. minor (data from Jiang et al. 2001, N = 14 calls recorded at 14.0°C) by having an average call repetition rate of 3.5 (3.5–3.6) calls/s (vs 4.0 [range not provided] calls/s in M. minor ), and having weak harmonics at 2.3 and 7.4 kHz (vs relatively clear harmonics at approximately 7.2 kHz in M. minor ); from M. qianbeiensis (data from Su et al. 2020, number of calls analysed not reported, calls recorded at 20.5°C) by having an average call duration of 46.5 (43.0–50.0) ms (vs 129.0–211.0] ms in M. qianbeiensis ) and an average dominant frequency of 3.6 (3.5–3.7) kHz (vs 2.25–3.00 kHz in M. qianbeiensis ); from M. shimentaina (data from Lyu et al. 2020, N = 96 calls recorded at 18.0–20.0°C) by having an average call duration of 46.5 (43.0–50.0) ms (vs 85.0 [64.0–101.0] ms in M. shimentaina ) and an average dominant frequency of 3.6 (3.5–3.7) kHz (vs 4.9 [4.7–5.2] kHz in M. shimentaina ); and from M. rubrimera (data from Tapley et al. 2017, N = 60 calls recorded at 21.0–22.9°C) by having an average dominant frequency of 3.6 (3.5–3.7) kHz (vs 3.3 [3.2–3.4] kHz in M. rubrimera ), an average call duration of 46.5 (43–50) ms (vs 73.3 [62.0–85.0] ms in M. rubrimera ), and a call repetition rate of 3.5 (3.5–3.6) calls/s (vs 3.3 [3.1–3.4] calls/s in M. rubrimera ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.