Polyonchobothrium polypteri ( Leydig, 1853 ) Lühe, 1900
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3309.1.1 |
|
DOI |
https://doi.org/10.5281/zenodo.6174509 |
|
persistent identifier |
https://treatment.plazi.org/id/038A9703-0A2D-FFA8-FF7D-75280416FA42 |
|
treatment provided by |
Plazi |
|
scientific name |
Polyonchobothrium polypteri ( Leydig, 1853 ) Lühe, 1900 |
| status |
|
Polyonchobothrium polypteri ( Leydig, 1853) Lühe, 1900
( Figs. 23–27 View FIGURES 23 – 32 , 33–40 View FIGURES 33–40 )
Syns: Tetrabothrium polypteri Leyding, 1853 ; Onchobothrium (Polyonchobothrium) septicolle Diesing, 1854 ; Anchistrocephalus polypteri (Leyding, 1853) Monticelli, 1890 ; Ptychobothrium armatum Fuhrmann, 1902 View in CoL ; Ancistrocephalus polypteri (Leyding, 1853) Hesse, 1922 ; Polyonchobothrium pseudopolypteri Meggitt, 1930 ; Oncobothriocephalus armatum ( Fuhrmann, 1902) Yamaguti, 1959 ; Polyoncobothrium polypteri (Leyding, 1853) Yamaguti, 1959 .
Type host: Polypterus bichir Lacépède ( Polypteriformes : Polypteridae ).
Other definitive hosts: Polypterus endlicheri Heckel ; Polypterus senegalus Cuvier.
Type locality: Nile River in Egypt.
Distribution: Congo basin – Democratic Republic of the Congo (Brazzaville); Gambia basin – Senegal; Lake Chad – Chad; Turkana basin – Kenya, Lake Turkana ( Polypterus spp. occur only sporadically in saline part of the lake and P. p ol y pt e r i is therefore probably restricted to the freshwater part of the Turkana Lake, i.e. Omo River delta and upstream and adjacent northernmost part of the lake); Niger basin – Nigeria, Ivory Coast, Mali; Nile basin – Egypt, Ethiopia, the Sudan; Ogoué basin – Gabon; Zambezi basin – Malawi.
Prevalence: Nile basin – the Sudan, 8–25%, n = 21, intensity 15–50 (present study), 43%, n = 312, 10–70 ( Khalil 1969); Turkana Lake – Kenya, 75%, 8, intensity up to 50 (present study); Niger basin – Nigeria, 94%, n = 84, intensity 1–226 (mostly juveniles) ( Shotter & Medaiyedu 1978).
Life cycle: Not known, but plerocercoids of P. polypteri have been found in the following fish of different families, which may serve as second intermediate or paratenic hosts: Auchenoglanis occidentalis (Valenciennes) ( Siluriformes : Claroteidae ); Barbus bynni ( Cypriniformes : Cyprinidae ); Lates niloticus (Linnaeus) ( Perciformes : Latidae ); Mormyrops anguilloides (Linnaeus) (Mormyriformes: Mormyridae ); Schilbe uranoscopus Rüppell ( Siluriformes : Schilbeidae ); Sarotherodon galilaeus (Linnaeus) ; Stigmatochromis woodi (Regan) ; and Oreochromis niloticus (Linnaeus) ( Perciformes : Cichlidae ) (see below).
Type material: Not known to exist. To enable future comparison of the species with other taxa, the specimen found in P. bichir (field No. T 169/08) from Lake Turkana – Omo River delta , Todonyang, Kenya is designated as neotype and it is deposited in IPCAS (No. C-464 ).
Material studied: Type material: syntype of Ptychobothrium armatum Fuhrmann, 1902 (one slide and vial with 9 scolexes and several pieces of strobila) ex Turdus parochus from Egypt (ZBM E.2361) ; probably syntype of Onchobothrium septicolle Diesing, 1854 ex P. bichir from Egypt collected by Kollar in 1847 or 1852 ( NMW 2612–3) ; vouchers: ex P. endlicheri from Brazzaville, Republic of the Congo ( MHNG 41938 –9; RMCA 30156 ) ; ex P. endlicheri from Mali ( MNHNP C75) ; ex P. senegalus from Sierra Leone ( BMNH 1965.2.24.46–53, 1977.6.28.3–4) ; ex P. bichir from Kainji Dam, Nigeria ( BMNH 1970.8.24.38) ; ex “ Silurus sp.” from Bamba, Mali ( MHNG 45401 ) ; Polyonchobothrium clarias ex Chrysichthys thonneri Steindachner from Gabon ( RMCA 33752) ; plerocercoids: Polyonchobothrium sp. ex Auchenoglanis occidentalis (Valenciennes) from unknown locality collected by McClelland (RVC C1106–7) ; ex Barbus bynni from unknown locality collected by McClelland (RVC C1105) ; ex Mormyrops anguilloides (Linnaeus) from unknown locality collected by McClelland (RVC C1103) ; ex Schilbe uranoscopus from unknown locality collected by McClelland (RVC C1109) ; new material (see Appendix 1 for details): around 100 worms collected from 7/8 (7 of 8 examined) Polypterus bichir from Kenya, Lake Turkana and the Sudan, Sennar Dam; 1/ 3 P. endlicheri from the Sudan, White Nile, Kostí; 1/ 13 P. senegalus from the Sudan. The new material is deposited in BMNH (Nos. 2012.3.120.1–13), IPCAS (No. C-464), MHNG (Nos. 62880, 82040–82047), USNPC (Nos. 105392–105394, 105401–105403) and ZMB (Nos. 7515–7516).
Published records: Leydig (1853); Klaptocz (1906); Hesse (1922); Janicki (1926); Joyeux & Baer (1928); Meggitt (1930); Ukoli (1965); Khalil (1969, 1973); Shotter & Medaiyedu (1978); Troncy (1978); Jones (1980).
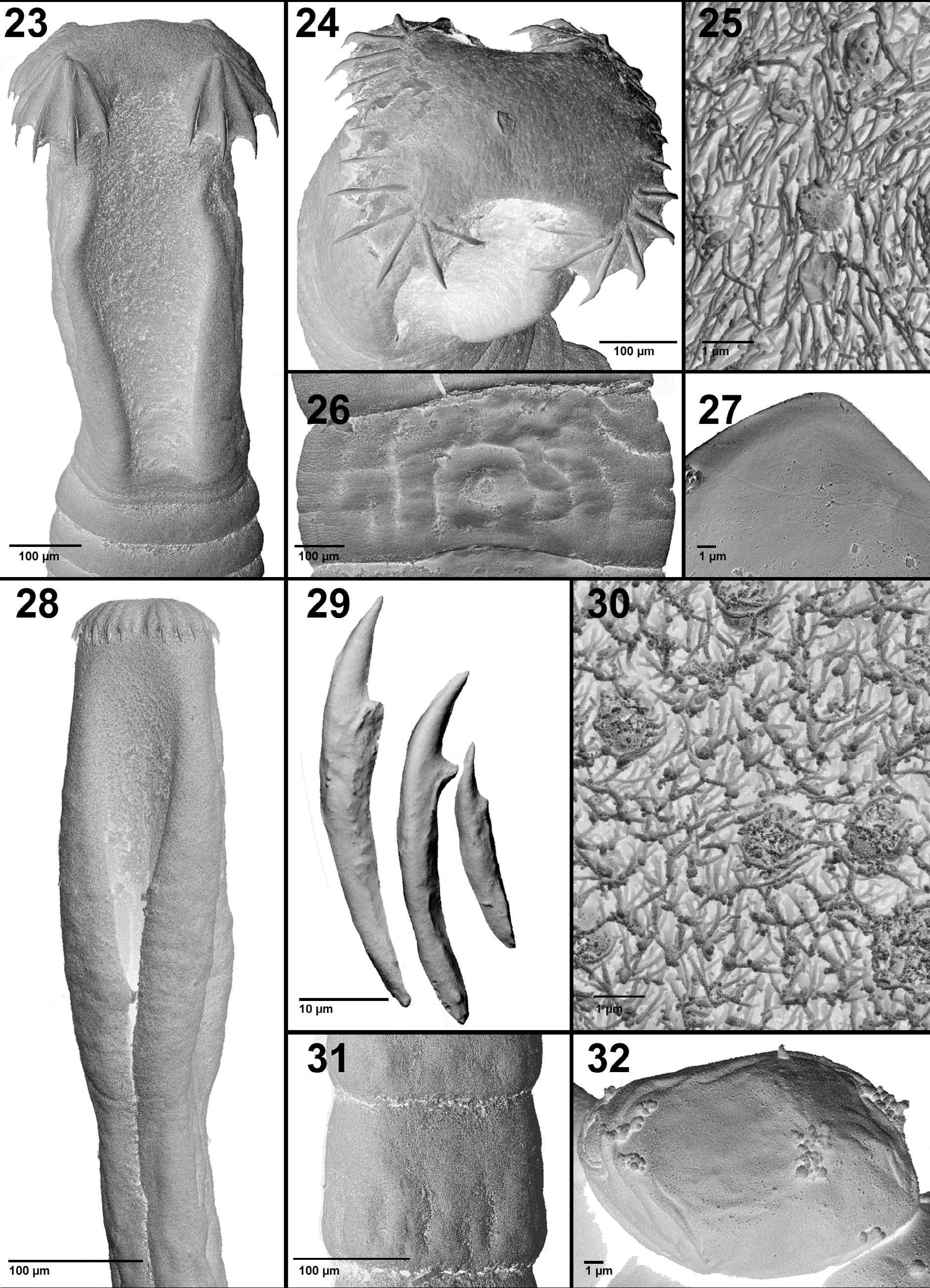
Re-description (based on 20 complete worms from Ethiopia, Kenya and the Sudan): Bothriocephalidea , Bothriocephalidae . Strobila up to 10 cm long (up to 20 cm according to Khalil, 1969); maximum width 2.3 mm. External and internal segmentation present; segments wider than long, markedly craspedote ( Fig. 33 View FIGURES 33–40 ).
Two pairs of longitudinal osmoregulatory canals; dorsal canals narrow (diameter up to 16); ventral canals wide (diameter up to 20), connected by transverse anastomoses. Longitudinal musculature well developed, muscle fibres diffused ( Fig. 40 View FIGURES 33–40 ). Surface of strobila covered with capilliform filitriches.
Scolex elongate ( Figs. 23 View FIGURES 23 – 32 , 34 View FIGURES 33–40 ), 700–1,490 (1,065 ± 198) long by 260–430 (350 ± 44) wide (n = 19). Apical disc prominent, wider than scolex proper, usually four-lobed in apical view, 350–510 (410 ± 45) wide by 145–255 (200 ± 33) long (n = 17), armed with 27–35 (32 ± 2; n = 12) large hooks, 14–165 (106 ± 37; n = 314) long ( Jones 1980 reported hooks up to 190 μm long), arranged usually in four quadrants (6–9 hooks in each quadrant). Hooks variable in size in each quadrant, smallest being on periphery and increasing to middle of quadrant, with largest hook 120–165 (152 ± 13; n = 10) in centre ( Figs. 24 View FIGURES 23 – 32 , 35, 36 View FIGURES 33–40 ). Bothria elongate, shallow, 580–1,020 (790 ± 37) long by 100–235 (163 ± 40) wide (n = 13) ( Figs. 23 View FIGURES 23 – 32 , 34 View FIGURES 33–40 ). Surface of scolex covered with capilliform filitriches and tumuliform globular structures (diameter around 1) ( Fig. 25 View FIGURES 23 – 32 ). Neck absent, first segments appear immediately posterior to scolex ( Figs. 23 View FIGURES 23 – 32 , 33, 34 View FIGURES 33–40 ).
Immature segments 80–247 long by 270–2130 wide; length/width ratio 0.06–1.04: 1 (n = 45) ( Fig. 33 View FIGURES 33–40 ). Mature segments wider than long, 125–300 (178 ± 56) long by 1,400–2,300 (1,830 ± 386) wide; length/width ratio 0.06– 0.60: 1 (n = 12) ( Fig. 39 View FIGURES 33–40 ). Gravid segments wider than long, 170–810 (415 ± 240) long by 775–1,680 (1,155 ± 254) wide; length/width ratio 0.12–0.76: 1 (n = 13) ( Fig. 33 View FIGURES 33–40 ).
Testes medullary, oval, 30–65 (48 ± 9; n = 18) in number per segment (up to 72 according to Jones 1980), 37– 81 (57 ± 14) long by 22–41 (34 ± 7) wide (n = 14), forming 2 narrow longitudinal bands (17–38 testes per band), confluent between segments, absent medially and near lateral margins ( Fig. 39 View FIGURES 33–40 ). Cirrus-sac large, thick-walled (thickness of sac wall 3–8), pyriform, oblique, with proximal part curved anterolaterally, 46–182 (111 ± 34) long by 51–179 (130 ± 30) wide (length/width ratio 0.70–1.06: 1) (n = 20), pre-equatorial to equatorial (at 30–51% of length of mature segment from its anterior margin; n = 10) ( Figs. 38–40 View FIGURES 33–40 ). Internal seminal vesicle absent. Vas deferens forms numerous loops lateral to cirrus-sac; internal sperm duct strongly coiled; cirrus unarmed, opening into genital atrium ( Figs. 26 View FIGURES 23 – 32 , 38–40 View FIGURES 33–40 ). Numerous prostatic glands around anterior part of cirrus-sac ( Figs. 38–40 View FIGURES 33–40 ). Genital pore dorsal, median, near anterior margin of segment, transversely elongate ( Fig. 39 View FIGURES 33–40 ).
Ovary slightly asymmetrical, compact, transversely elongate, 20–50 (40 ± 10) long by 100–550 (410 ± 150) wide (n = 10) ( Fig. 39 View FIGURES 33–40 ). Vagina a straight, thick-walled wide tube, 22–37 (28 ± 4; n = 15) in diameter, opens posterior to cirrus-sac into genital atrium; vaginal sphincter absent ( Fig. 38 View FIGURES 33–40 ). Vitelline follicles cortical, numerous, small, spherical, 19–41 (28 ± 5; n= 15) in diameter, form 2 wide longitudinal bands confluent between segments, separated medially to form ventral and dorsal bands ( Fig. 39 View FIGURES 33–40 ), rarely connected by several follicles in postovarian region.
Uterine duct winding, forms numerous tightly coiled loops, filled with eggs, enlarged in gravid segments ( Fig. 33 View FIGURES 33–40 ). Uterus thin-walled, median, spherical, enlarged in gravid segments, occupying 23–36% of segment surface ( Fig. 33 View FIGURES 33–40 ). Uterine pore thick-walled, opens anterior to midlength of uterus. Eggs oval, thin-walled, operculate, may be embryonated, 30–50 (40 ± 7) long by 20–45 (30 ± 6) wide (n = 20), fully formed oncosphere 22–37 (28 ± 4) long by 18–30 (23 ± 4) wide (n = 15) ( Figs. 27 View FIGURES 23 – 32 , 37 View FIGURES 33–40 ).
Remarks: Polyonchobothrium polypteri was described as Tetrabothrium polypteri based on worms found in Polypterus bichir from the Nile River in Egypt by Leydig (1853), who described only scolex morphology. Taxonomic history of the species, which was transferred to several genera, was reviewed by Jones (1980), who redescribed the taxon on the basis of tapeworms collected by L. F. Khalil in three species of bichirs ( P. bichir , P. endlicheri and P. senegalus ) from the White Nile at Jebel-Awlia (south of Khartoum, the Sudan). This redescription was detailed, but it seems that tapeworms studied by Jones (1980) may have been relaxed too long in the water, because their segments were unnaturally long in relation to their width (see figs. 14 and 15 in Jones 1980). In our new material from Polypterus spp. from Kenya and the Sudan, which was observed alive, segments were invariably much wider than long and markedly craspedote.
Jones (1980) provided erroneous measurements of the eggs (396–444 μm by 264–288 μm), which was apparently caused by an incorrect position of the decimal point, and reported the eggs to be unoperculate when laid. However, we observed operculate eggs in the new material from the Sudan ( Figs. 27 View FIGURES 23 – 32 , 37 View FIGURES 33–40 ).
Kuchta et al. (2008a, b) considered Polyonchobothrium to be monotypic, with P. polypteri representing its type and only species, because species previously placed in Polyonchobothrium (see Kuchta & Scholz 2007 for list of synonyms) actually belong to other genera ( Kirstenella , Senga and Tetracampos ). Polyonchobothrium differs from other bothriocephalidean genera in scolex morphology, especially in the possession of a prominent apical disc, usually divided into four separate lobes, each of them armed with 6–9 massive hooks up to 190 μm long.
Polyonchobothrium polypteri is considered here to be a specific parasite of bichirs ( Polypteridae ), which represent an ancient lineage of ray-finned fish (Actinopterygii) endemic to Africa. Polypterids are phylogenetically distant from teleosts and other freshwater fish, such as paddlefish, sturgeons, gars and bowfins ( Suzuki et al. 2010). Adult worms were found in other hosts, such as Chrysichthys thanneri (present study; RMCA 33752) or “ Silurus sp.” (present study, Joyeux & Baer 1928; MHNG 45401), but they may represent just atypical hosts.
Fuhrmann (1902) described Ptychobothrium armatum from a trush reported under the name Turdus parochus from Egypt. However, trush of this name has never been described, which casts doubts upon the actual host of these specimens. Examination of the type material (ZBM E.2361) has shown that Polyonchobothrium polypteri was misidentified. The most probable explanation of this unusual finding of a specific parasite of bichir in a passeriform bird is mislabelling of samples. Bichi & Yelwa (2010) reported P. polypteri in clariid catfish ( Clarias gariepinus ) in Nigeria. This finding may represent misidentification of Tetracampos ciliotheca , which is a typical parasite frequently infecting that host (see below).
Larvae (plerocercoids), juvenile or immature specimens of P. polypteri have been found by the present authors in the intestine of several unrelated fish, such as claroteid catfish, mormyrids, barbels, Nile perch and tilapias, which may serve as paratenic or postcyclic hosts ( Appendix 1).
Seasonality in the occurrence and maturation of P. polypteri has been indicated by previous authors, because immature specimens were found in spring (March and April by Jones, 1980) in the Sudan and from July to September in Nigeria ( Ukoli 1965), whereas fully mature specimens were present only in autumn (October) in the Sudan ( Jones 1980). Even though immature worms dominated in all newly collected samples, material from Kenya and the Sudan was represented by mixture of immature, mature and gravid worms in March 2006 ( the Sudan), September 2008 and 2009 ( Kenya). In November 2008 ( the Sudan) only immature worms were found.
Shotter & Medaiyedu (1977) reported the highest prevalence and intensity of infection P. polypteri in bichirs from Nigeria in fish of the total length of 300–350 mm. Based on a high proportion of immature worms to mature ones (3,879 versus 866 specimens, i.e. ratio 4.5: 1), these authors supposed that many juvenile worms did not reach maturity, possibly due to effective immune reaction of fish hosts.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Polyonchobothrium polypteri ( Leydig, 1853 ) Lühe, 1900
| Kuchta, Roman, Burianová, Alena, Jirkú, Miloslav, Chambrier, Alain, Oros, Mikuláš, Brabec, Jan & Scholz, Tomáš 2012 |
Oncobothriocephalus armatum ( Fuhrmann, 1902 ) Yamaguti, 1959
| (Fuhrmann, 1902) Yamaguti 1959 |
Polyoncobothrium polypteri
| (Leyding, 1853) Yamaguti 1959 |
Polyonchobothrium pseudopolypteri
| Meggitt 1930 |
Ancistrocephalus polypteri (Leyding, 1853)
| Hesse 1922 |
Ptychobothrium armatum
| Fuhrmann 1902 |
Anchistrocephalus polypteri
| (Leyding, 1853) Monticelli 1890 |
Onchobothrium (Polyonchobothrium) septicolle
| Diesing 1854 |
Tetrabothrium polypteri
| Leyding 1853 |