Nigella sativa subsp. organs
|
publication ID |
https://doi.org/ 10.1016/j.phytochem.2022.113290 |
|
persistent identifier |
https://treatment.plazi.org/id/038D6D68-FFDE-FFC7-FFE0-CB9EAA1B779B |
|
treatment provided by |
Felipe |
|
scientific name |
Nigella sativa subsp. organs |
| status |
|
2.1. Terpenoids concentrations in N. sativa organs
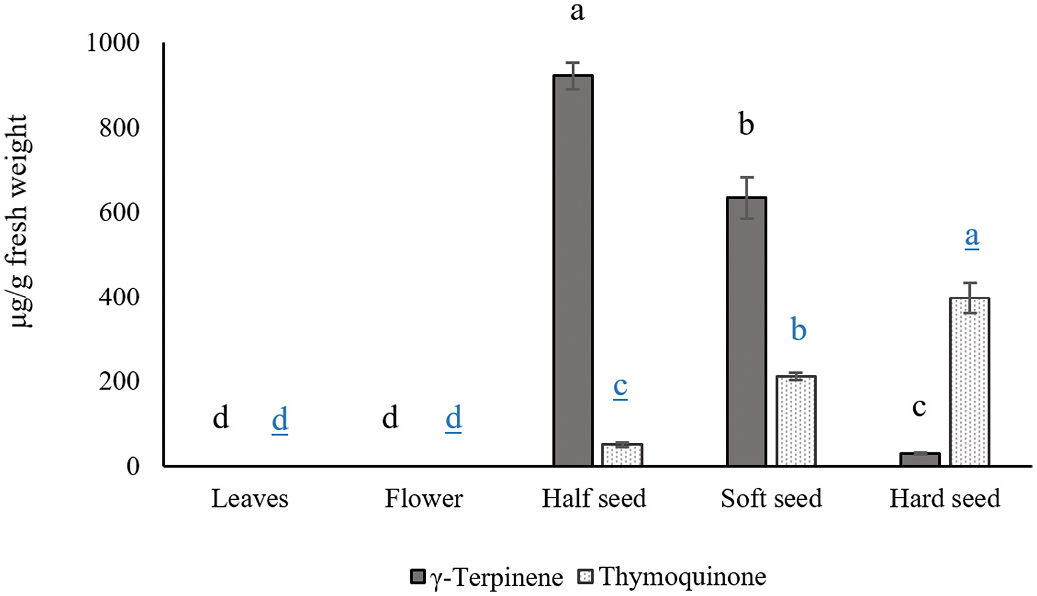
In order to check the chemical composition of terpenoids in different organs of N. sativa including leaves, flowers, and seeds, GC-MS analysis was conducted. Seed tissue was sampled in several stages of maturation, including half green-and-black seeds (appearing as dark green seeds), soft black seeds and hard black seeds. There were significant differences between organs in terms of terpenoids composition. No volatile terpenes were detected in leaves and flowers of N. sativa , while we identified 14, 14 and 17 components from half-black, soft black and hard black seeds, respectively ( Fig. 2 View Fig : Supplementary Table S1). The terpene blend consists of monoterpenes (more than 99%) and sesquiterpenes (less than 1%) but changed during seed maturation. Seeds in the half black stage contain monoterpene hydrocarbons (94.59%), monoterpene ketones (3.46%), monoterpene alcohols (1.82%) and sesquiterpenes (0.11%). Seeds in the soft black stage contain monoterpene hydrocarbons (84%), monoterpene ketones (13.53%), monoterpene alcohols (2.34%), a monoterpene ester (0.06%) and sesquiterpenes (0.05%), whereas seeds in the hard black stage contain monoterpene hydrocarbons (68.92%), monoterpene ketones (28.15%), monoterpene alcohols (1.49%), a monoterpene ester (0.1%) and sesquiterpenes (0.94%). The composition of the major monoterpenes in half black seeds included γ- terpinene (62.54%), α- thujene (14.25%), p -cymene (5.07%), α- terpinene (3.62%), β- pinene (3.54%), thymoquinone (3.46%), α- pinene (2.80%), limonene (1.79%), carvacrol (1.69%), myrcene (0.62%), α- phellandrene (0.36%), and terpinen-4-ol (0.13%). No thymohydroquinone and carene were detected in half black and soft black seeds but they were present in low concentrations in hard black seeds (carene 0.05%, thymohydroquinone 0.14%). The highest amount of bornyl acetate was observed in hard black seeds (0.11%), followed by soft black seeds (0.06%) but none was detected in half black seeds. Thymohydroquinone and thymoquinone levels were highest in hard black seeds. The sesquiterpene components were higher in hard black seeds (0.94%) than half black (0.11%) or soft black seeds (0.05%). While α- longipinene was only detected in hard black seeds (0.16%), β- longipinene was observed in hard black seeds (0.78%), followed by half black seeds (0.11%) and soft black seeds (0.05%).
2.2. 2. Correlation between the precursors in the biosynthetic pathway of thymoquinone
To improve our understanding of the developmental regulation of γ- terpinene and thymoquinone, we conducted an ANOVA analysis with the developmental stages of seeds for the γ- terpinene and thymoquinone concentrations (P <0.01). A significantly higher amount of γ- terpinene was observed in half-black seeds (62.54%), than in soft black seeds (40.33%) and hard black seeds (2.09%). The highest amount of thymoquinone was observed in hard black seeds (28.15%), followed by significantly less in soft black seeds (13.53%) and half-black seeds (3.46%) (Supplementary Table S1). This suggests that γ- terpinene is converted to thymoquinone during seed maturation. A correlation analysis with all proposed intermediates of the pathway was conducted to study this hypothesis in more detail ( Table 1). Pearson’ s correlation coefficients indicated a negative correlation between γ- terpinene and its potential products p -cymene, carvacrol, thymohydroquinone, and thymoquinone. The value of the Pearson’ s correlation coefficient of γ- terpinene with thymoquinone, p -cymene, thymohydroquinone, and carvacrol were 0.47, 0.46, 0.43 and 0.22, respectively. A positive correlation was observed between the p -cymene, carvacrol, thymohydroquinone and thymoquinone which are prospective intermediates and products. Apparently, these metabolites are regulated coordinately. Thymoquinone displayed a strong positive correlation with p -cymene with a coefficient of 0.95.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.