Diorhabda carinata ( Faldermann, 1837 )
|
publication ID |
https://doi.org/10.11646/zootaxa.2101.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/038C2C5B-AC47-FFF4-3B91-F9C4F51AD4E9 |
|
treatment provided by |
Felipe |
|
scientific name |
Diorhabda carinata ( Faldermann, 1837 ) |
| status |
|
Diorhabda carinata ( Faldermann, 1837)
larger tamarisk beetle
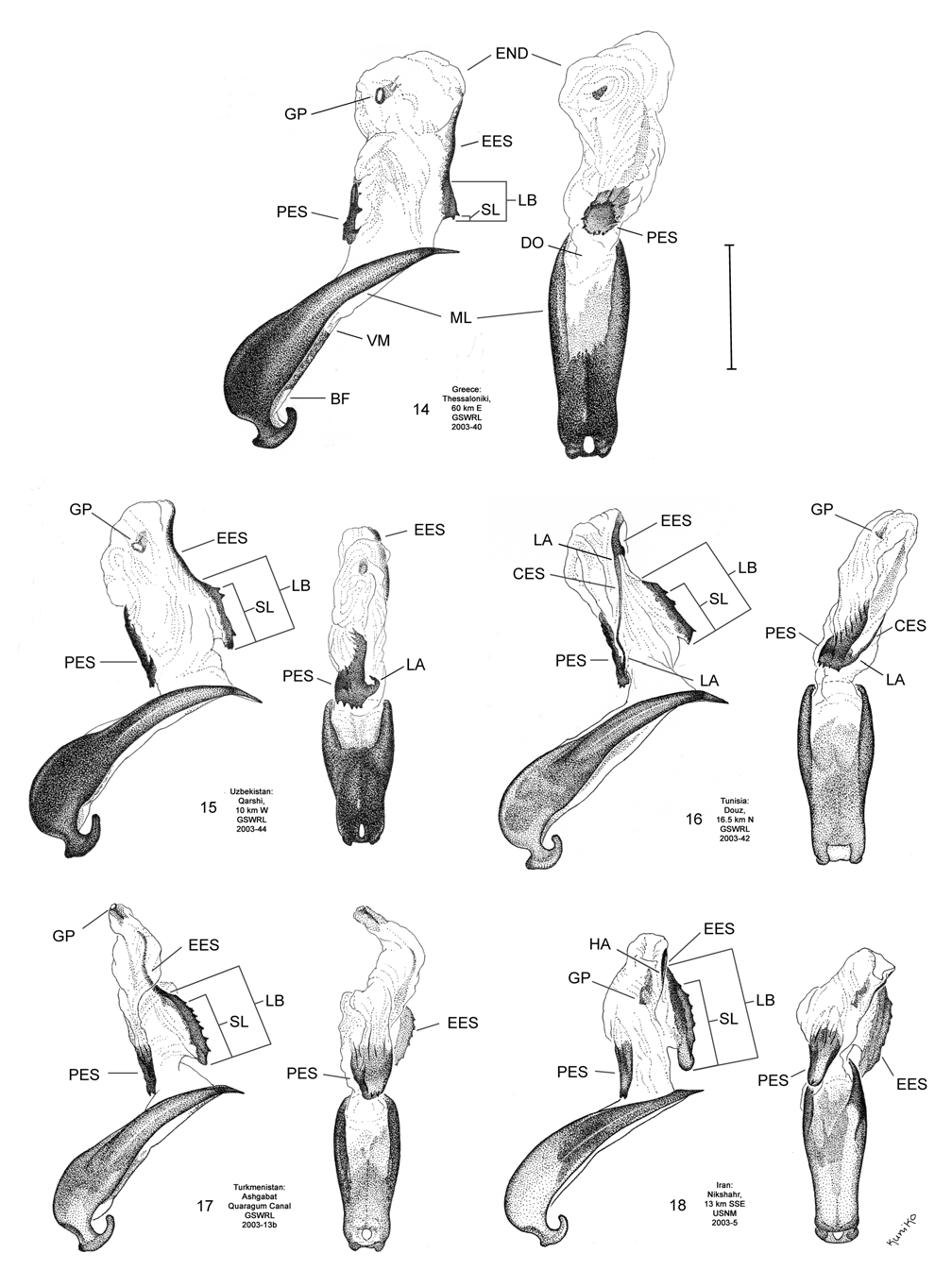
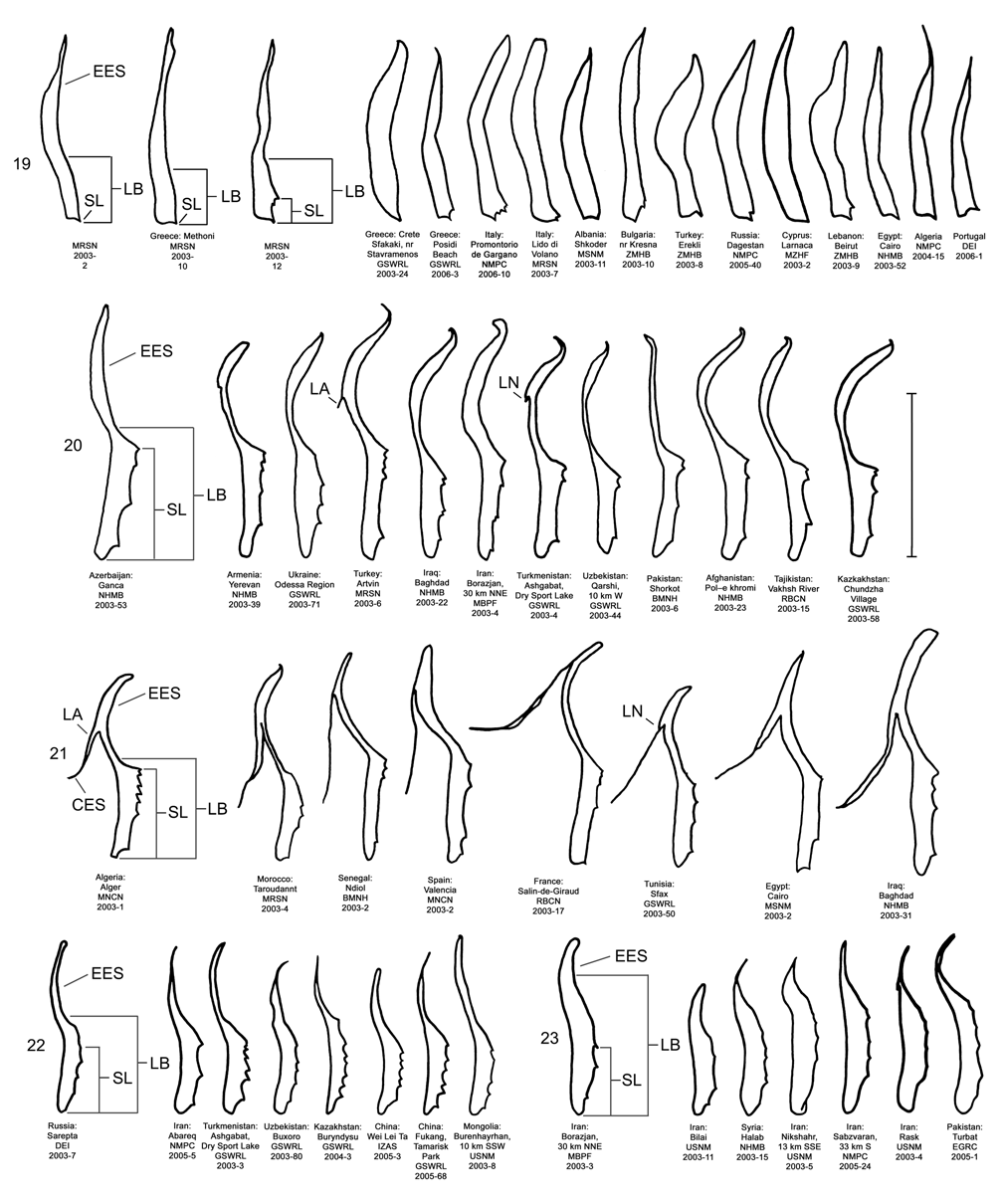
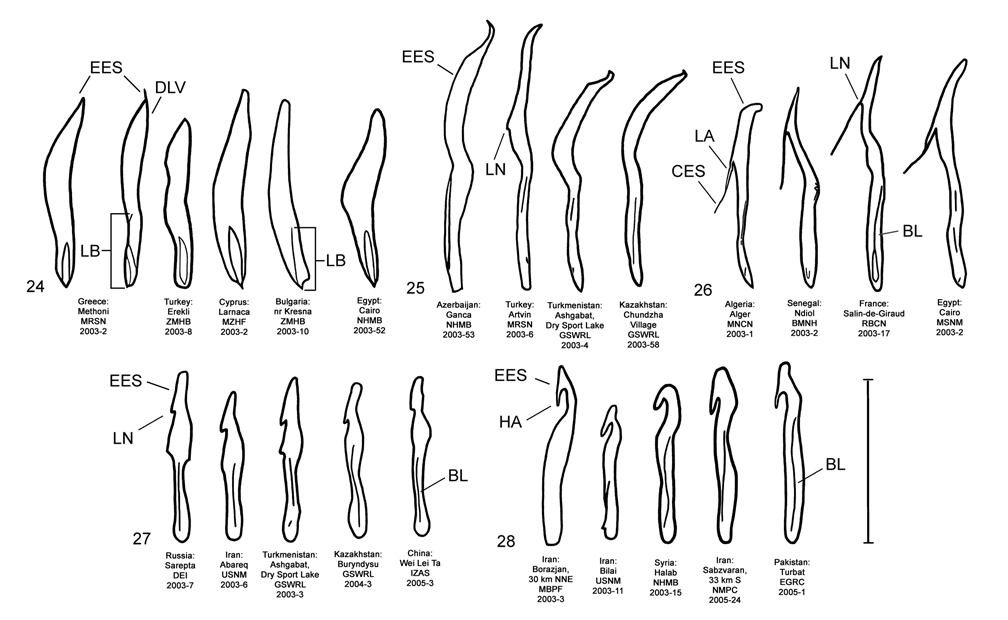
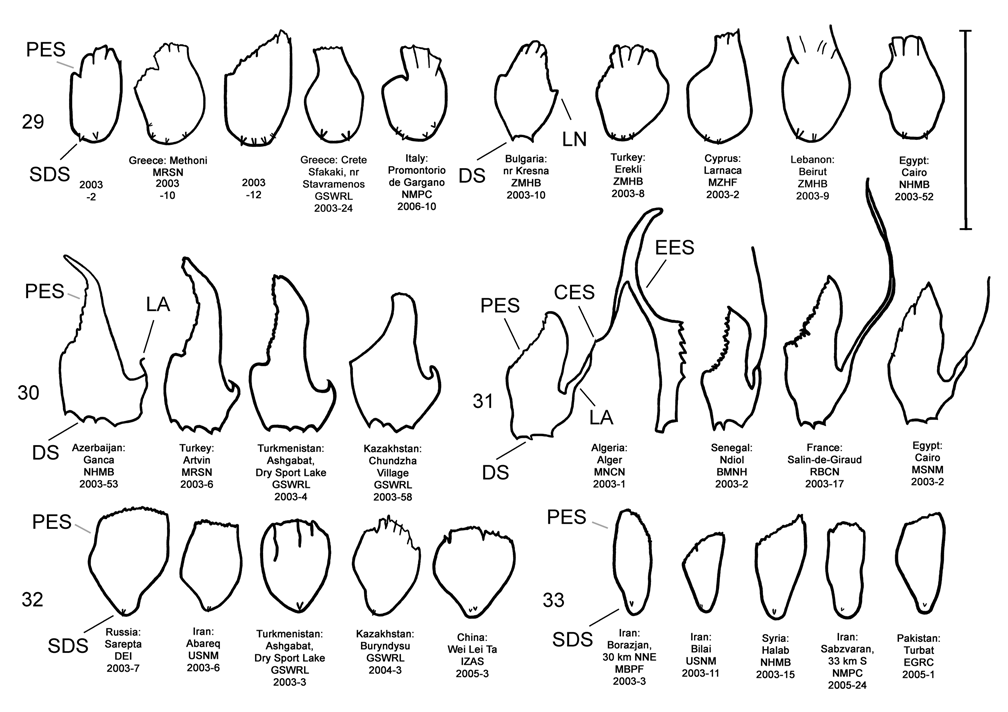
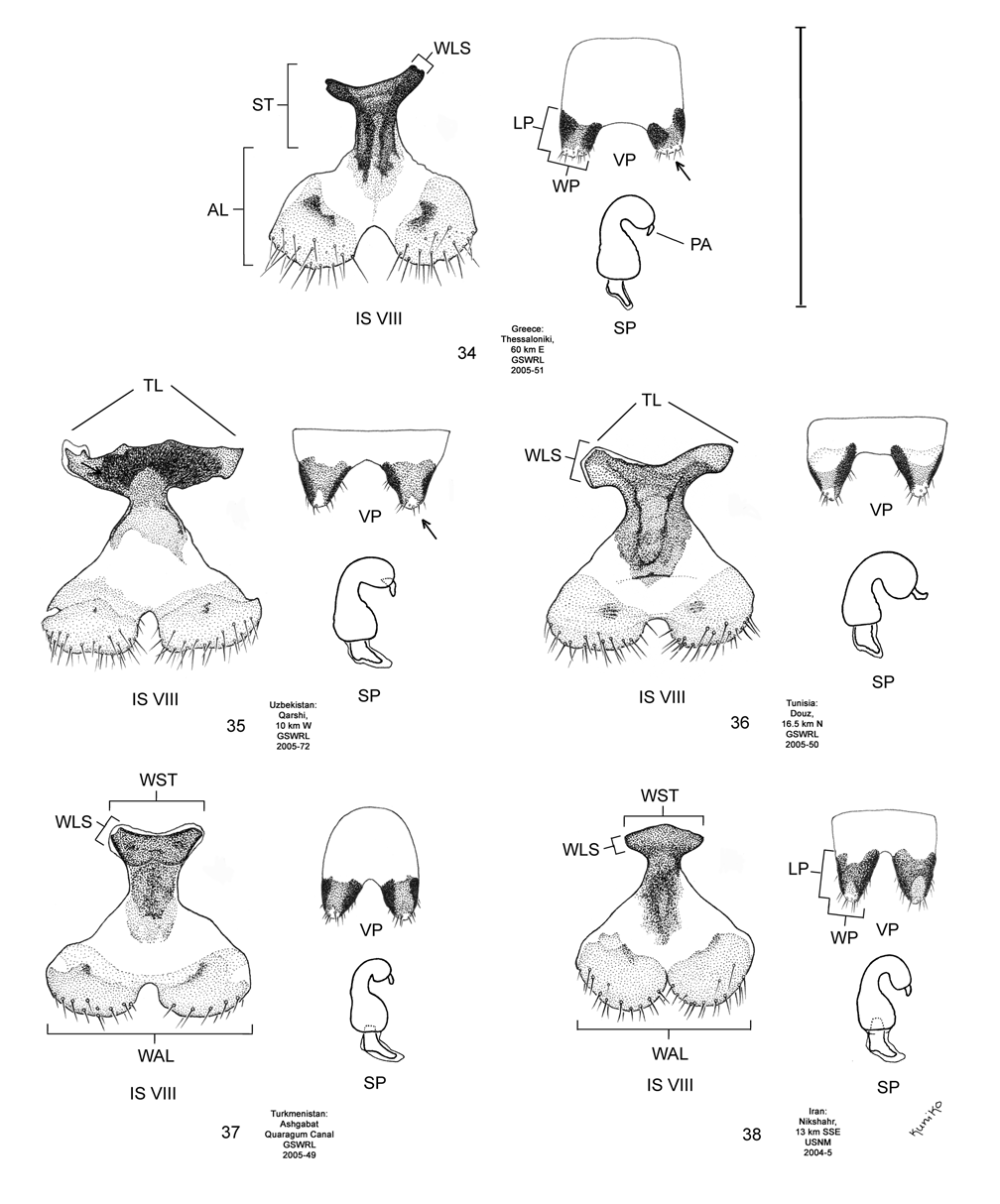
( Figs. 3, 4 View FIGURES 1–9 , 15 View FIGURES 14–18 , 20 View FIGURES 19–23 , 25 View FIGURES 24–28 , 30 View FIGURES 29–33 , 35 View FIGURES 34–38 , 40 View FIGURES 39–43 )
Galeruca carinata Faldermann, 1837:329 ( Type locality: Transcaucasus region [ Georgia, Armenia and Azerbaijan]; as Galleruca ).
Galeruca elongata: Reiche and Saulcy, 1858:42 (part, l’Immerétie [western Georgia], Syria, Turkey, as Galleruca ); Joannis, 1866:83 (part, monograph; Syria, as Galleruca ).
Diorhabda elongata: Weise, 1883:316 (part, established genus; Transcaucasus); Holdhaus, 1920:45 (Assur [Ash Sharqat], Iraq); Ogloblin, 1936:79 (part, Iran, Syria, Turkey, Transcaucasus, central Asia); Rusanov, 1949:118 (part, Central Asia; as Diorrhabda); Kyrzhanovskiy, 1952:198 (part, Turkmenistan), 1965:392 (part, middle Asia); Pavlovskii and Shtakelberg, 1955:566 (part, southwest Europe, as Diorrhabda); Yakhontov and Davletshina, 1955:58 (part, biology; Amu Darya Delta, northern Uzbekistan); Sinadsky, 1957:950, 1963:84, 1968:64 (part, biology; Amu Darya Valley, Uzbekistan; as Diorrhabda); Medvedev, 1959:118 (part, Turkmenistan); Yakhontov, 1959:338 (part, biology; Uzbekistan); Lozovoi, 1961:86 (part, eastern Georgia); Kulinich, 1962:73 (part, biology; Tajikistan); Pripisnova, 1965:83 (part, biology, Tajikistan); Kulenova, 1968:171 (part, southeastern Kazakhstan); Lopatin and Tadzhibaev, 1972:591 (part, Tajikistan); Lopatin, 1977a:282 (part, Asia), 1981:375 (Robate–Ghozlog [Robat–e Qozlog], Iran); Davletshina et al., 1979:79 (part, southwest Kyzyl– Kum Desert, central Uzbekistan); Habib and Hasan, 1982:19 (host range; northern Pakistan); Tomov, 1984:377 (part, Artvin, Turkey); Samedov and Mirzoeva, 1985:712 (part, Azerbaijan); Mirzoeva, 1988:?, 2001:48 (part, Azerbaijan); Lopatin and Kulenova, 1986:129 (part, Kazakhstan); Myartseva, 1995:4, 1999:1; 2001:1 (part, biology; Turkmenistan); Kovalev, 1995:78 (part, south-central Palearctic); Richter and Myartseva, 1996:316 (parasitoid, Turkmenistan); Gruev and Tomov, 1998:70 (part, Transcaucasus, mid- Asia); Aslan, 1998:287 (Ezurum Ili, province of northeast Turkey); Aslan et al., 2000:30 (part, eastern Turkey); Anonymous, 2001:52 N (part, Turkmenistan); DeLoach et al., 2003a:230 (part, Uzbekistan [Qarshi]), 2003b:126 (part, host range; Pakistan, Uzbekistan, Turkmenistan, Georgia, Azerbaijan), (2008, in prep.) (part, Qarshi, Uzbekistan); Khamraev, 2003:11 (part, Uzbekistan); Milbrath et al., 2003:225 (part, Qarshi, Uzbekistan); Riley et al., 2003:69,189 (part, catalog of North America [introduced]); Warchalowski, 2003:328 (part, taxonomic keys; Caucasus and Central Asia); Bieṅkowski, 2004:76 (part, keys; eastern Europe, Caucasus, Iran, Iraq, Central Asia, Kazakhstan); DeLoach and Carruthers, 2004a:13, 2004b:311 (part, Uzbekistan [Qarshi]); Gök and Duran, 2004:17 (part, Turkey); Lopatin et al., 2004:127 (part, central Asia); Gates et al. 2005:28 (parasitoid; Ashgabat, Turkmenistan); Dudley, 2005a:13, 2005b:42 N (part, biological control; ex: Uzbekistan); Milbrath and DeLoach, 2006a:32, 2006b:1379 (part, host specificity; Qarshi, Uzbekistan); Dudley et al., 2006:137 (part, host range; Qarshi, Uzbekistan); Milbrath et al. (2007) (part, biology; Qarshi, Uzbekistan); DeLoach (2008); Bean and Keller (in prep.) (part, diapause induction; Qarshi, Uzbekistan); Thompson et al. (in prep.) (part, laboratory hybridization; Qarshi, Uzbekistan).
Diorhabda elongata var. carinata: Heyden et al., 1891:375 (part, catalog for Europe and Caucasus); Weise, 1893:635, 1132 (part, Transcaucasus); Jacobson, 1901:137 (part, Amu Darya (river), Turkmenistan, as Dirrhabda).
Diorhabda elongata ab. carinata: Weise, 1924:78 (part; world catalog; Transcaucasus; Turkmenistan, Amu Darya); Winkler, 1924 –1932:1307 (part, Palearctic catalog, Transcaspian region); Warchalowski, 2003:328 (part, taxonomic keys, Caucasus and Central Asia).
Diorhabda rybakowi: Mityaev, 1958:86 (part, biology, Kazakhstan, as rybakovi).
Diorhabda elongata carinata: Bechyné, 1961:256 (Pol–e khomri [Polichromi], Afghanistan; central Asia); Lopatin, 1963:355 ( Afghanistan); Wilcox, 1971:63 (world catalog, Afghanistan); Medvedev, 1983:123 (zoogeography, Afghanistan); 1985:44 ( Afghanistan); Lopatin et al., 2004:127 (east Kazakhstan, northwest China); Dalin et al. (in press) (host range; Qarshi, Uzbekistan).
Diorhabda elongata sublineata: Gressitt and Kimoto, 1963a:407 (part, Asia Minor, W. Asia); Wilcox, 1971:63 (part, world catalog, Asia Minor, W. Asia).
Diorhabda carinata: Berti and Rapilly, 1973:881 (restored species, Transcaucasus), DeLoach et al., 2003b:126.
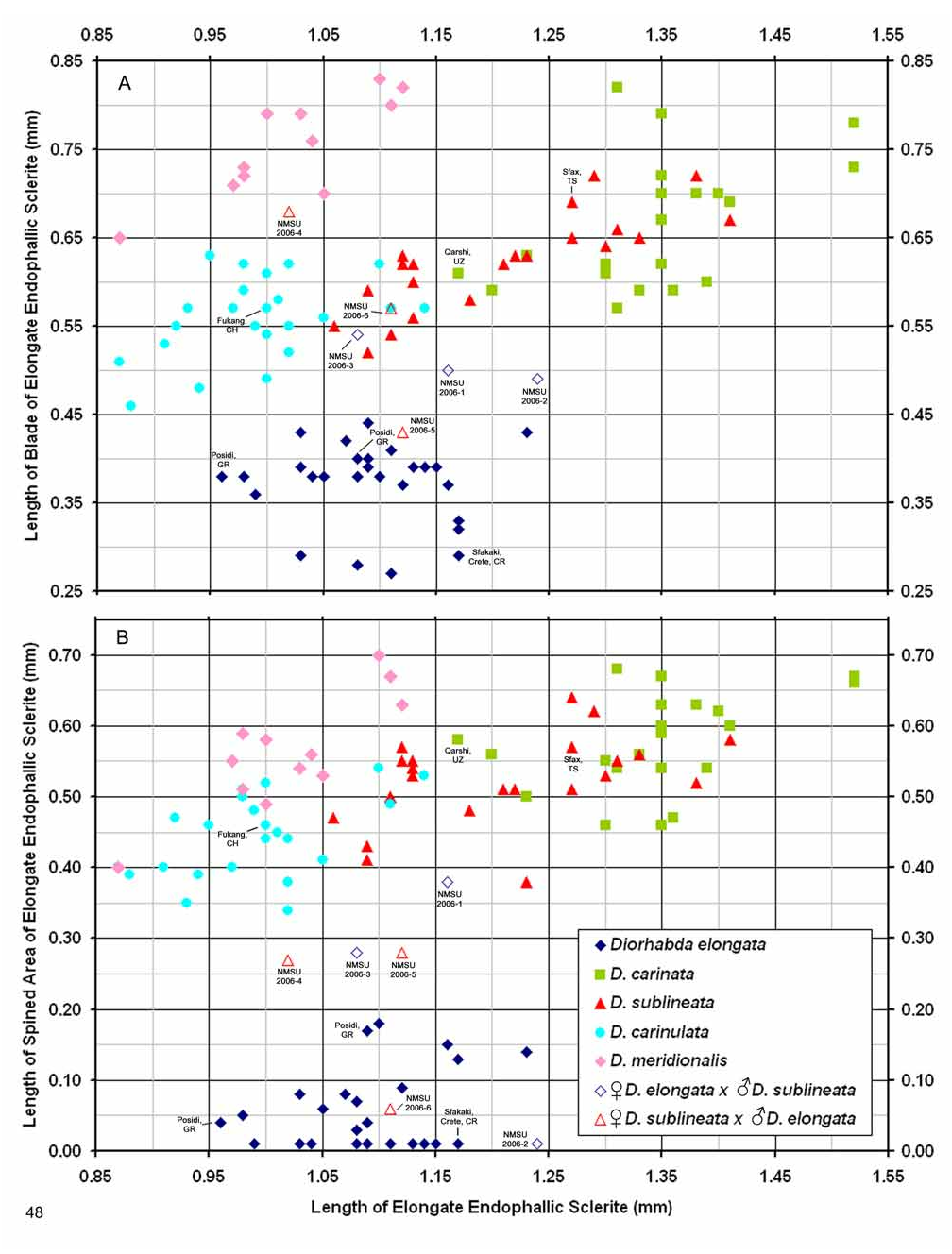
Male. Genitalia. Diorhabda carinata can be distinguished from all other members of the D. elongata group by a combination of characters of the palmate endophallic sclerite (PES) and the lack of a connecting endophallic sclerite (CES). The palmate endophallic sclerite (PES) in D. carinata always bears a strong lateral appendage (LA) and the distal margin is truncate-serrate with two to six (commonly four to five) usually distal spines, a maximum of one spine being subdistal ( Figs. 15 View FIGURES 14–18 , 30 View FIGURES 29–33 ). In contrast, the PES of D. carinulata ( Figs. 17 View FIGURES 14–18 , 32 View FIGURES 29–33 ) and D. meridionalis ( Figs. 18 View FIGURES 14–18 , 33 View FIGURES 29–33 ) lacks a lateral appendage (but may bear a lateral notch) and the distal margins of the PES are narrowly rounded and generally smooth with one or two small subdistal spines that sometimes project beyond the distal margin. The PES of D. elongata also lacks a lateral appendage and is usually rounded with mostly subdistal spines and a maximum of two distal spines ( Figs. 14 View FIGURES 14–18 , 29 View FIGURES 29–33 ). Diorhabda sublineata bears a CES connecting the PES to the elongate endophallic sclerite (EES) ( Figs. 16 View FIGURES 14–18 , 21 View FIGURES 19–23 , 31 View FIGURES 29–33 ), but the CES is lacking in D. carinata ( Figs. 15 View FIGURES 14–18 , 20 View FIGURES 19–23 , 30 View FIGURES 29–33 ) (in some darkly sclerotized specimens of D. carinata , a faint lateral line is seen where a CES would be found). In D. carinata , the spined area of the EES extends greater than or equal to 0.34 times the length of the EES ( Figs. 15 View FIGURES 14–18 , 20 View FIGURES 19–23 ). In contrast, the spined area of the EES is confined to less than or equal to 0.16 times the length of the sclerite in D. elongata ( Table 3; Figs. 14 View FIGURES 14–18 , 19 View FIGURES 19–23 , 24 View FIGURES 24–28 , 48 View FIGURE 48 ). In D. carinata , spines of the EES are often irregularly spaced along the blade with conspicuous gaps ( Fig. 20 View FIGURES 19–23 ). In D. carinulata and D. meridionalis , spines along the EES are usually evenly and closely spaced along the blade ( Figs. 22–23 View FIGURES 19–23 ). The EES of D. meridionalis additionally bears a hooked apex that is absent in D. carinata .
Measurements. See Tables 2 and 3.
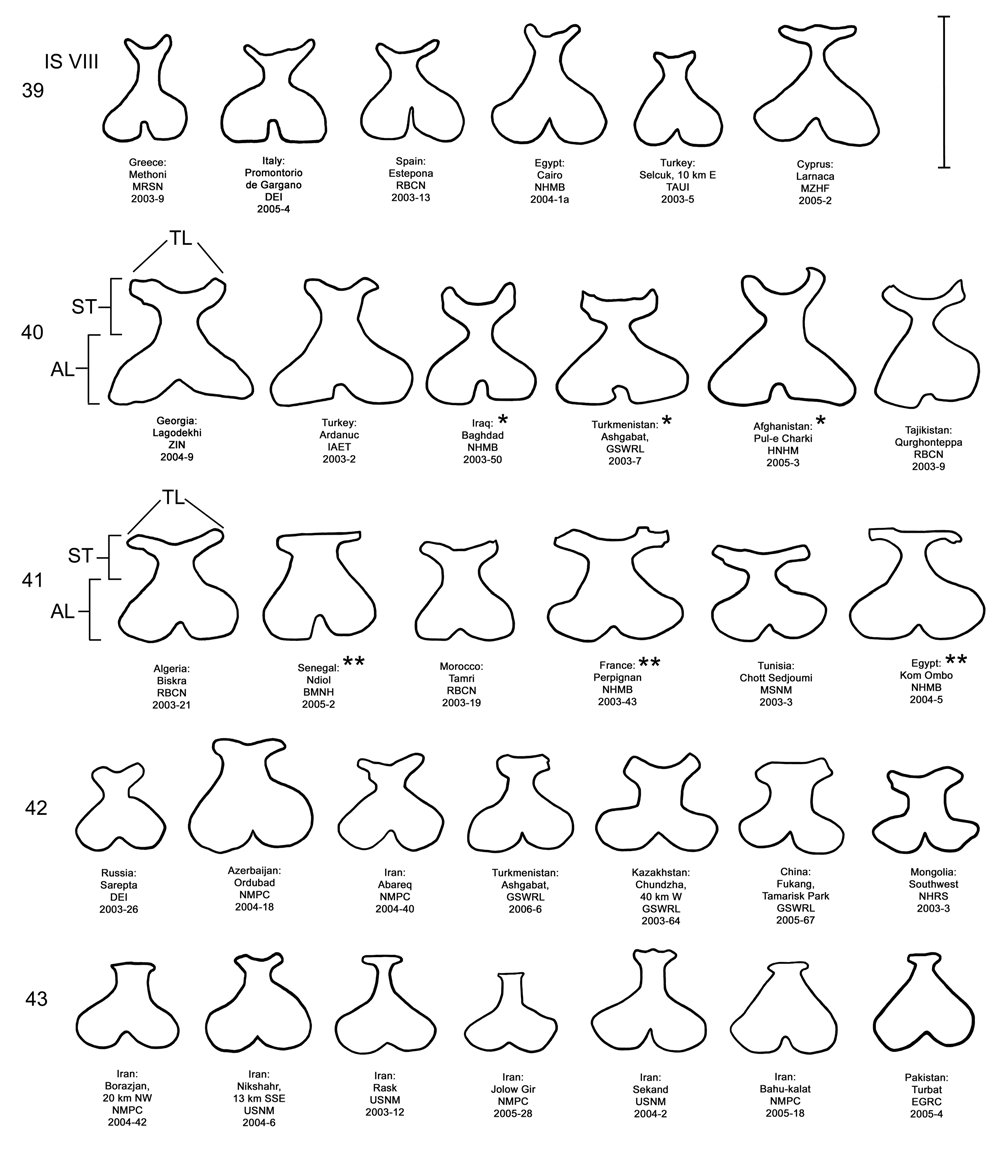
Female. Genitalia. Female D. carinata may be distinguished from all other members of the D. elongata group except D. sublineata by their triangulate vaginal palpi (VP) that are wider than long with a width to length ratio (LP/WP) of 0.50–0.89 (n = 21) ( Fig. 35 View FIGURES 34–38 , Table 4). In contrast, the vaginal palpi are broadly rounded with length to width ratio of 0.94–1.36 in the sympatric D. carinulata ( Fig. 37 View FIGURES 34–38 ) and D. meridionalis ( Fig. 38 View FIGURES 34–38 ; Table 4). The vaginal palpi are also broadly rounded in D. elongata ( Fig. 34 View FIGURES 34–38 ). In addition, the width of the widest lobe of the stalk (WLS) of internal sternite VIII (IS VIII) is usually larger in D. carinata (range 0.11–0.17 mm; Fig. 35 View FIGURES 34–38 ) compared to D. elongata (range 0.06–0.11 mm; Fig. 34 View FIGURES 34–38 ). Some female D. carinata can be distinguished from D. sublineata by having the tips of both lobes (TL) of the stalk of IS VIII strongly curved inward with the tips either pointed or rounded ( Fig. 40 View FIGURES 39–43 — Baghdad, Ashgabat, and Pul-e Charki), a combinations of characters never found in D. sublineata ( Figs. 36 View FIGURES 34–38 , 41 View FIGURES 39–43 ). Some female D. sublineata can be distinguished from D. carinata by having the tips of the lobes of the stalk of IS VIII either not curved or curved outward toward the apical lobe with the tips either rounded or quadrate ( Fig. 41 View FIGURES 39–43 —Ndiol, Perpignan, Kom Ombo). Female D. carinata and D. sublineata with at least one lobe of the stalk of IS VIII curved only slightly inward ( Figs. 35–36 View FIGURES 34–38 , 40 View FIGURES 39–43 —Lagodekhi and Ardanuc, 41—Biskra and Tamri) are indeterminable to species, except on the basis of geographic distribution outside of the area of known sympatry in Iraq.
Measurements. See Tables 2 and 4.
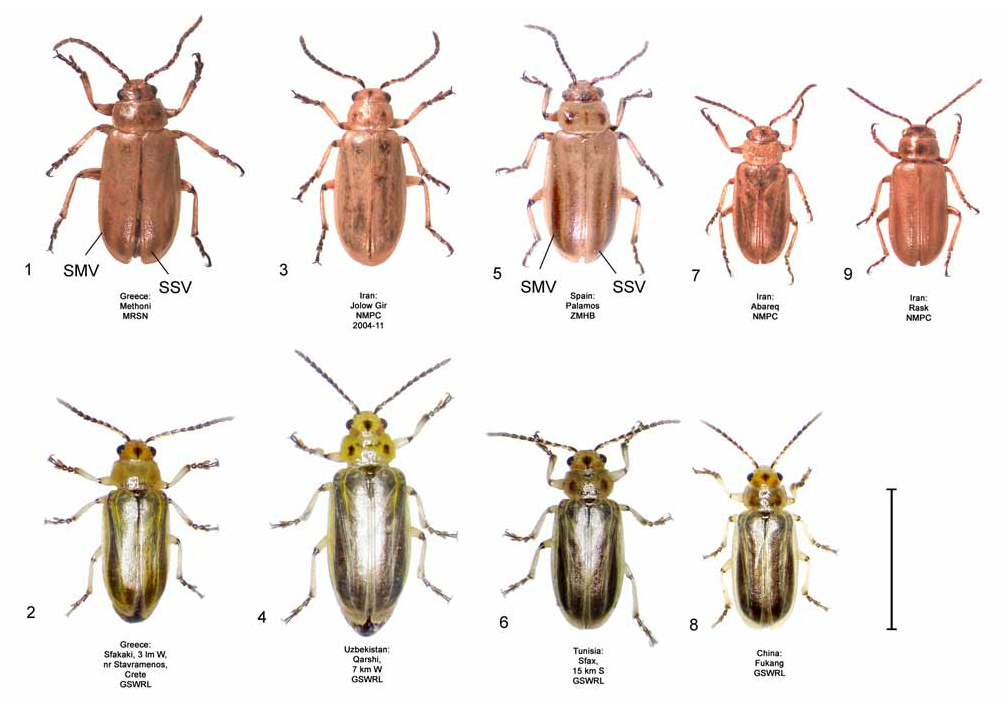
Coloration. Subsutural and submarginal elytral vittae are often present in D. carinata ( Fig. 9 View FIGURES 1–9 ), and the vittae may extend well into the basal half of the elytra, such as in D. sublineata ( Fig. 5 View FIGURES 1–9 ). In contrast, the elytral vittae, if present, are confined to the apical half of the elytra in D. elongata ( Fig. 1 View FIGURES 1–9 ). The elytral vittae are less often present in D. carinata ( Fig. 3 View FIGURES 1–9 ) compared to D. sublineata . Live specimens of D. carinata tend to have less greenish-yellow tinting in veins of the elytra ( Fig. 4 View FIGURES 1–9 ) than do D. elongata ( Fig. 2 View FIGURES 1–9 ). In contrast, D. sublineata ( Fig. 6 View FIGURES 1–9 ) and D. carinulata ( Fig. 8 View FIGURES 1–9 ) both lack greenish-yellow tinting.
Type material. According to Berti and Rapilly (1973), the Faldermann type material of D. carinata , consisting of a male holotype, is deposited in the Mniszech Collection at MNHN. We studied the original description by Faldermann (1837), based on an unspecified number of specimens, the illustration of the endophallus of the male holotype by Berti and Rapilly (1973), and topotypes from the Transcaucasus.
Material examined. 122♂♂ dissected (diss.), 67♀♀ diss., 87♂♂, 125♀♀. AFGHANISTAN: 1♂ diss., 3♂♂, Gerab [Sare Gearbid Mt.; 33.89667°N, 66.64583°E], Orurgan [Velayat–e Oruzgan], 1,300 m, 12-VI- 1970, O. Kabakov, ZIN [2004-02] [from series listed as Diorhabda elongata carinata from Uruzgan, Herab by Medvedev (1985)]; 1♂ diss., Polichromi [Pol-e khromi; 35.948889°N, 68.714167°E], 28-V-1956, H.G. Amsel, [from series listed as D. e. carinata by Bechyné (1961)], NHMB [2003-23]; 1♀ diss., Polichromi [Pol–e khromi], 700 m, 5-VI-1956, H.G. Amsel, NHMB [2005-05]; 2♂♂ diss., 1♀ diss., 4♂♂, 6♀♀, Pul–e–Charkhi [Pul–e Charki; 34.541944°N, 69.348889°E], 22–24 km east northeast Kabul, 1,780 m [elev.], 19-VI-1974, L. Papp, No. 163, D. elongata det. V. Tomov, HNHM [2004-09, 2005-03, 04]; 1♂ diss., Tangi–Gharuh a. Kabul –Fluss [Tangi Gharu Pass, Kabul River; 34.62°N, 69.62°E], 1,600 m [elev.], 8-VII- 1952, J. Klapperich, D. elongata det. K. Lopatin, HNHM [2004-05] [from series listed as D. e. carinata by Lopatin (1963)]; ARMENIA: 1♂ diss., 1♀, Eriwan [ Yerevan; 40.181111°N, 44.513611°E], Caucasus, NHMB [2003-39]; 1♂, Eriwan, Caucasus, 1893, Horváth, D. elongata det. K. Lopatin [ HNHM]; AZERBAIJAN: 1♂ diss., 2♂♂, 16♀♀, Aresch [a district of the 19 th century Russian Caucasus located in present-day northern Azerbaijan according to Frisch (2007)], Caucasus, Schekownikow, D. e. ab. carinata det. Le Moult, IRSNB [2006-08]; 1♀ diss., Dzhafarkhan [ 39.9431°N, 48.4917°E], 23-V-1939, AMA [2004- 01]; 2♂♂ diss., 2♂♂, 2♀♀, Elisabetpol [ Ganca; 40.682778°N, 46.360556E], Caucase, Babadjanides, D. elongata [ 1♂]; O. Leonhard [collection; 1♂], DEI [2003-08, 2005-09]; 2♂♂ diss., 2♂♂, 2♀♀, Elisabetpol, Caucasus, D. elongata det. K. Lopatin [ HNHM], NHMB [2003-53], ZMAN [2008-20], DEI [ 1♂], HNHM [ 1♂, 1♀]; 1♂ diss., Geoktapa [Geok–Tapa stream; 39.188056°N, 48.679444°E], 18-VII-1901, P. Schmidt, rice fields, ZIN [2004-22]; 1♀ diss., Geok–Tapa [stream], Transcaucasus, G. C. Champion, BMNH [2003-10]; 1♂ diss., Karandonly [Qaradonlu; 39.7914°N, 48.0428°E], on banks of Araks [Aras] River, 29-V-1911, P. Schmidt, ZIN [2004-04]; 1♂ diss., Khankendy [ Xankandi; 39.8153°N, 46.7519°E], Erivansк.g [Erivanskaya gubernia; Xankandi was formerly of Armenia, and now it is in the contested Nagorno-Karabakh zone], Maljushenco, NHMB [2003-42]; 1♂ diss., Mingechaur [ Mingacevir; 40.77°N, 47.0489°E], 19-IV-1959, AMA [2004-03]; 1♂ diss., Ordubad [ 38.9081°N, 46.0278°E], Araxesthal [Aras River], Caucasus, Col[lection] Matcha, NMPC [2004-53]; 1♀, Ordubad, Caucasus, Col[lection] Matcha, NMPC; 4♂♂ diss., 2♂♂, 2♀♀, Ordubat [ Ordubad], [19]14, Dr. Veselý, NMPC [2004-10, 55, 2005-38, 39]; 1♂ diss., Ordubat [ Ordubad], VI-1910, Javůrek, Coll[ection] Dr. J. Veselý, D. elongata, NMPC [2004-07]; 2♂♂ diss., Qobustan [ 40.08416°N, 49.41583°E], 18-IV-1984, N. Mirzoeva, AMA [2004-04, 05]; 1♂ diss., Sabirabad [ 40.0128°N, 48.4789°E], 6-VI-1929, AMA [2004-02]; CHINA: Xinjiang Uygur Zizhiqu: 1♂ diss., 1♂, 1♀, Kuldja [Yining; 43.9000°N, 81.35000°E], Tien-Shan, Sven Hedins Exp. Ctr. Asien, D. elongata, NHRS [2008-01]; 1♀ diss., 1♀, Ili [Ili Kazakh Autonomous Prefecture, includes Yining], V-[19]06, Collectie C. & O. Vogt Acq. 1960, ZMAN [2008-18]; GEORGIA: 1♂ diss., 1♀ diss., 1♂, Eldari [ 41.28833°N, 46.46305°E], 18-IV-1910, G. Vinogradov–Nikítin, ZIN [2004-06, 23]; 1♀ diss., Eldari, 3-VIII-1984, G. Jacobsen, ZIN [2004-03]; 1♂ diss., 1♀ diss., Lagodekhi [ 41.8228°N, 46.2756°E], 15-V-1910, L. Miokosiewicz, ZIN [2004-09, 24]; 2♂♂ diss., 2♀♀ diss., 2♂♂, Owtshaly [Avchala; 41.79028°N, 44.8261°E], 24-VI-1879, G. Sievers, ZIN [2004-08, 11, 25, 26]; 1♂ diss., 1♂, 1♀, Mtzchet [Mts'khet'a; 41.8439°N, 44.7164°E], Caucasus, [18]79, Leder (Reitter), ZIN [ 1♂ diss., 2004-19], NMPC; 1♂ diss., 1♀, Tflis [T’blisi; 41.725°N, 44.79083°E], Caucasus, Leder (Reitter), D. elongata, Coll. Reitter, HNHM [2004-01], NMPC; IRAN: 2♂♂ diss., 2♀♀ diss., Borazjan, 30 km north northeast [ 29.53333°N, 51.40000°E; 10 km N Dalaki, Hableh Rud River; Tamarix sp. present ( Hoberlandt 1983)], 18-19-IV-1977, Exped. Nat. Mus. Praha Loc. No. 298, NMPC [2004-41, 48, 2005-36], MPBF [2003-04]; 2♂♂ diss., Jeiugir ( 33° 27' N, 49° 01' E) [sic; Jolow Gir; 32.96667°N, 47.81611°E; coordinates corrected from relation to Sarab–e Jahangir], 20 km southeast Sarab–e Jahángir [ 32.326994°N, 48.51111°E], Lorestán, 8-10-X-1998, Chvojka, D. elongata det. A. Warchalowski, NMPC [2004-11, 2006-02]; 2♂♂ diss., 4♀♀, Omidiyeh, 34 km south southeast [ 30.55°N, 49.91667°E; Rud-e Zohreh river, Tamarix sp. present ( Hoberlandt 1983)], 16-17-IV-1977, Exped. Nat. Mus. Praha Loc. No. 292, NMPC [2004-33, 2005-34]; 1♂ diss., 1♂, Golhak [Qolhak; 35.7803°N, 51.4919°E], 1400m [elev.], near Teheran [ Tehran], VI-VIII, 1961, J. Klapperich, D. elongata det. I.K. Lopatin, HNHM [2003-02]; 1♀ diss., Robat–e Qozlog [Robate–Ghozlog; 36.7°N, 54.61667°E; valley of small river; Tamarix not recorded ( Hoberlandt 1974)], [ 10 km] south of Gorgan, 500 m [elev.], 26-VII-1970, Exped. Nat. Mus. Praha Loc. No. 74, D. elongata det. I.K. Lopatin 1964, NMPC [2004-04] [from series listed as D. elongata by Lopatin (1981)]; 1♂ diss., 1♀, Semnan to Dameqan [Damghan] road, 35° 46' 13" N, 53°, 44' 47" E [ 35.77028°N, 53.74639°E], 1,975 m elev., 13-V-2000, J. Gaskin, on Tamarix, No. 870, GSWRL [2003-38]; 1♂ diss., 1♂, 3♀♀, Shushtar [ 32.05°N, 48.85°E; river Karun; Tamarix sp. present ( Hoberlandt 1983)], 13-IV-1977, Exped. Nat. Mus. Praha Loc. No. 287, NMPC [2004-35a]; 2♀♀ diss., Wildlife Park, vicinity of Dasht, 650 m [elev.] [ 37.38°N, 55.89°E; Mohammad Reza Shah Wildlife Park (= Golestan Biosphere Reserve), east tributary of the river Rud-i Gorgan; Tamarix not recorded; coordinates adjusted based on elevation and forest description ( Hoberlandt 1974)], 27-30-VII-1970, Exped. Nat. Mus. Praha Loc. No. 77, NMPC [2004-17, 20]; IRAQ: 4♂♂ diss., 5♀♀ diss., 4♀♀, Assur [Ash Sharqat; 35.45861°N, 43.25722°E], Mesopotamia, 1910, Pietschmann, D. elongata [ 1♀ diss.], Mesopot. Exp. Nat. O. V. 1910, NHMB [2003-06, 21, 33, 34, 35, 36, 47, 51, 2004-04] [from series listed as D. elongata by Holdhaus (1920)]; 1♂ diss., Assur [Ash Sharqat] Mesopotamia, Coll. F. Hauser, NHMB [2006-01]; 1♂ diss., Baghdad [ 33.33861°N, 44.37722°E], summer 1923, B.W. G. Hingston, B.M. 1923-486, BMNH [2005-03]; 2♂♂ diss., 2♀♀ diss., 3♀♀, Baghdad, IV-1936, Frey, NHMB [2003-07, 22, 32, 50]; 4♂♂ diss., 1♀ diss., 5♀♀, 4♂♂, Baghdad, Kálalová, NMPC [2004-06, 09, 13, 54, 56]; 1♀ diss., Khanikin [Khaniqin; 34.34694°N, 45.40056°E], 9-IV-1936, Frey, D. persica Faldermann det. H. Bollow 1938, NHMB [2003-37]; KAZAKHSTAN: 1♀ diss., Aulie–Ata [Taraz; 42.9°N, 71.3667°E], Turkestan, K. Arris, D. sulphureus Reitter det. H. Bollow 1939, D. e. subsp. carinata Fald. det. J. Bechyné 1955, NHMB [2003-30]; 2♀♀, Aulie Ata, Syr Darja, NMPC; 1♂ diss., Chilik [Shelek] near, Chilik River Valley [ 43.6003°N, 78.2963°E], 6-VI-1999, I.D. Mityaev and R. Jashenko, on Tamarix ramosissima , voucher shipment GSWRL-1999-8, GSWRL [2004-01] [ 28 specimens from Lot GSWRL-1999-1 identified as D. elongata by A. Konstantinov on 20-VII-1999 at USDA Systematic Entomology Laboratory ( SEL), SEL Lot # 9904498]; 2♂♂ diss., Chundzha Village, Charin [Charyn] River [ 43.5161°N, 79.2437°E], Gorodinski, V-1994, D. elongata det. D. Sassi 2000, GSWRL [2003-58, 59]; 1♂ diss., Sargotay [Sarytogay] Locality [ 43.43240°N, 79.15040°E], Charyn River, 700 m [elev.], 2-VII-1907, A.G. Jacobson, ZIN [2005-01]; 1♀ diss., Shelek, near, 1-VIII-1999, I.D. Mityaev and R. Jashenko, on Tamarix ramosissima , voucher shipment GSWRL-1999-15, GSWRL [2005-94]; KYRGYZSTAN: 1♂ diss., 2♂♂, Kugart–su River [Kugart stream], Ferghana Valley [ 40.8667°N, 72.8833°E], 8-V-1925, F. Dobrzhanskii, ZIN [2004-10]; PAKISTAN: 1♀ diss., Kund [ 33.9306°N, 72.2289°E], 4-VI-1976, CIBC [Commonwealth Institute of Biological Control], larvae feeding on T. aphylla , D. elongata , Tam-6/76-147-II, 2364, C.I.E. coll. A 9076, CABP [2003-01] [from series listed as D. elongata by Habib and Hasan (1982)]; 1♀ diss., Nomal [ 36.07305°N, 74.25444°E], 25-VI-1962, CIBC, adult feeding on T. cf. indica [as T. cf. troupii ], D. elongata, CIBC Wol-6/62-465-III, 947, CIE. Coll. No. 18503, CABP [2003-02]; 1♂ diss., 1♀ diss., Shorkot [ 31.90972°N, 70.87722°E], west Pakistan, 17-IV- 1967, M. Nararullah, on Tamarix sp. , D. elongata N.A. Aslam det. 1970, D. elongata det. M.L. Cox 1982, CIE Coll. No. 3509, Comm. Inst. Ent. B.M. 1981-315, BMNH [2003-06, 07]; SYRIA: 2♀♀ diss., 1♀, Alep [po] [ Halab; 36.20278°N, 37.15861°E], Syrie, NHMB [2003-14, 44]; TAJIKISTAN: 1♀ diss., 1♀, Dushanbe [ 38.5600°N, 68.7739°E], May–June 1966, Král, NMPC [2005-32]; 1♂ diss., 2♂♂, 1♀, Khodzhent [Qayraqqum; 40.2647°N, 69.7894°E], Samarkand Ob[last], 23-IV-1903, Val'nev, ZIN [2004-13]; 1♂ diss., 3♂♂, 1♀, Ghissar and Karateghin Mt. Ranges [approximate location; 39.06°N, 69.82°E], ZIN [2004-27]; 1♂ diss., Hissar [Hisor; 38.48194°N, 68.59694°E], Buchara, [ D.] sulphurea, MSNM [2003-16]; 1♂ diss., 1♂, Karatack [Karatag; 38.617°N, 68.333°E], Buchara, Stauding, [ D.] sulphurea m.n.sp., D. sulphurea Reitt., DEI [2003-11]; 2♂♂ diss., 4♂♂, 4♀♀, Karatag, west Buchara, 916 m [elev.], 1898, F. Hauser, D. [ e.] a. carinata [ 2♂♂ diss., 1♂, 1♀ of DEI], DEI [2005-12], NHMB [2005-07; 3♂♂, 2♀♀ not diss.], BMNH [ 1♀]; 1♀ diss., Karatag, west Buchara, D. [ e.] v. carinata, NMPC [2005-33]; 1♀ diss., Kurgan–Tsu BG [Qurghonteppa or Kurgan–Tyube; 37.83639°N, 68.78028°E], Ruβland, 28-IV-1989, D. elongata R. Beenen det. 2002, RBCN [2003-09]; 2♂♂ diss., 1♀, Nurek [Norak; 38.38833°N, 69.325°E], Dušanbe [ Dushanbe] environs, 25-VI- 1976, Josef Král, NMPC [2004-38, 39]; 2♂♂, 2♀♀, diss., Sary-pul [ 38.4167°N, 70.1333°E], Mts. Karategin, 1,482 m [elev.], 1898, F. Hauser, D. e. v. carinata R. det [ 1♂ of BMNH], NHMB [2003-29], BMNH [2005-04 and 1♀]; 1♂ diss., Vachsch [Vakhsh] River [approximate location: 37.5629°N, 68.5275°E], 23-IV-1992, RBCN [2003-15]; TURKEY: 1♀ diss., 3♂♂, 7♀♀, Aǧri [ Agri], 9 km west on road to Horasan [ 39.7393°N, 42.9235°E], 1,600 m [elev.], Aǧri Prov., 1-VIII-2000, C. Morkel, on Asteraceae , D. elongata det. D. Erber 2001, ZMHB [2006-01]; 1♂ diss., Aralich [Aralik; 39.872778°N, 44.519167°E], Caucasus, 1893, Horváth, [ D.] e. var. sublineata det. K. Lopatin 1958, [ HNHM 2003-09]; 1♂ diss., 1♀ diss., Ardanuç [Ardanuc; 41.12861°N, 42.05916°E], 600m, Artvin [Prov.], 9-VII-1998, I. Aslan, D. elongata det. I. Aslan, IAET [2003- 01, 02] [from series listed as D. elongata by Aslan et al. 2000]; 1♂ diss., 2♀♀, Artvin [ 41.18222°N, 41.81944°E], 2-VII- 1975, 700 m [elev.], Osella, MRSN [2003-06]; 1♂ diss., 1♂, 1♀, Artvin, 13–15-VIII- 1976, Bohao, ex coll. Josef Kral, Prag, 1987–1989, coll. U. Arnold, Berlin, D. elongata carinata R. Beenen det 1993, RBCN [2008-01]; 1♀ diss., Artvin, 12 km east [ 41.1329°N, 41.8978°E], 26-V-1990, P. Kanaar, D. elongata ssp. carinata R. Beenen det. 1992, RBCN [2003-06]; 1♀ diss., 6♀♀, Siirt, southwest, on riverbank [ 37.8192°N, 41.8679°E], ca. 550 m [elev.], southeast Anatolia, 28-VII-1985, Heinz, D. e. carinata det. Beenen 1996 [ 2♀♀], D. elongata det. Beenen 1998 [ 1♀ diss., 4♀♀], ZMHB [2006-04]; TURKMENISTAN: 2♂♂ diss., 1♀ diss., Ashgabat, 2-IX-2002, S.N. Myartseva, on T. ramosissima, GSWRL [2003-02, 04, 07]; 2♂♂ diss., Ashgabat [ 37.95°N, 58.36667°E], Dry Sport Lake, 26-IX-1996, S. N. Myartseva, on Tamarix sp. [prob. T. aralensis J. Gaskin ], [parasitoid] wasp found in abdomen [ 1♂], GSWRL [2005-36, 102] [ 7 specimens from Lot GSWRL( CJD)-1996-4 identified as D. elongata by S.M. Clark on 28-X-1996 at West Virginia Dept of Agric.] [ 8 specimens (no. 9204) from Lot GSWRL( CJD)-1997-1 identified as Diorhabda n. sp. by A. Konstantinov on 21-V-1997 at USDA SEL, SEL Lot # 9704023] [parasite Baryscapus diorhabdivorus Gates & Myartseva ( Hymenoptera : Eulophidae ) found in 1 adult in lot GSWRL-2005-01 identified by M. Gates on 28-VII-2005 at USDA SEL]; 1♀ diss., 4♂♂, 7♀♀, Ashgabat, Dry Sport Lake, 11- V-1997, S. N. Myartseva, on T. ramosissima [prob. T. aralensis J. Gaskin ], voucher shipment GSWRL( CJD)- 1997-11, 9005–9012, 9020–9022, D. elongata det. I. Lopatin 1999 [ 4♂♂, 7♀♀], GSWRL [2005-66] [ 8 specimens (nos. 9200, 9201) from Lot GSWRL( CJD)-1997-1 identified as Diorhabda n. sp. by A, Konstantinov on 21-V-1997 at USDA SEL, SEL Lot # 9704023]; 4♂♂ diss., 2♀♀ diss., Ashgabat, Dry Sport Lake, 11-VI-1997, S. N. Myartseva and P. Boldt, on T. ramosissima [prob. T. aralensis J. Gaskin ], USDA lab colony at Temple, Texas, voucher (1997, J.L. Tracy), voucher shipment GSWRL( CJD)-1997-12, D. elongata det. I. Lopatin 1999 [ 1♂ diss.], 9004, GSWRL [2003-11, 2005-09, 59, 60, 61, 62]; 7♂♂ diss., 7♂♂, 5♀♀, Ashgabat, Dry Sport Lake, 17-18-VIII-1998, A Knutson, Andrey Averin and Allen Knutson [ 7♂♂], on T. ramosissima [prob. T. aralensis J. Gaskin ], defoliating shrubs, voucher shipment GSWRL-1998-15, D. elongata det. I. Lopatin 1999 [ 1♂ diss., 5♂♂, 3♀♀], 8993–8996, 8998–9003, GSWRL [2005-25; 2008-12, 13, 14, 15, 16, 17] [photo of damage to tamarisk in DeLoach et al. (2003b), Fig. 3 View FIGURES 1–9 ]; 1♀ diss., 6♂♂, 2♀♀, Ashgabat, Dry Sport Lake, 26-IX-2002, S.N. Myartseva, on Tamarix spp. , GSWRL [2006-10]; 1♂ diss., 3♀♀ diss., Ashgabat, Karakum [Quaragum] Canal, 12-IX-2002, S.N. Myartseva, on T. ramosissima, GSWRL [2003-A1, A2, A3, A4]; 2♀♀ diss., Cardruj [Turkmenabat; 39.10139°N, 63.575°E], Buchara, [ D.] e. var. sublineata det. K. Lopatin 1958, HNHM [2003-13, 2004-02]; 1♂ diss., 2♂♂, Chardzhuy [Turkmenabat], 21- VI-[19]04, Z. Fisher, ZIN [2005-03]; 2♂♂ diss., Cemenibit [ 35.4489°N, 62.3958°E], 22 km north Kujka, 1- IV-1992, Snilek, EGRC [2005-02, 07]; 1♀ diss., Chule [Chuli] Canyon, 53 km west Ashgabat ( 10, 852 km odom.) [ 38.08°N, 57.77°E], 25-V-1995, on Tamarix ramosissima, C.J. DeLoach, GSWRL [2004-13]; 1♂ diss., Danata Village [ 39.08389°N, 55.1617°E], 157 m [elev.], 31-V-2000, J. Gaskin, on T. aucheriana [det. J. Gaskin], Coll. No. 1044, GSWRL [2003-55]; 3♂♂ diss., 3 ♀♀ diss., Svintsovyi Rudnik Village [Svintsovyy Rudnik; 37.88472°N, 66.45138°E], Kughitangolarja [Kugitang] River, Kughitang Mts., 3-V-1989, Osipov Coll., GSWRL [2003-66, 67, 68, 69, 2004-06, 07]; 1♂ diss., 1♀ diss., 1♂, 1♀, Tedzhen [Tejen; 37.37861°N, 60.49611°E], D. elongata, Coll. Reitter, HNHM [2004-03, 04]; 1♀ diss., 1♀, Tschardsui [Turkmenabat; see Cardruj], Turkestan, D. sulfurea Rt. det. Dr. Fleischer, NMPC [2005-03], 1♀, Tschardsui [Turkmenabat], Trans-Caspia, 4358b, coll[ection] Kǒuřil P5/46/62, NMPC; UKRAINE: 1♂ diss., 1♀ diss., Izium v[illage] [geocoordinates not locatable], Odessa Reg. [mapped approximate location in Odessa region as Izmayil: 45.3500°N, 28.8330°E], southwest Ukraine, 15-VII-1974, Osipov Coll., GSWRL [2003-71, 2004-09]; UNITED STATES OF AMERICA (introduced): Texas: Hutchinson Co.: 4♂♂ diss., 1♀, Johnson Ranch (cage 7) on Canadian River near Borger [approx., 35.7294°N, - 101.4126°W], 26-VI-2007, E. Jones, on T. ramosissima / T. chinensis , voucher [source: 7 km W of Qarshi, Uzbekistan], GSWRL [2007-54, 55, 56, 57]; Baylor Co.: 1♂ diss., 2♂♂, 1♀, Lake Kemp, west side, Waggoner Ranch site B, 33.709267°N, - 99.276395°E, 7–9-X-2008, Charles Randal, on T. ramosissima / T. chinensis , voucher [source: 7 km W of Qarshi, Uzbekistan], GSWRL [2008-25]; UNKNOWN COUNTRY: 1♂ diss., Attica [location of Attiki, Greece; considered as mislabeled], Reitter, ZMAN [2008-05]; 1♂ diss., 1♀, Araxes [Aras River of Armenia, Azerbaijan and Iran], VI-[19]10, J. Vesely, Coll[ection] Dr. J. Veselý, NMPC [2004-23]; 2♂♂, 2♀♀, Araxes, coll. Purkynĕ, ZMAN; 1♀ diss., Araxesthal [Aras River], Reitter, HNHM [2003-12]; 1♂ diss., Dulicata [geocoordinates not locatable, possibly Dul'ta, Uzbekistan], Turkestan, NHMB [2003-03]; 1♂ diss., Ferolash [geocoordinates not locatable], Caucasus, D. elongata, DEI [2005-10]; UZBEKISTAN: 2♂♂ diss., 3♀♀ diss., Buxoro, 5 km northwest [ 39.8228°N, 64.39134°E], along the road to Gazli, 27-IX-2002, R. Sobhian, on Tamarix, USDA [Buchara] lab colony at Albany, California, voucher (2002-2003, D. Bean), shipment EIWRU-2002-1010, GSWRL [2003-39, 2005-52, 55, 57, 58]; 1♂ diss., 1♀ Ferghana [Farg`ona; 40.3933°N, 71.7794°E], V-1961, L. Medvedev, D. elongata det. L. Medvedev, GSWRL [2004-12]; 3♂♂ diss., 3♀♀ diss., 6♂♂, 4♀♀, Karshi [Qarshi], 10 km west [ 38.92278°N, 65.73307°E], 7-IV-1999, A. Kirk & R. Sobhian, on Tamarix , D. elongata det. I.K. Lopatin 1999 [ 3♂♂ diss., 3♀♀ diss., 4♂♂, 3♀♀], 9052–9064, GSWRL [2003- 44, 2005-71, 72, 2007-02, 2008-18, 2008-19]; 4♂♂ diss., 5♀♀ diss., Qarshi, 7 km west [ 38.90721°N, 65.76314°E], 26-IX-2002, R. Sobhian, on Tamarix, USDA lab colony at Temple, Texas, voucher (2003, L. Milbrath [nos. 1298-1300]), USDA lab colony at Albany, California, voucher ( 1♂, 3♀♀, 26-VI-2002 and 20- I-2003, D. Bean), shipment EIWRU-2002-1010, GSWRL [2003-16, 18, 72, 73, 2005-69, 70, 85, 88, 89] [released in north Texas in 2006] [used in biological studies of Milbrath and DeLoach (2006a, 2006b), Milbrath et al. (2007), Herr et al. (in prep.), Bean and Keller (in prep.), and Thompson et al. (in prep.)]; 1♂ diss., 2♂♂, Namangan City [ 40.99527°N, 71.6725°E], Ferghana Valley, 30-III to 15-IV-1903, Yankovskii, ZIN [2004-07]; 1♀ diss., 4♀♀, Samarkand [Samarqand; 39.6542°N, 66.9597°E], Turkestan or[iental], daroval Král, NMPC [2006-03]; 1♂ diss., Sansar [Kirk; 39.7167°N, 68.0167°E], Turkestan, 1892, Glasnov, NHMB [2004-02]; 2♂♂ diss., 4♀♀, 2♂♂, Saratoff. [Saratovskiy; 40.7667°N, 68.6667°E], Turkestan, NHMB [2003-24, 2005-06]; 2♂♂ diss., 1 ♀ diss., 6♂♂, 5♀♀, Tashkent [Toshkent], 140 km south [approximate location: 40.29057°N, 68.91332°E], 10-IV-1999, Kirk & Sobhian, on Tamarix spp. , D. elongata det. I. Lopatin 1999 [ 1♂ diss., 1♀ diss., 6♂♂, 3♀♀], 9067–9077, GSWRL [2003-51, 52, 2004-16]; 1♂ diss., 7♂♂, 9♀♀, Tashkent [Toshkent], 50 km east [ 41.3206°N, 69.8273°E], 11-IV-1999, Kirk & Sobhian, on Tamarix spp. , D. elongata det I. Lopatin 1999 [ 6♂♂, 6♀♀], 9078, 9080–9090, GSWRL [2003-81].
Distribution. General. The native distribution of D. carinata ranges from Ukraine, eastern Turkey and Syria east to northwest China, Kyrgyzstan and Pakistan, extending as far south as southern Iran (Maps 3, 6). Further collections could give an exact location for D. carinata within the Odes'ka Oblast' of Ukraine and possibly expand its range to include the coast of the Black Sea in eastern Romania and southern Russia, and the semi-arid regions of northwestern India.
Confirmed Records. We have dissected specimens listed above (see Materials Examined) and confirmed the presence of D. carinata in the following countries with previous literature records (Map 3): Armenia (as Transcaucasus, Faldermann 1837), Azerbaijan (as Transcaucasus, Faldermann 1837), Georgia (as Transcaucasus, Faldermann 1937), Turkmenistan (as D. e. var. carinata ) ( Jacobson 1901), Afghanistan (as D. e. carinata ) ( Bechyné 1961; Lopatin 1963; Wilcox 1971; Medvedev 1983, 1985), Kazakhstan, and China (as D. e. carinata ) ( Lopatin et al. 2004). Specimens were dissected from three of four regions of Afghanistan listed with D. e. carinata by Medvedev (1983) (central, Badakshan, and Kabul-Jalalabad) and close to the fourth region (western) at Cemenibit, Turkmenistan. Diorhabda carinata were dissected from three series identified in publications as D. e. carinata in Afghanistan: Polichromi [Pol–e khromi] ( Bechyné 1961), Tangi–Gharuh [Tangi Gharu Pass] at Kabul River ( Lopatin 1963), and Gerab Orugan [Sare Gearbid Mt, Velayat–e Oruzgan] ( Medvedev 1985).
New Records. We have dissected D. carinata from the following countries for which we find no previous specific reports of D. carinata in the literature: Turkey, Iran, Iraq, Syria, Uzbekistan, Kyrgyzstan, Tajikistan, Pakistan, Ukraine, and the United States of America ( Texas; introduced; Map 7, see Potential in Tamarisk Biological Control below for additional details). With the exception of Ukraine and Kyrgyzstan, past reports of D. elongata occur from all these countries that should refer, at least in part, to D. carinata (see synonymy above). Yakhontov and Davletshina (1955) site a potential report of D. elongata in Ukraine by Degtarev (1928), but we were unable to obtain this reference for verification. We dissected D. carinata from four series with published identifications of D. elongata: Assur [Ash Sharqat], Iraq ( Holdhaus 1920); Robat–e Qozlog [Robate–Ghozlog], Iran ( Lopatin 1981); Kund, Pakistan (Habib and Hasan 1982); and Artvin, Turkey ( Aslan et al. 2000).
Unconfirmed Records. We cannot confirm a listing of D. carinata (as D. e. var. carinata ) from Russia ( Heyden 1891), but suspect it may be present along the Caspian Sea in the Dagestan region of southern Russia (Map 8b). We consider records of D. e. var. carinata from France ( Laboissière 1934) and D. e. ab. carinata from Italy ( Porta 1934) as erroneous (see lists of material examined with these identifications by Laboissière for specimens from France and Italy under D. sublineata and D. elongata , respectively). We consider as mislabeled a single male specimen bearing the label of “ Attica, Reitter” ( Greece) from ZMAN (2008-05).
We dissected only D. carinata from all seven available locations in Afghanistan (four) and Pakistan (three) (Map 3). Therefore we consider as D. carinata five unconfirmed records of D. elongata from northern Pakistan (Habib and Hasan 1982; three records) and D. e. carinata from Afghanistan ( Medvedev 1985; two records), but D. carinulata may also be present at some locations. Specimens of D. carinata were dissected from all nine available localities in Azerbaijan and D. carinulata was also dissected from two of these same localities. Consequently, we accept all nine unconfirmed collection records of D. elongata in Azerbaijan (Samedov and Mirzoeva 1985) as D. carinata , but D. carinulata may also be present at some of these localities. Mirzoeva (2001) reported that D. carinata (as D. elongata ) was found in all four major regions of Azerbaijan and was most common in the Great Caucasus and Small Caucasus. Specimens dissected from all five available locations in eastern Georgia were D. carinata , and we consider Lozovoi’s (1961) report of D. elongata from eastern Georgia to be D. carinata . We dissected D. carinata from all five available locations in Turkey east of 41°E, including three locations in Arvin Ili. The closest occurrence of D. elongata to D. carinata in Turkey is in Malatya, which is 316 km north northwest of D. carinata in Siirt. Therefore, we regard two unconfirmed records of D. elongata in Artvin Ili and neighboring Ezurum Ili to also be D. carinata . We dissected D. carinata from nine and D. carinulata from one of the ten available locations in Tajikistan. Therefore we consider nine unconfirmed records in Tajikistan to be D. carinata , but D. carinulata may also be present at some sites. We regard a report of D. elongata at Mikhaylovka [Sarykemer], Kazakhstan ( Kulenova 1968) to be D. carinata because of its proximity to a collection of D. carinata from Auliye–Ata [Taraz], Kazakhstan.
Below are 28 unconfirmed locality records that we consider as D. carinata , but are listed as D. e. carinata in Afghanistan (2) and D. elongata in Azerbaijan (9), Kazakhstan (1), Pakistan (3), Tajikistan (9), and western Turkey (4) (Map 3):
AFGHANISTAN: 2 spms., Banu [Banow], southwest [ 35.63420°N, 69.25260°N], Baglan [Velyat–e Baghlan], 2,000 m [elev.], 14-VIII-1972; 1 spm., Samti [west; 37.5854°N, 69.9592°N], Pan’ [Panj] River, Badakhshan [Velyat–e Badakhshan], 1,100 m [elev.], IV-1971 ( Medvedev 1985); AZERBAIJAN: Akstafa [ Agstafa; 41.1189°N, 45.4539°E], 25-V-1958; Belokany [ Balakan; 41.7258°N, 46.4083°E], 3-V-1972; Dzhul'fa [ Culfa; 38.9500°N, 45.6319°E], 13-V-1933; Matsekh [Matsex; 41.6553°N, 46.5811°E], 3-V-1972; Mingachaur [ Mingacevir; 40.7700°N, 47.0489°E], 23-V-1946 [ D. carinata dissected from same location]; Neftechala [ Neftcala; 39.3586°N, 49.2469°E], 28-V-1980; Saatly [two possible geocoordinates], 26-VI-1969; Sumgait [ Sumqayit; 40.5897°N, 49.6689°E], 12-VII-1935; Yevlakh [ Yevlax; 40.6172°N, 47.1500°E], 12-VI- 1903 (Samedov and Mirzoeva 1985); KAZAKHSTAN: Mikhaylovka [Sarykemer; 43.0000°N, 71.5000°E] ( Kulenova 1968); PAKISTAN: Chitral [ 35.8419°N, 71.7819°E], Tamarix spp. ; Gilgit [ 35.9167°N, 74.3000°E], Tamarix spp. ; Islamabad [ 33.7000°N, 73.1667°E], Tamarix spp. (Habib and Hasan 1982);
TAJIKISTAN: Baljuan [Baljuvon; 38.3108°N, 69.6669°E], 11-V-1951, E.P. Luppova; Dushanbe [ 38.5600°N, 68.7739°E] [ D. carinata dissected from same location], P.N. Kulinich; Kal'aiKhumb, behind and on right side, on Pyanj River [Darya–ye Panj] [ 38.4489°N, 70.81529°E], 6-VII-1960, L.V. Soboleva; Khoja Obigarm [ 38.9003°N, 68.8008°E], P.N. Kulinich; Kondara [ 38.1190°N, 68.8283°E] canyon, VI-VII-1956, P.N. Kulinich ( Kulinich 1962); Kurgan–Tyube [Qurghonteppa; 37.8364°N, 68.7803°E] environs [ D. carinata dissected from same location] (Lopatin 1959); Pyanj [Panj; 37.2383°N, 69.0969°E] environs, P.N. Kulinich ( Kulinich 1962); Shaartuz [Shahrtuz; 37.2594°N, 68.1347°E] (Lopatin 1959); Vaidar village [Vaydara; 38.9667°N, 70.1833°E], 20-V-1961, L.V. Soboleva ( Kulinich 1962); TURKEY: Artvin [ 41.1822°N, 41.8194°E], 5-VII-1994, I. Aslan [ D. carinata dissected from same location]; Sarigol [ 40.9553°N, 41.4989°E], 5-VII-1994, I. Aslan; Yusufeli [ 40.8167°N, 41.5500°E], 5-VII-1994, I. Aslan ( Aslan et al. 2000); 2♂♂, 3♀♀, Uzundere [ 40.5314°N, 41.5531°E], Ezurum Ili, 1,100 m [elev.], 7-VI-1995 ( Aslan 1998).
Discussion. Taxonomy. Galeruca carinata Faldermann (1837) (as Galleruca carinata ) was described from the Transcaucasus and synonymyzed under G. elongata by Reiche and Saulcy (1858). Weise (1893) proposed the variety D. elongata var. carinata under which he incorrectly synonymyzed G. carinulata ( Desbrochers 1870) of southern Russia. Weise (1924) later proposed the aberration D. elongata ab. carinata and this has been followed by several taxonomists ( Winkler 1924 –1932, Laboissière 1934, Warchalowski 2003). Bechyné (1961) proposed the subspecies D. e. carinata with an implied range from the Transcaucasus to central Asia and Afghanistan. Several other taxonomists followed Bechyné in reporting D. e. carinata from Afghanistan ( Lopatin 1963, Wilcox 1971, Medvedev 1983), Kazakhstan, and China ( Lopatin et al. 2004). Berti and Rapilly (1973) studied the endophalli of type specimens of D. carinata and D. carinulata and restored their species status, removing them from synonymy with one another and, by implication, with D. elongata . However, Berti and Rapilly (1973) omit D. elongata in their discussion and provide no distributional data for D. carinata . We find that the external characters they provide regarding elytral carinae and the shape of the pronotum are variable and insufficient for species diagnosis (further discussed below). In addition, they provide no information on variability of the endophallus. This lack of data has apparently contributed to the lack of recognition of D. carinata as a species in recent taxonomic treatments ( Riley et al. 2003, Warchalowski 2003, Bieṅkowski 2004, Lopatin et al. 2004).
We dissected 25 males from 16 locations of the type locality of D. carinata in the Transcaucasus ( Georgia, Armenia and Azerbaijan) with endophalli matching that of the type specimen of D. carinata as illustrated by Berti and Rapilly (1973) (Map 3). One male and two female D. carinulata were dissected from two of the same locations with D. carinata in Azerbaijan. Three key characters of the endophallus of D. carinata that distinguish it from other members of the D. elongata group can be seen in the illustration of the holotype ( Fig. 18 View FIGURES 14–18 of Berti and Rapilly 1973) and our illustration ( Fig. 15 View FIGURES 14–18 ): (1) the presence of spines irregularly spaced along the distal blade of the elongate endophallic sclerite; (2) a lateral appendage on the palmate endophallic sclerite; and (3) the lack of a connecting endophallic sclerite between the palmate and elongate sclerites. We are certain that specimens we studied with endophalli matching that of D. carinata ( Figs. 15 View FIGURES 14–18 , 20 View FIGURES 19–23 , 25 View FIGURES 24–28 , 30 View FIGURES 29–33 ) form a single species conspecific with D. carinata . We find additional characters in the endophallic sclerites and female genitalia (vaginal palpi and internal sternite VIII) of D. carinata throughout its range from west to central Asia that distinguish it from D. elongata , D. carinulata and other members of the D. elongata group ( Figs. 15 View FIGURES 14–18 , 20 View FIGURES 19–23 , 25 View FIGURES 24–28 , 30 View FIGURES 29–33 , 35 View FIGURES 34–38 , 40 View FIGURES 39–43 ; Map 3). The distinctive genitalic characters of D. carinata are maintained in the same areas where D. elongata , D. carinulata , D. meridionalis , and D. sublineata occur, and this is strong evidence for reproductive isolation between these species (see Biogeography below; Map 1, Table 8). Therefore, we firmly support Berti and Rapilly in restoring D. carinata to species status, removing it from synonymy with both D. elongata and D. carinulata .
If D. carinata were an interbreeding subspecies of D. elongata , we should see intermediate morphologies of the distinguishing characters found in the two endophallic sclerites and the vaginal palpi. Such intermediate forms should increase along a geographic gradient approaching range contact of these species in Turkey, Georgia and Syria. For example, we should see intermediate forms of the palmate sclerite in a progression from broadly rounded (in D. elongata ; Fig. 29 View FIGURES 29–33 ) to truncate serrate (in D. carinata ; Fig. 30 View FIGURES 29–33 ). However, no intermediate forms of palmate sclerites were seen in field collections of 157 male D. elongata and 122 male D. carinata , including 21 male D. elongata in Turkey and Syria and 11 male D. carinata in Turkey and Georgia. Similarly, intermediate forms in vaginal palpi ranging from broadly rounded (as in D. elongata ; Fig. 34 View FIGURES 34–38 ) to triangulate (as in D. carinata ; Fig. 35 View FIGURES 34–38 ) were not seen in 85 female D. elongata or 67 female D. carinata , including 14 female D. elongata from Turkey and 9 female D. carinata from Turkey, Georgia, and Syria (Map 1). The lack of intermediate forms is evidence of reproductive isolation between D. elongata and D. carinata . Further evidence for reproductive isolation between D. carinata and several members of the D. elongata group is also found in previously discussed differences in component ratios of putative aggregation pheromones and reduced hybrid egg viability.
Faldermann (1837) gave a length of 7 mm for the male type of D. carinata which is too large to have included the sympatric D. carinulata in which males only reach 6.09 mm ( Table 2). Male D. carinata commonly exceed 7 mm in length (range 5.12–7.34 mm). The application of the name “ D. elongata ” to both D. carinata and D. carinulata where these species are sympatric can be seen in the given range in length of 4.5–8 mm for males and females of “ D. elongata ” in taxonomic keys of Russia ( Ogloblin 1936) and central Asia ( Medvedev 1959, Lopatin 1977 b, Lopatin and Kulenova 1986). Overall ranges in length for both sexes are 4.63–6.99 mm for D. carinulata and 5.05–8.44 mm for D. carinata ( Table 2).
We find that external characters used in separating D. carinata from sibling species are inadequate and that genitalic characters must be used. Weise (1893) noted black markings on the pronotum and abdominal sternites as characters distinguishing D. carinata (as D. e. var. carinata ) from D. elongata , but this character is highly variable in both taxa. Black spots on the pronotum were also noted as a distinguishing character for D. carinata (as D. e. ab. carinata ) by Laboissière (1934) and Porta (1934), who also noted black spots on the femora of the leg. Warchalowski (2003) notes black spots on both the pronotum and head as distinguishing characters for D. carinata (as D. e. ab. carinata ). However, we find that black spots on the head, pronotum, and femora variably occur in both D. elongata and D. carinata , making these characters unsuitable for species diagnosis. Bechyné (1961) noted two differences between D. e. carinata and the Mediterranean form, D. e. elongata : (1) D. carinata reaches a greater length, ca. 7 mm and (2) the sublateral carina approaches the lateral margins of the elytra more closely towards the apex than in D. e. elongata . However, both of these external characters are variable and overlap between D. carinata and D. elongata (although it is uncommon for D. elongata to exceed 7 mm in length). We have dissected specimens of D. carinata that were misidentified by taxonomists using external diagnostic characters as D. elongata in eleven countries and D. e. var. sublineata in Turkey and Turkmenistan (see Material examined).
Berti and Rapilly (1973) proposed that the following five external characters of D. carinata can be used to distinguish it from D. carinulata : (1) length of ca. 6.7 mm, (2) posterior angles on the pronotum are more pronounced and farther from the base, (3) pronotum is broader, (4) only a lateral carina is clearly present on the elytra, and (5) punctuation on the elytra is simple. They give the following contrasting characters for D. carinulata : (1) length of ca. 4.9 mm, (2) posterior angles on pronotum are less pronounced and closer to the base, (3) pronotum is narrower, (4) in addition to a lateral carina on the elytra, a median carina (along a clear humeral furrow) and a sutural carina are seen, and (5) punctation on the elytra is almost confluent in places. In keys for separating D. carinulata from D. elongata (considered as including D. carinata ), Warchalowski (2003) used three of the above characters: body length, the posterior angle of the pronotum, and number of elytral carinae. However, we find all these external characters as too interspecifically variable for species diagnosis, and the only taxonomically reliable characters provided by Berti and Rapilly (1973) are their well illustrated differences in the morphology of the endophalli of D. carinata , D. carinulata , and D. meridionalis .
We consider D. e. carinata as regarded by Bechyné to be synonymous with D. carinata . Because of the incorrect synonymization of D. carinulata (as G. carinulata ) under D. elongata var. carinata by Weise (1893), Wilcox (1971) included D. carinulata under the name D. e. carinata . But Bechyné (1961) did not mention D. carinulata in his description of D. e. carinata and the larger stated size of ca. 7 mm would exclude almost all D. carinulata ( Table 2). We examined a specimen of D. carinata from the series that Bechyné referred to D. e. carinata from Pol–e khomri (as Polichromi), Afghanistan. Also, a specimen of D. carinata was dissected from Taraz (= Auliye–Ata), Kazakhstan with a determination label of D. e. carinata by Bechyné in 1955.
We dissected genitalia from four possibly 50–100 year old specimens of D. carinata from central Asia with the following four identification labels: “ Diorhabda sulphurea Reitt ” (Karatack [Karatag], Tajikistan; DEI), “ D. sulfurea Rt ” (Tshcardsui [Turkmenabat], Turkmenistan; NMPC), “ sulphurea ” (Hissar [Hisor], Tajikistan; MSNM), and “ Diorhabda sulphureus Reitter det. H. Bollow 1939” (Aulie–Ata [Taraz], Kazakhstan; also with label “ D. e. carinata Fald. det. J. Bechyné 1955”; NHMB). The specimen from Karatag, Tajikistan also bore the label “m.n.sp. [manuscript nova species] sulphurea ” indicating that “ D. sulphurea ” was an unpublished manuscript name, probably of the German coleopterist Edmund Reitter who actively published ca. 1870–1915. We find no publication of the manuscript name “ D. sulphurea ”. In any case, Faldermann’s (1837) description of D. carinata precedes Reitter’s works.
In the field in central and southwest Asia, it would be useful to distinguish live adults of D. carinata from the sympatric and partially syntopic species D. carinulata and D. meridionalis . Differences in minimum and maximum sizes reached by males and females of D. carinata compared to D. carinulata and D. meridionalis can aid in identification of some specimens (see Table 2). In some living females, the internal sternite VIII appears as a dark Y-shaped area visible through the translucent last visible abdominal sternite (see Lewis et al. 2003b, Fig. 1G View FIGURES 1–9 ). Female D. carinata often have the lobes of internal sternite VIII both pointed and curved inward ( Fig. 40 View FIGURES 39–43 — Baghdad, Ashgabat), a condition not seen in D. carinulata and D. meridionalis ( Figs. 42–43 View FIGURES 39–43 ). As discussed below, a high incidence of Diorhabda egg masses on Tamarix bark ( Milbrath et al. 2007) also can indicate the presence of D. carinata .
Common Name. The vernacular name “larger tamarisk beetle” refers to the statistically significant larger mean size of D. carinata compared to all other species in the D. elongata group. Among the D. elongata group, only D. carinata exceeds 7 mm in length in males and 8 mm in length in females ( Table 2). Diorhabda carinata is especially larger than the two species with which it is moderately to partially sympatric and syntopic, D. carinulata and D. meridionalis .
Biology. Host Plants. Because of the misapplication of the name D. elongata to both D. carinata and D. carinulata over a wide area of west and central Asia, we cannot conclusively distinguish biological literature that refers to D. carinata alone. Diorhabda carinata is more commonly collected than D. carinulata in the following areas where we consider field observations to primarily involve D. carinata (see Unconfirmed Records above): eastern Georgia ( Lozovoi 1961), Azerbaijan (Samedov and Mirzoeva 1985), southern Turkmenistan ( Myartseva 1995, 1999, 2001), and Tajikistan ( Kulinich 1962).
All previous reports of hosts for D. carinata were made under the name D. elongata . D. carinata (as D. elongata ) feeds upon Tamarix ramosissima and T. smyrnensis (as T. hohenackeri Bunge ) in eastern Georgia ( Lozovoi 1961), T. meyeri Boissier and T. smyrnensis in Azerbaijan (Samedov and Mirzoeva 1985), and T. ramosissima , T. hispida Willdenow and T. arceuthoides Bunge in Tajikistan ( Kulinich 1962) (see Unconfirmed Records above) ( Table 1). We also examined specimens of D. carinata collected from T. ramosissima near Shelek (Chilik), Kazakhstan by I.D. Mityaev and R. Jashenko, and in Chuli Canyon, Turkmenistan by C.J. DeLoach. Tamarix sp. is recorded as a host for material we examined of D. carinata collected from Shorkot, Pakistan. We report the following four new host records from our dissected material: T. aralensis Bunge from Dry Sport Lake, Ashgabat, Turkmenistan collected by S. Myartseva; T. aucheriana (Decaisne) Baum from Village Danata, Turkmenistan collected by C.J. DeLoach; T. aphylla from Kund, Pakistan (as D. elongata in Habib and Hasan [1982]); and T. ramosissima / T. chinensis from near Borger and Seymour, Texas. We also examined an adult collected on T. cf. indica Willdenow (as T. cf. troupii Hole ) from Nomal, Pakistan. Dalin et al. (in press) found that D. carinata (as D. e. carinata ) from Uzbekistan preferred T. parviflora to a similar degree as T. ramosissima in multiple-choice field cage studies in southern California, making T. parviflora a potential new host. Diorhabda carinata shares four of its eight known Tamarix spp. hosts with D. carinulata : T. ramosissima , T. arceuthoides , T. hispida , and T. aralensis ( Table 1). Diorhabda carinata was collected syntopically from the same trees with D. carinulata on T. aralensis at Dry Sport Lake (nr Ashgabat), Turkmenistan, and T. ramosissima near Shelek, Kazakhstan and Quaragum Canal (nr Ashgabat), Turkmenistan.
In no-choice larval host suitability studies, D. carinata larvae from Uzbekistan can only survive to adulthood on plants of the order Tamaricales , including Tamarix (Tamaricaceae) and, to a generally lesser degree, on three North American Frankenia spp. (Frankeniaceae) : F. salina , F. johnstonii , and F. jamesii (Milbrath and DeLoach 2006a, Herr et al. in prep.). Multiple-choice adult oviposition studies in field cages (Milbrath and DeLoach 2006a) reveal that the three North American Frankenia spp. provide little attraction for oviposition by D. carinata compared to Tamarix . In field cage no-choice studies, oviposition by D. carinata on F. jamesii and F. johnstonii was not different from non-host coyote willow ( Salix exigua ) and adults experienced increased mortality compared to T. ramosissima × T. chinensis treatments (Milbrath and DeLoach 2006a). Among invasive North American Tamarix spp. in large field cages, adult D. carinata oviposited as much on T. aphylla as on T. ramosissima , T. parviflora or T. chinensis × T. canariensis / T. gallica , and oviposited significantly more on T. aphylla than on T. canariensis / T. gallica . However, in a later similar multiple-choice field cage test among Tamarix spp. , Milbrath and DeLoach (2006b) found that D. carinata oviposited significantly less on T. aphylla than on all other Tamarix , including T. ramosissima × T. chinensis , T. ramosissima × T. canariensis / T. gallica , T. canariensis / T. gallica and T. parviflora . In less discriminating no-choice field cage studies, D. carinata accepted T. aphylla for oviposition to the same degree they accepted T. ramosissima × T. chinensis (Milbrath and DeLoach 2006b) . Tamarix aphylla is at moderate risk of damage by D. carinata in the field and it is difficult to predict to what degree D. carinata would damage T. aphylla , especially in the absence of other Tamarix spp. (Milbrath and DeLoach 2006b). Frankenia is at low risk to damage from D. carinata (Milbrath and DeLoach 2006a) . Risk of damage to both T. aphylla and Frankenia by D. carinata is probably much lower when these plants are not in the proximity of preferred Tamarix spp. (e.g., Blossey et al. 2001).
Ecology and Phenology. Kulinich (1962) found D. carinata (as D. elongata ) damaging leaves and young shoots of tamarisk in Tajikistan, where it occurs from May to September. According to our data from examined material, D. carinata was collected as early as 23 April in Tajikistan. In southern Tajikistan, D. carinata (as D. elongata ) can have four generations ( Pripisnova 1965). Samedov and Mirzoeva (1985) report D. carinata (as D. elongata ) as “frequently badly damaging” bushes of both T. meyeri and T. smyrnensis in Azerbaijan, where it has three generations from April to October. Lozovoi (1961) commonly found D. carinata (as D. elongata ) on tamarisk throughout eastern Georgia from 1959–1960, but never in sufficient quantity to damage the plants. We have seen collection records from 18 April to 3 August in Georgia.
Diorhabda carinata severely defoliated tamarisk at Dry Sport Lake, near Ashgabat, Turkmenistan in August of 1998, and this was photographed by A. Knutson (for photo, see DeLoach et al. 2003b, Fig. 3 View FIGURES 1–9 ) (see Material examined, specimen no. GSWRL 2005-25). We also found D. carinulata at this site in 1997 and 1998, but it was in much lower numbers than D. carinata . Our collaborator, Myartseva (1999) made season long observations of D. carinata (as D. elongata ) at Dry Sport Lake in 1999. Five generations were observed, with the overwintered adults emerging and copulating in mid-March, the first eggs appearing in April, and the fifth generation adults emerging in late August and going into diapause. In July and August, temperatures often reached ca. 40°C during many days in which adults and larvae were absent, and this absence may be associated with a period of possible aestivation in the pupal or adult stage. Collection dates from our examined material range from 1 April to 12 September in Turkmenistan, 7 April to 26 September in Uzbekistan, and 9 April to 10 October in Iraq and Iran.
Milbrath et al. (2007) found that adult D. carinata (as D. elongata from Qarshi, Uzbekistan) overwintering at Temple, Texas had ca. 60–80% survival from early November through the beginning of March but survival dropped precipitously to ca. 10% by mid-March when the tamarisk leaves were just budding. Overwintered adults began ovipositing in late March giving rise to five generations and a partial sixth generation. Fifth generation adults emerging in early September oviposited for several weeks before ceasing oviposition in November when they appeared to enter diapause.
Five generations were also found in the field at Lake Kemp near Seymour, Texas in 2008 where adults were released in late April and the fifth generation adults emerged in the field by late September. Hundreds of adults could be easily found feeding in this area through 9 October. In late July, a group of ca. 2,000 adults emerged and congregated on a group of large tamarisks and over the course of 12 days appeared to migrate en masse east northeast for 46 meters, congregating on certain tamarisk trees, first at 18 meters and depositing eggs and then moving another 46 meters. (C. Randal, pers. comm.).
Milbrath et al. (2007) observed that D. carinata may deposit as much as one-third of its egg masses on the bark of trunks and branches of tamarisk. In contrast, D. carinulata , D. sublineata , and D. elongata , all deposit their eggs on tamarisk leaves, with the exception of D. sublineata rarely ovipositing on tamarisk stems ( Milbrath et al. 2007). A high incidence of Diorhabda eggs found on the bark of trunks and branches of tamarisk can indicate the presence of D. carinata in areas of sympatry with other tamarisk beetles.
Development and Reproduction. Milbrath et al. (2007) found that, at 28°C, D. carinata (as D. elongata from Qarshi, Uzbekistan) had a development time of 18.6 days from egg to adult (with 73% survival), a fecundity of 233 eggs, and a population doubling time of 5.7 days. These values were all very similar to those found for D. carinulata (Turpan and Fukang) , D. elongata (Crete) , and D. sublineata ( Tunisia) (all as D. elongata ) in the same study.
Natural Enemies. The tachinid Erynniopsis antennata (Rondani) is reported as a parasite of larvae and pupae of D. elongata in Ashgabat, Turkmenistan (Richter and Myartseva 1996). Diorhabda elongata does not occur in Turkmenistan and this record probably should refer to D. carinata which is generally much more abundant than D. carinulata in Ashgabat. An adult parasitoid wasp, Baryscapus diorhabdivorus Gates & Myartseva ( Hymenoptera : Eulophidae ), was found inside an adult D. carinata collected from Ashgabat, Turkmenistan in late September. Previous reports of adult B. diorhabdivorus from both larvae and adults of D. elongata in Ashgabat ( Gates et al. 2005) should also refer to D. carinata . The ground beetle, Lebia holomera Chaudior ( Coleoptera : Carabidae ), preys especially upon leaf beetles of the genus Diorhabda in south-central Asia and in the eastern Caucasus ( Kyrzhanovskiy 1965), areas where D. carinata is common. The protozoan mircosporidian parasite, Nosema sp. , was found in adult D. carinata originating from two collections at Ashgabat, Turkmenistan (shipments GSWRL(CJD)1996-28 [identified by T. Poprawski] and GSWRL(CJD)- 1997-12 [identified by G.M. Thomas]). The fungal pathogen B. bassiana was also found in adult D. carinata from Ashgabat, Turkmenistan (shipment GSWRL(CJD)1996-28) (identified by T. Poprawski).
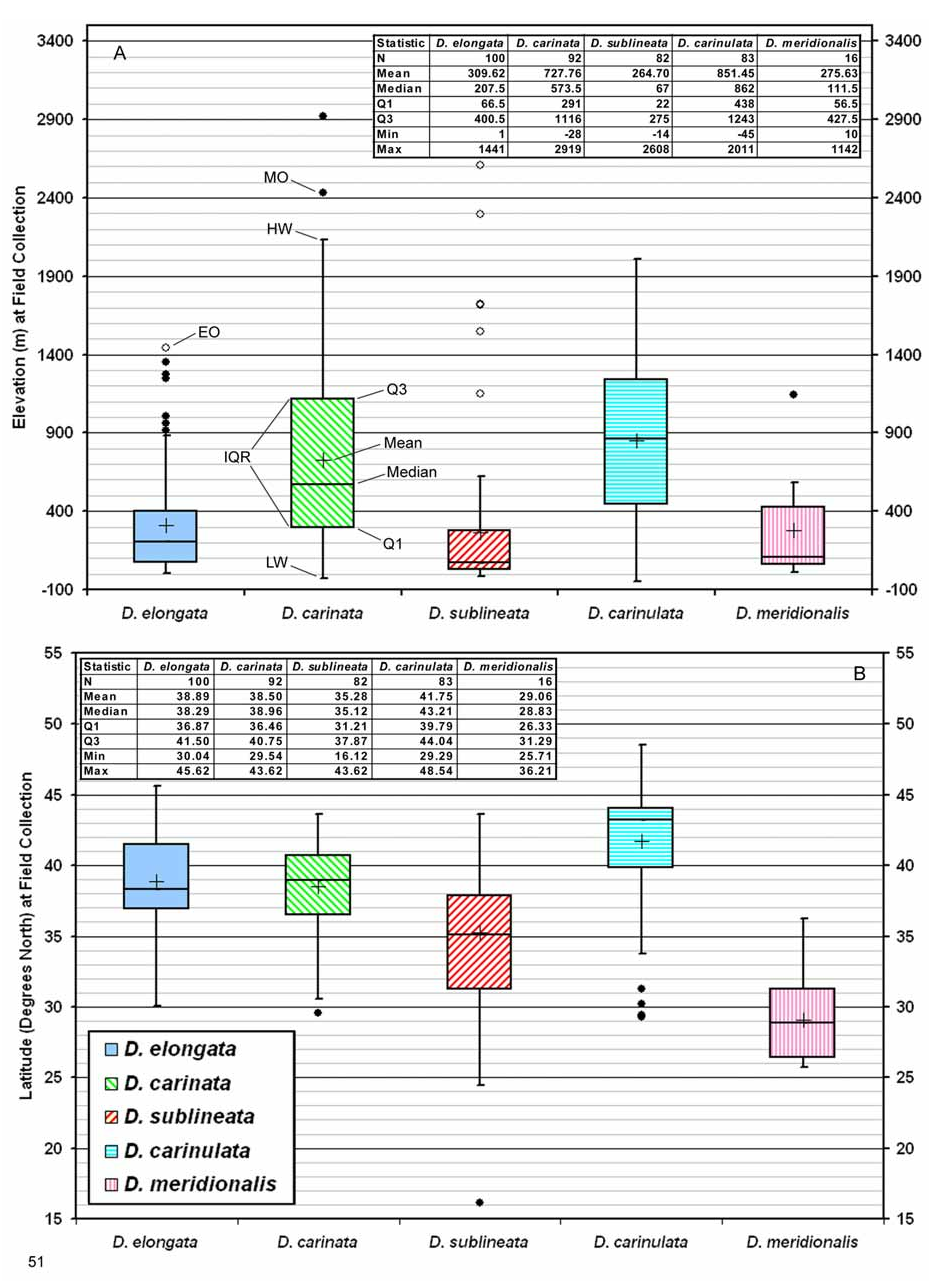
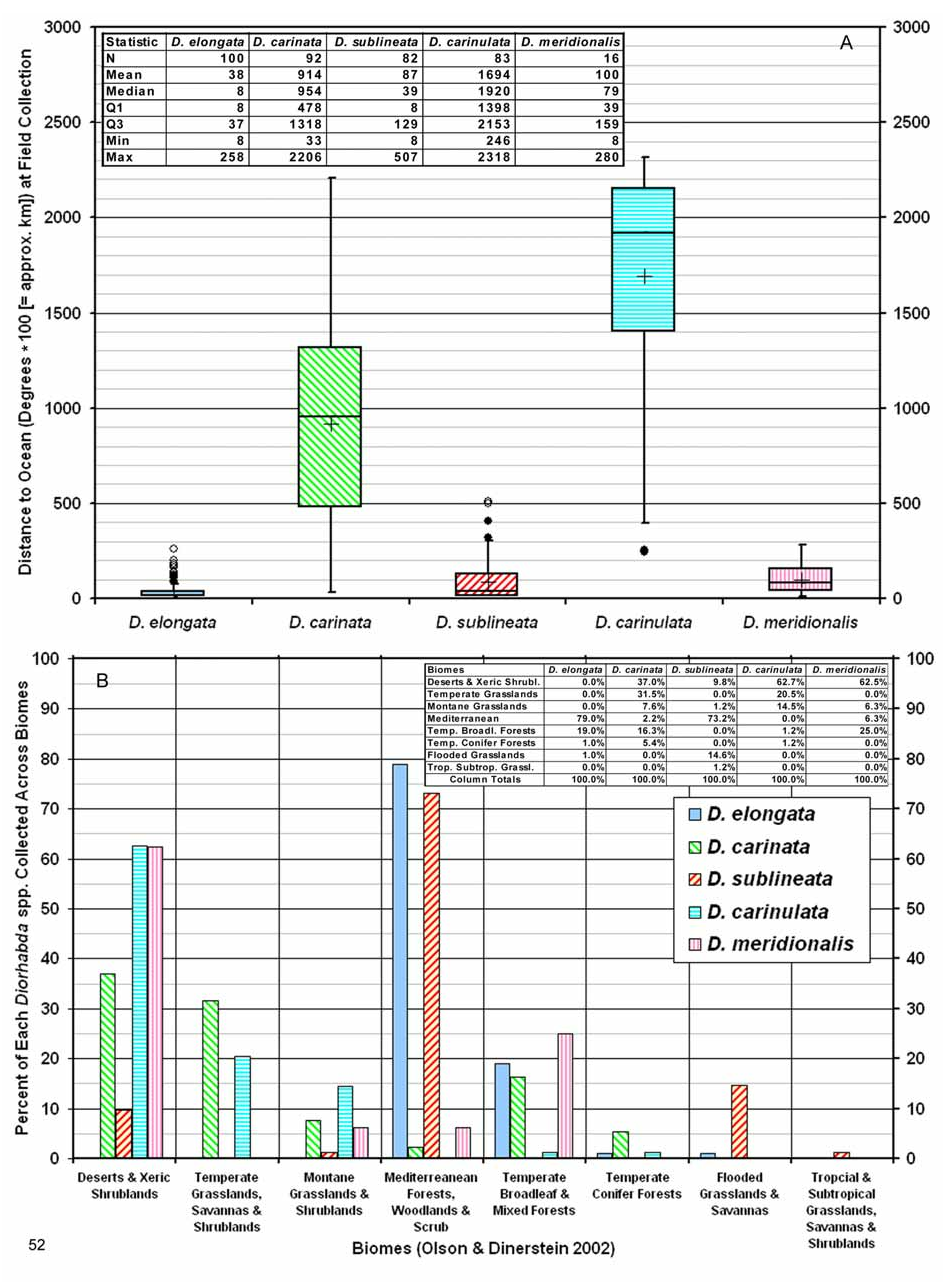
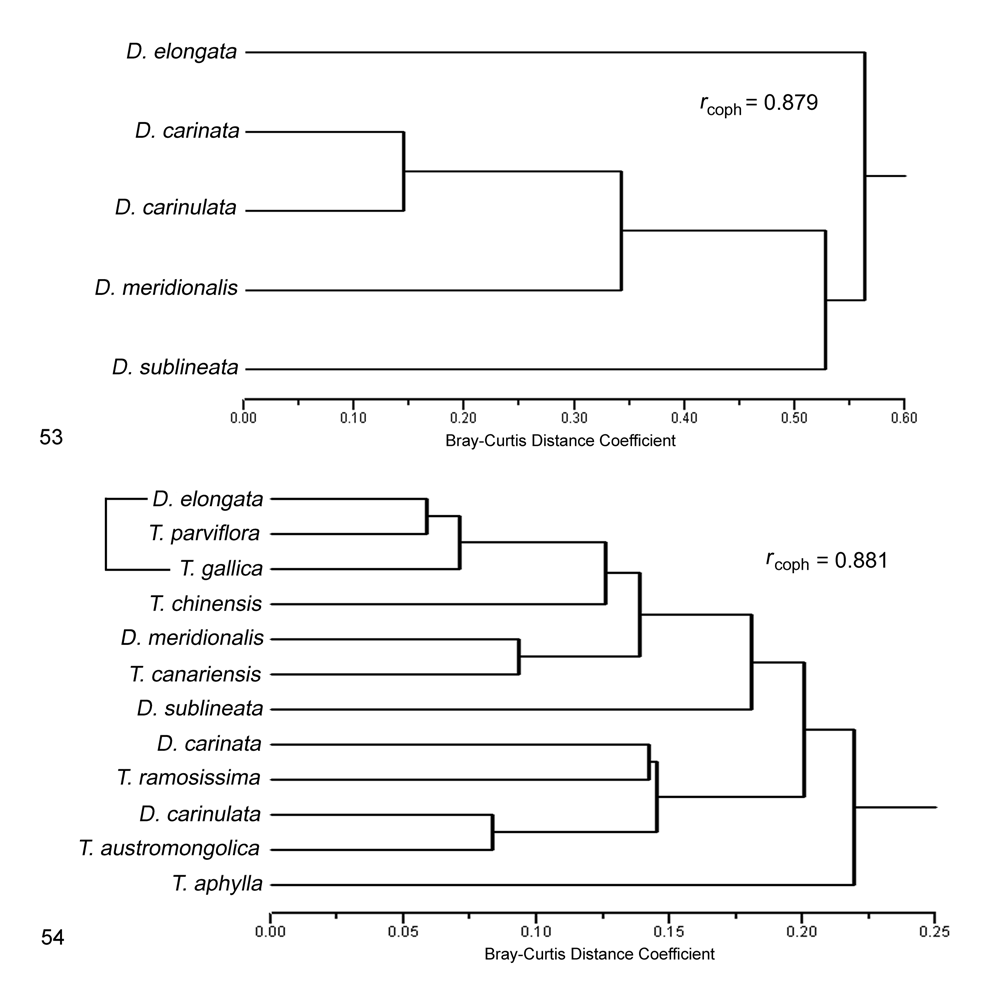
Biogeography. Comparative. Diorhabda carinata differs from other tamarisk beetles by the following combination of biogeographic characteristics: (1) primarily continental with distribution usually greater than 500 km from the oceans and ranging to 2,900 m elevation; (2) usually found in warm temperate desert and grassland biomes; and (3) latitudinal range of 30– 44°N and most common from 36– 41°N ( Table 7, Figs. 51–52 View FIGURE 51 View FIGURE 52 ). Diorhabda carinata is moderately sympatric with D. carinulata , their ranges overlapping in a large area that includes Azerbaijan and central Asia (northern Iran, Turkmenistan, Uzbekistan, Kazakhstan, Tajikistan, Kyrgyzstan, and western China), where both species have been referred to as D. elongata (see synonymy above; e.g., Ogloblin 1936, Yakhontov and Davletshina 1955, Kulinich 1962, Sinadsky 1968, Lopatin 1977a, Samedov and Mirzoeva 1985, Lopatin et al. 2004) (Map 1; Table 8). Diorhabda carinulata differs from D. carinata in ranging further north to 49°N and in being more common from 42– 44°N. Conversely, D. carinata is more commonly collected than D. carinulata in some more southern areas from 35– 42°N, especially in the deserts of the Transcaucasus and grasslands of Tajikistan. However, D. carinulata appears to be more common than D. carinata from 31– 34°N in deserts of eastern Iran (Map 6). Further studies are needed to better understand the differing climatic preferences of these species. Diorhabda carinata and D. carinulata are also syntopic in some areas, having been collected together in the same series from the same tamarisk trees at three localities: Dry Sport Lake and Quaragum Canal, near Ashgabat, Turkmenistan, and Shelek, Kazakhstan. Diorhabda carinata and D. carinulata were also collected together in the same series (and probably also the same tamarisk trees) at Ordubad, Azerbaijan, and three location in Uzbekistan: Buxoro ( 5 km northwest), Qarshi ( 10 km west), and 140 km south of Toshkent. These species were collected in the same locations but differing series at Qobustan, Azerbaijan; Golestan Biosphere Reserve, Iran; Sarytogay Forest, Kazakhstan; and Yining, China. Care must be taken to separate D. carinata and D. carinulata in laboratory colonies begun from areas of sympatry and syntopy in Central Asia.
Diorhabda carinata is partially sympatric with D. meridionalis in western Iran and Syria (Maps 1, 6; Table 8). These species are probably also syntopic over some areas, having been collected together in the same series at Halab, Syria and at four locations in western Iran: Jolow Gir, 34 km SE of Omidiyeh, Shushtar, and 30 km NNE of Borazjan. Diorhabda meridionalis differs from D. carinata in being maritime and common further south at 26– 31°N ( Figs. 51–52 View FIGURE 51 View FIGURE 52 ). In the small sample sizes in which D. carinata and D. meridionalis were collected together from 29.5– 36°N, D. carinata was a little more abundant than D. meridionalis north of 30°N while D. meridionalis was a little more abundant south of 30°N (at 30 km NNE Borazjan). Diorhabda carinata is most similar to D. meridionalis and D. carinulata in terms of inhabited biomes ( Fig. 53 View FIGURES 53–54 ).
Diorhabda carinata is marginally sympatric with D. sublineata , their distributions meeting in Baghdad, Iraq. Diorhabda sublineata differs from D. carinata in being primarily maritime with a strong presence in the Mediterranean biome, and in ranging further south to 16°N where it is most common from 31– 35°N. Diorhabda carinata is most biomically different from D. sublineata , the species with which it is most morphologically similar ( Tables 9 and 10; Fig. 53 View FIGURES 53–54 ). Diorhabda carinata is probably marginally sympatric with D. elongata in eastern Turkey, western Syria, and, possibly, southern Russia, Georgia and Azerbaijan (Map 1). Diorhabda elongata differs from D. carinata in being maritime with a strong presence in the Mediterranean biome ( Figs. 51–52 View FIGURE 51 View FIGURE 52 ).
Descriptive. Diorhabda carinata is primarily found in the south central Palearctic realm, but two collection locations in northern Pakistan (Shorkot and Islamabad) fall within the borders of the Indo-Malayan realm (Maps 1, 3, 6). Most collections originate between 37° and 41°N in two biomes of Central Asia: the Deserts and Xeric Shrublands (ca. 0–800 m elevation) and Temperate Grasslands, Savannas and Shrublands (ca. 250–1,600 m) (Map 3, Table 9). Reports of D. carinata damaging tamarisk ( Kulinich 1962, Samedov and Mirzoeva 1985, Myartseva 1999) originate from these two biomes in this region. Primary ecoregions for D. carinata in the Temperate Grasslands, Savannas and Shrublands biome from 37– 41°N include the Gissaro–Alai Open Woodlands (ca. 250–450 m) and the Alai–West Tian Shian Steppe (ca. 400–1,600 m) in south central Kazakhstan and western Uzbekistan, Tajikistan and Kyrgyzstan (Map 3). Primary ecoregions with D. carinata in the Deserts and Xeric Shrublands biome from 37– 41°N include the Azerbaijan Shrub Desert and Steppe (ca. 0–400 m) and the Central Asian Southern Desert in south central Kazakhstan, southern Uzbekistan and Turkmenistan (ca. 200–350 m). Diorhabda carinata occurs more frequently in the Temperate Conifer Forests biome than any other Diorhabda species , where it may be found from 33– 41°N in northwestern Iran, eastern Turkey and northern Pakistan (ca. 550–1,600 m; Maps 3 and 6; Table 9). Other biomes with D. carinata from 37– 41°N include the Temperate Broadleaf and Mixed Forests of northern Iran and northeast Turkey (ca. 350–550 m) and Montane Grasslands and Shrublands (Kopet Dag Woodlands and Forest Steppe ecoregion) along the border of Iran and Turkmenistan (ca. 200–850 m).
The westward distribution limit of D. carinata in Turkey and Ukraine corresponds well with the westward distribution limit of one of its host plants, T. ramosissima (Map 3). The distribution of the host T. meyeri , which ranges across deserts from Azerbaijan eastwards to Uzbekistan and Afghanistan ( Rusanov 1949, Baum 1978; not shown), corresponds to the center of distribution of D. carinata in central Asia. The distribution of the host plant T. aralensis approximately coincides with the distribution of D. carinata in deserts of its southern range in Iran and Iraq as well as over its north-central range in Turkmenistan, Uzbekistan and Tajikistan (Map 3). The northern distribution of the host T. aucheriana also coincides with the range of D. carinata in Iraq and Iran (Map 3). The north central distribution of the host T. arceuthoides ( Rusanov 1949, Baum 1978, Browicz 1991; not shown) coincides with the heavy areas of occurrence of D. carinata in its northeastern range in Tajikistan and southern Uzbekistan. Tamarix arceuthoides is a very common Tamarix from eastern Iraq to Tajikistan ( 100–3,000 m elevation) that occurs more commonly in mountainous rocky or pebbly substrates than does T. ramosissima ( Rusanov 1949, Liu 1987, Browicz 1991) and prefers lower salinity habitats compared to T. aucheriana ( Leonard 1992) .
We find no reports of damage by D. carinata from the southern portion of its range, at 29– 37°N (Map 6). Habib and Hasan (1982) studied Tamarix insect herbivores in northern Pakistan, but did not report any damage by D. carinata (as D. elongata ) from 33– 36°N. South of 37°N, D. carinata is primarily found in the Deserts and Xeric Shrublands biome of Iraq, Afghanistan, and Pakistan (ca. 50–1,500 m), but it also occurs in the Temperate Broadleaf and Mixed Forests and Temperate Conifer Forests biomes of western and northern Iran (ca. 1,150 –1,900 m) (Maps 3 and 6; Table 9).
Potential in Tamarisk Biological Control. Summary. The larger tamarisk beetle is apparently establishing near Seymour, Texas (Map 7). Diorhabda carinata may be the most effective tamarisk beetle for control of T. ramosissima / T. chinensis in the temperate grasslands biome, including the Western Short Grasslands and Central and Southern Mixed Grasslands, and temperate conifer forest biome which includes the Arizona Mountains Forests of New Mexico and Arizona (Map 13). Diorhabda carinata may co-dominate with D. carinulata in some temperate warm desert areas such as the Trans-Pecos Chihuahuan and Mojave deserts and southern portions of the Great Basin Shrub Steppe and Colorado Plateau shrublands (Map 13).
Discussion. The larger tamarisk beetle damages Tamarix in west to central Asia from latitude ca. 38– 41°N and ca. - 10–1,000 m elevation in Azerbaijan (Samedov and Mirzoeva 1985), Turkmenistan ( Myartseva 1999), and Tajikistan ( Kulinich 1962). Diorhabda carinata attacks T. ramosissima throughout the northern portion of its range in Georgia ( Lozovoi 1961), Turkmenistan (present study), Tajikistan ( Kulinich 1962) and Kazakhstan (present study), and damages T. smyrnensis , a close relative of T. ramosissima , in Azerbaijan (Samedov and Mirzoeva 1985). We expect D. carinata would attack and probably damage and control T. ramosissima / T. chinensis in North America. It has a moderate risk of damaging T. aphylla (Milbrath and DeLoach 2006b) and very low risk of damaging Frankenia (Milbrath and DeLoach 2006a) , and both these risks are probably much reduced at less proximity to preferred Tamarix spp. (e.g., Blossey et al. 2001).
Diorhabda carinata appears to be establishing on Lake Kemp, near Seymour, Texas where it was released in April, 2008 and defoliated more than 0.2 ha by August and was common over a 0.8 ha area (C. Randal, pers. comm.; Maps 7 and 13). Diorhabda carinata defoliated portions of a few saltcedar trees in the summer of 2008 following its spring release at Matador Wildlife Management Area (WMA) near Paducah, Texas (Mike Janis, Texas Parks and Wildlife Department, Matador WMA, pers. comm.). It appeared to be weakly established on the Canadian River near Borger, Texas where it was first released (under permit as D. elongata ) in July 2006, but populations could not be found in 2008 (J. Michels and E. Jones, pers. comm.). D. carinata was also released in west Texas at Roaring Springs, Rotan, and Guthrie in the summer of 2008, but it has not yet established at these sites (A. Knutson, pers. comm.). D. carinata was caged in the Mojave Desert at Camp Cady, California in 2008 and permissions are being sought for release at this site in 2009 (T. Dudley, University of California, Santa Barbara, CA, pers. com.).
Northern climatypes of larger tamarisk beetles originating from the Desert and Xeric Shrublands and Temperate Grasslands and Shrublands biomes between 37– 41°N probably have the greatest potential to damage tamarisk in corresponding latitudes and biomes within 0–1,000 m elevation in North America (Map 13). Areas of primary ecoregions matching these criteria are the Western Short Grasslands in western Kansas, portions of the Great Basin Shrub Steppe and Colorado Plateau shrublands, and some of the extreme northern Mojave Desert (Map 13). Elevations above 1,100 m from 37– 41°N should be suitable, but in some cases suboptimal, for D. carinata . Milbrath et al. (2007) found that a northern climatype of D. carinata from 38°N in Uzbekistan suffered higher overwintering mortality in early March at 31°N in Temple, Texas than did D. sublineata and D. elongata . Higher mortality in the northern D. carinata climatype may have been related to asynchronization in the breaking of adult quiescence (not diapause, which probably ends in early winter in all the tamarisk beetles) with bud break in tamarisk as signaled by late winter/early spring temperatures. At 31°N in Temple, higher temperatures in late winter/early spring may occur several weeks before tamarisk bud break (temperatures begin to gradually warm before bud break), but at 38°N these same higher temperatures may occur later in the season and be more closely associated with tamarisk bud break (temperatures may more sharply warm up before bud break). A more southern climatype of D. carinata (see below) may be better synchronized in responding to higher temperatures as a signal to ending of winter quiescence at 31°N.
Several North American ecoregions correspond to the most northern areas inhabited by D. carinata in its native distribution from 41– 45°N (Map 13). These regions include northern portions of the Western Short Grasslands and southern portions of the Wyoming Basin Shrub Steppe (Map 13). Much of these northern areas may be more suitable for D. carinulata .
Southern climatypes of D. carinata (Map 6) may be best suited to ecoregions in the Temperate Grasslands, Savannas and Shrublands biome between 31– 37°N (Map 13). These include the southern portions of the Western Short Grasslands in eastern New Mexico and northwest Texas and southwest portions of the Central and Southern Mixed Grasslands in north central and western Texas (Map 13). Several temperate warm desert regions in North America correspond to native habitats for southern D. carinata climatypes in the Desert and Xeric Shrublands biome between 31– 37°N. These desert ecoregions include southern portions of the Colorado Plateau Shrublands, portions of the Mojave Desert in southern California and Nevada, and the Trans-Pecos Chihuahuan Desert in Texas, New Mexico and northern Chihuahua, Mexico (Map 13). Larger tamarisk beetles might damage tamarisk in some of these southern grassland and desert ecoregions. Diorhabda carinata appears to be the best suited species to the Temperate Conifer Forests among the D. elongata group ( Tables 9 and 13). This would make D. carinata the best suited species to tamarisk invaded riparian areas of the Arizona Mountains Forests ecoregion of Arizona and New Mexico, such as along the Rio Hondo within the pinyon-juniper woodlands near Hondo, New Mexico (Map 13). Southern maritime subtropical desert areas of 31– 29°N, such as the Sonoran Desert, may not be as suitable for D. carinata as for other more southern sibling species (Map 13, Table 7).
| ZIN |
Russian Academy of Sciences, Zoological Institute, Zoological Museum |
| NHMB |
Natural History Museum Bucharest |
| HNHM |
Hungarian Natural History Museum (Termeszettudomanyi Muzeum) |
| DEI |
Senckenberg Deutsches Entomologisches Institut |
| ZMAN |
Instituut voor Taxonomische Zoologie, Zoologisch Museum |
| NMPC |
National Museum Prague |
| SEL |
Marie Selby Botanical Gardens |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Diorhabda carinata ( Faldermann, 1837 )
| Tracy, James L. & Robbins, Thomas O. 2009 |
Diorhabda carinata
| DeLoach, C. J. & Lewis, P. A. & Herr, J. C. & Carruthers, R. I. & Tracy, J. L. & Johnson, J. 2003: 126 |
Diorhabda elongata sublineata
| Wilcox, J. A. 1971: 63 |
Diorhabda elongata carinata : Bechyné, 1961:256
| Lopatin, I. K. & Aleksandrovich, O. R. & Konstantinov, A. S. 2004: 127 |
| Medvedev, L. N. 1983: 123 |
| Wilcox, J. A. 1971: 63 |
| Lopatin, I. K. 1963: 355 |
| Bechyne, J. 1961: 256 |
Diorhabda rybakowi : Mityaev, 1958:86
| Mityaev, I. D. 1958: 86 |
Diorhabda elongata
| Warchalowski, A. 2003: 328 |
| Weise, J. 1924: 78 |
Diorhabda elongata var. carinata :
| Jacobson, G. G. 1901: 137 |
| Weise, J. 1893: 635 |
| Heyden, L. V. & Reitter, E. & Weise, J. 1891: 375 |
Diorhabda elongata : Weise, 1883:316
| Dudley, T. L. & Dalin, P. & Bean, D. W. 2006: 137 |
| Gates, M. & Myartseva, S. & Schauff, M. 2005: 28 |
| Dudley, T. L. 2005: 13 |
| Dudley, T. L. 2005: 42 |
| Lopatin, I. K. & Aleksandrovich, O. R. & Konstantinov, A. S. 2004: 127 |
| DeLoach, C. J. & Carruthers, R. I. & Rodriguez-del & Bosque, L. A. 2003: 230 |
| Khamraev, A. S. 2003: 11 |
| Milbrath, L. R. & Herr, J. C. & Knutson, A. E. & Tracy, J. L. & Bean, D. W. & Rodriguez-del-Bosque, L. A. & Carruthers, R. I. & DeLoach, C. J. 2003: 225 |
| Warchalowski, A. 2003: 328 |
| Myartseva, S. N. 2001: 1 |
| Anonymous 2001: 52 |
| Aslan, I. & Warchalowski, A. & Ozbek, H. 2000: 30 |
| Myartseva, S. N. 1999: 1 |
| Aslan, I. 1998: 287 |
| Myartseva, S. N. 1995: 4 |
| Kovalev, O. V. 1995: 78 |
| Tomov, V. 1984: 377 |
| Davletshina, A. G. & Avanesova, G. A. & Mansurov, A. K. 1979: 79 |
| Lopatin, I. K. 1977: 282 |
| Sinadsky, Y. V. 1968: 64 |
| Kulenova, K. Z. 1968: 171 |
| Pripisnova, M. G. 1965: 83 |
| Sinadsky, Y. V. 1963: 84 |
| Kulinich, P. N. 1962: 73 |
| Lozovoi, A. I. 1961: 86 |
| Medvedev, L. N. 1959: 118 |
| Yakhontov, V. V. 1959: 338 |
| Sinadsky, Y. V. 1957: 950 |
| Kyrzhanovskiy, O. L. 1952: 198 |
| Rusanov, F. N. 1949: 118 |
| Ogloblin, D. A. 1936: 79 |
| Holdhaus, K. 1920: 45 |
| Weise, J. 1883: 316 |
Galeruca elongata
| Joannis, M. L. 1866: 83 |
Galeruca carinata
| Faldermann, F. 1837: 329 |