Peltogaster reticulata Shiino, 1943
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4768.1.2 |
|
publication LSID |
urn:lsid:zoobank.org:pub:98FCA850-263F-4BB7-87D8-D838B29D128A |
|
DOI |
https://doi.org/10.5281/zenodo.3795496 |
|
persistent identifier |
https://treatment.plazi.org/id/038A3F58-FF91-FFAC-83C8-2205FD69FF81 |
|
treatment provided by |
Plazi |
|
scientific name |
Peltogaster reticulata Shiino, 1943 |
| status |
|
Peltogaster reticulata Shiino, 1943
Peltogaster reticulatus . Rybakov & Høeg 2002: 98, figs 13, 26.— Kashenko & Korn 2003 — Isaeva et al. 2005.
Peltogaster reticulata .— Yoshida et al. 2011: 853, 855, 857.— Yoshida & Naruse 2016: 215, 220.— Kornienko et al. 2018.
Morphological description. Host. Shield length of the males of Pagurus minutus View in CoL infested with the rhizocephalan ranged from 1.8–4.9 mm; females from 1.7–4.4 mm.
Bathymetrical range. In Vostok Bay (Peter the Great Bay, Sea of Japan), infested hermit crabs were found at a depth of 0– 6 m.
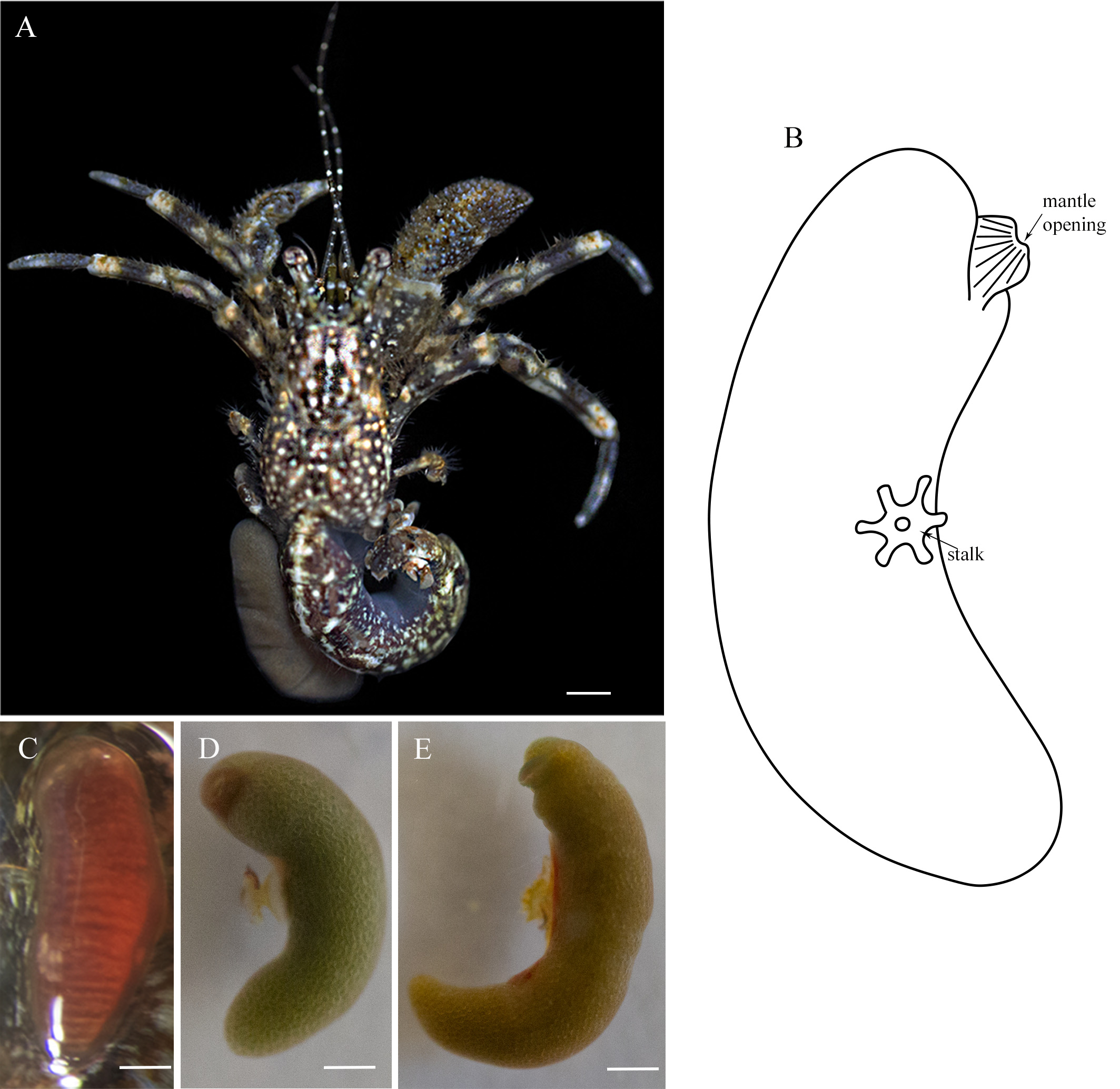
Location on the host. Most hermit crabs had only one externa. The externa was usually located on the left side of the pleon, between the second and third pleopods ( Fig. 1A View FIGURE 1 ); the second externa was placed towards the dorsal side.
Morphology of externa. The size of the externa ranged from 3.0–14.0 mm in length and from 1.0– 3.7 mm in width; the length was three to four times more than the width. The mature externa is elongated, cylindrical and strongly curved. The anterior end is broad and bilobed, with the mantle opening inserted between the two lobes. The mantle opening is slightly elevated as a tube-like projection and placed on the side facing the host. The shield is conspicuous, fusiform, having growth rings, extending anteriorly and posteriorly from the stalk, covering nearly 1/3 of the externa. A very short, narrow stalk, ~ 0.25 mm in diameter, is placed nearly in the middle of the externa ( Fig. 1B View FIGURE 1 ).
Coloration in life. The immature externa without embryos in the mantle cavity is red as well as the externa sterilized by Liriopsis pygmaea ( Fig. 1C View FIGURE 1 ). The mature externa with embryos in the mantle cavity is green, olive or brown in accordance with the stage of development ( Fig. 1D, E View FIGURE 1 ). The root system is green.
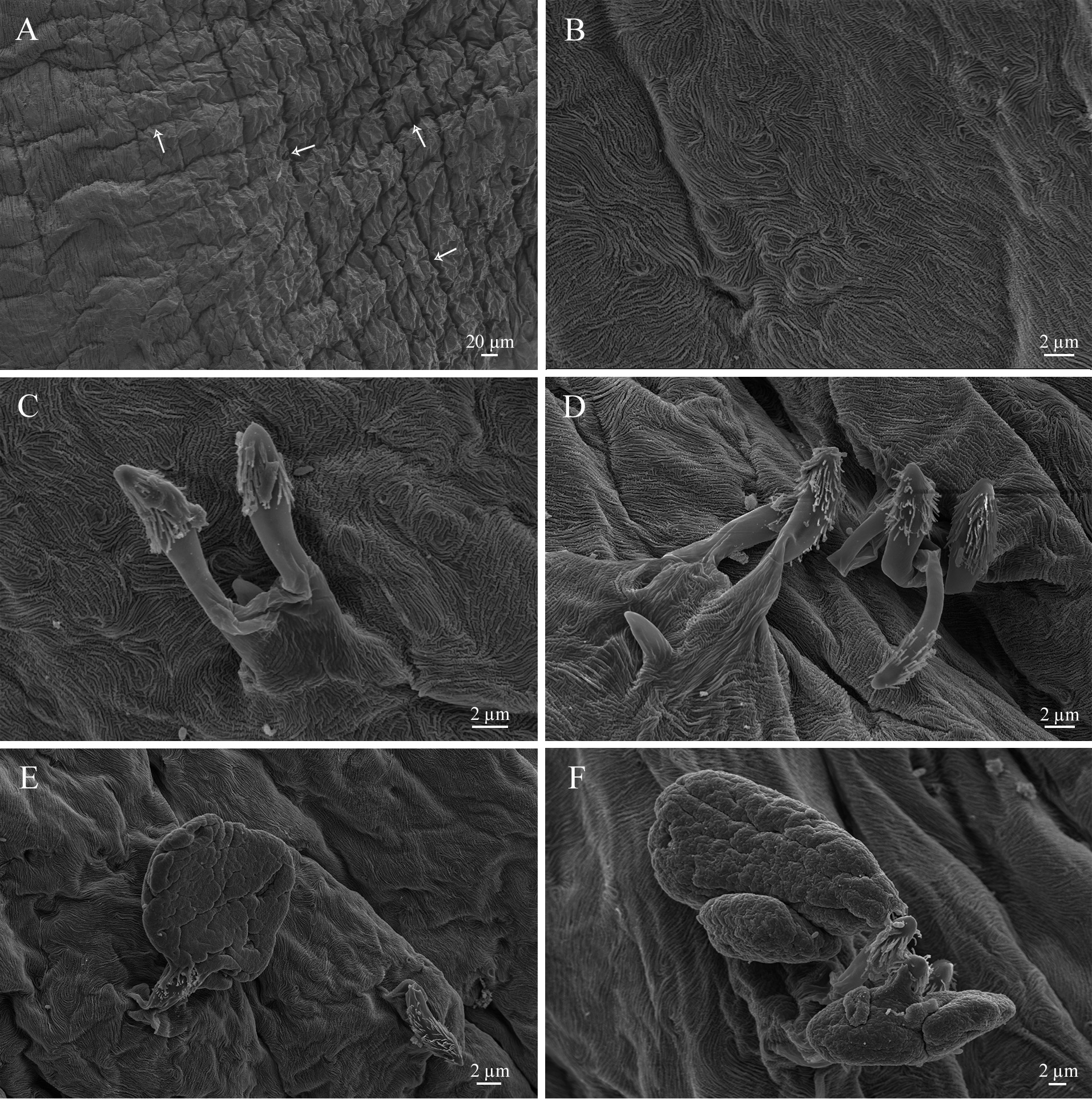
Cuticle. The external cuticle is thin, longitudinally wrinkled, without papillae or excrescences. Thin transverse ridges create an indistinct reticular pattern ( Fig. 2A View FIGURE 2 ). The internal cuticle is waved ( Fig. 2B View FIGURE 2 ) and covered with numerous retinacula. Lamp-brush retinacula present a group of 2–4 spindles of 14–16 µm in length covered distally with soft finger-like projection. Spindles often arise from a common base of 6–8 µm in height ( Fig. 2 View FIGURE 2 С, D). Numer- ous spindles were also placed inside flat, balloon-like structures of 25–35 µm in diameter and gradually released out of their envelopes ( Fig. 2E, F View FIGURE 2 ).
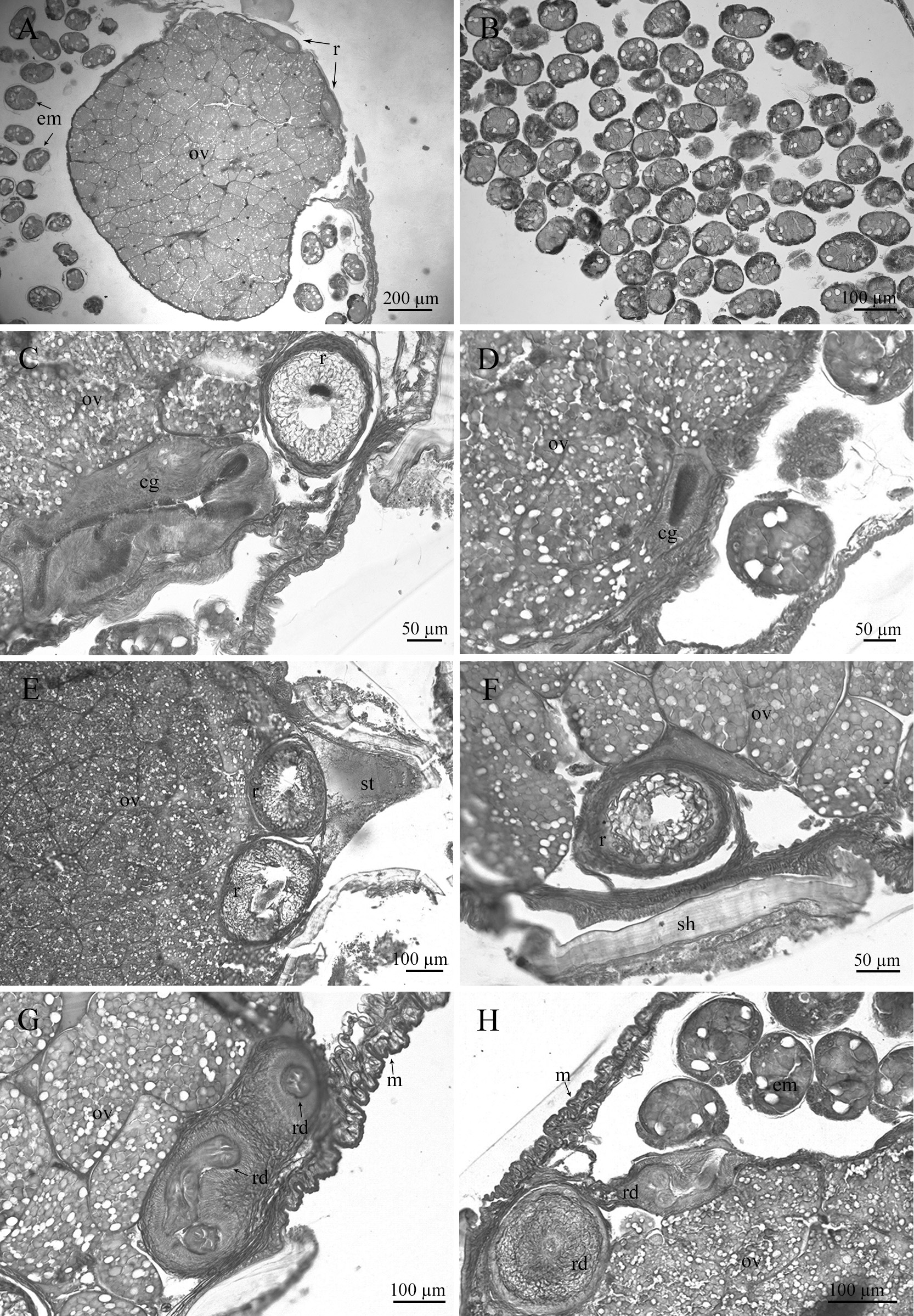
Anatomy of externa. A visceral sac with developing oocytes extends along the most part of the externa. It is boot-shaped in transverse section ( Fig. 3A View FIGURE 3 ). The mantle cavity is closely filled with embryos ( Fig. 3B View FIGURE 3 ). Colleteric glands are long, strongly folded tubes with a diameter from 300–450 to 100 µm ( Fig. 3C, D View FIGURE 3 ), placed anteriorly towards the stalk ( Fig. 3E View FIGURE 3 ). Two thin, tubular receptacles, with a diameter in transverse section of 140–260 µm, are placed inside the visceral sac ( Fig. 3A, C, E View FIGURE 3 ). The left receptacle begins anteriorly, being longer than right one ( Fig. 3F View FIGURE 3 ). The receptacles extend along the most part of visceral mass and gradually pass into receptacle ducts with a diameter of 80–130 µm. The posterior part of the receptacle duct is coiled, opening on the lateral surface of the visceral mass ( Fig. 3G, H View FIGURE 3 ). The mantle is 50–90 µm in thickness ( Fig. 3G, H View FIGURE 3 ).
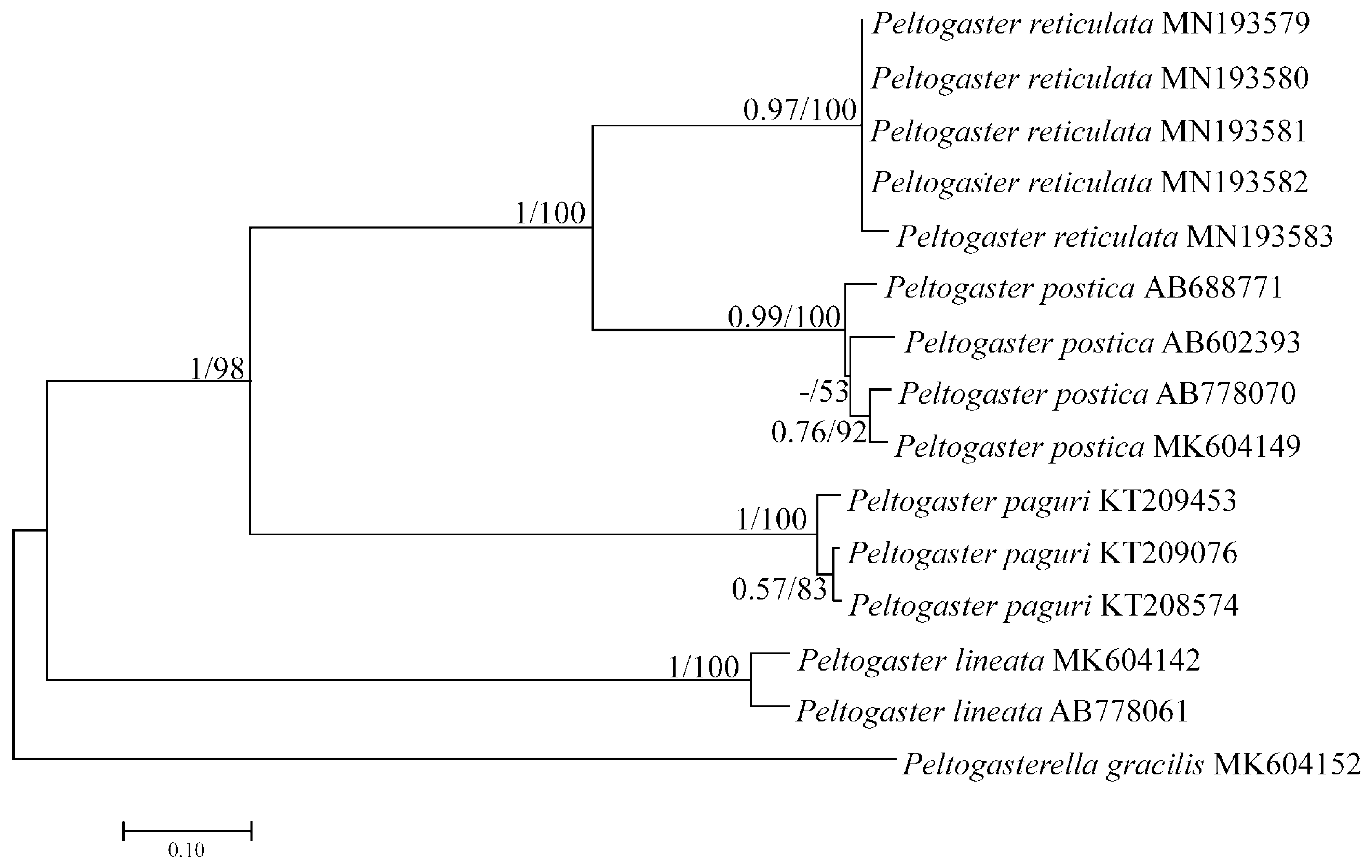
Molecular analysis. Clear differences between the sequences of congeneric peltogastrids were the basis for accurate identification of the species. Identification was confirmed by BI and NJ analyses with high support values between different species. Genetic analysis of P. reticulata infesting Pagurus minutus in Russian waters based on the comparison of partial mitochondrial COI sequence data showed that this rhizocephalan species differs from the known congeneric species, Peltogaster paguri Rathke, 1842 and P. lineata Shiino, 1943 . Sequences of P. reticulata form a monophyletic clade with the morphologically similar rhizocephalan species, P. postica . Monophyly of each species was supported by high bootstrap values ( Fig. 4 View FIGURE 4 ). Average interspecific and intraspecific distances are shown in Tables 1 and 2. The comparison of pairwise genetic distances indicates high differences between P. reticulata and P. postica . The interspecific divergence is 10 times higher than the intraspecific genetic distances. Therefore, the “barcoding gap” ( Meyer & Paulay 2005) can be observed for the studied taxa.
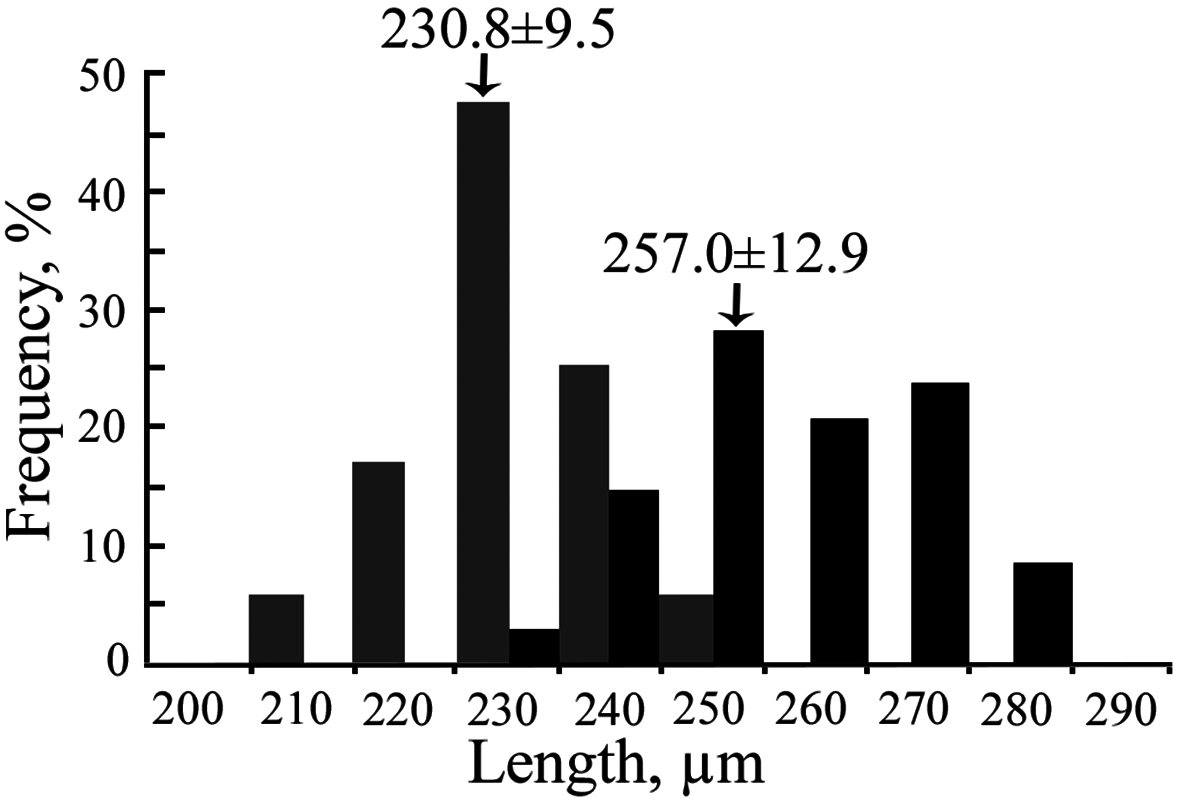
Larval development. The larval development includes five naupliar and one cypris stages. Development to the cypris stage took 3.5–4 days at a water temperature of 22–23ºC, and about 6 days at 18ºC. The lecithotrophic nauplii slightly increase in size during the development and decrease after the nauplius-cyprid moult. Male larvae are slightly larger than female ones. Male cyprids varied from 230 to 280 µm, females from 210 to 250 µm in length. The sizes of male and female larvae slightly overlap ( Fig. 5 View FIGURE 5 ). In summer, the larval sex ratio was male-biased; in autumn it was female-biased.
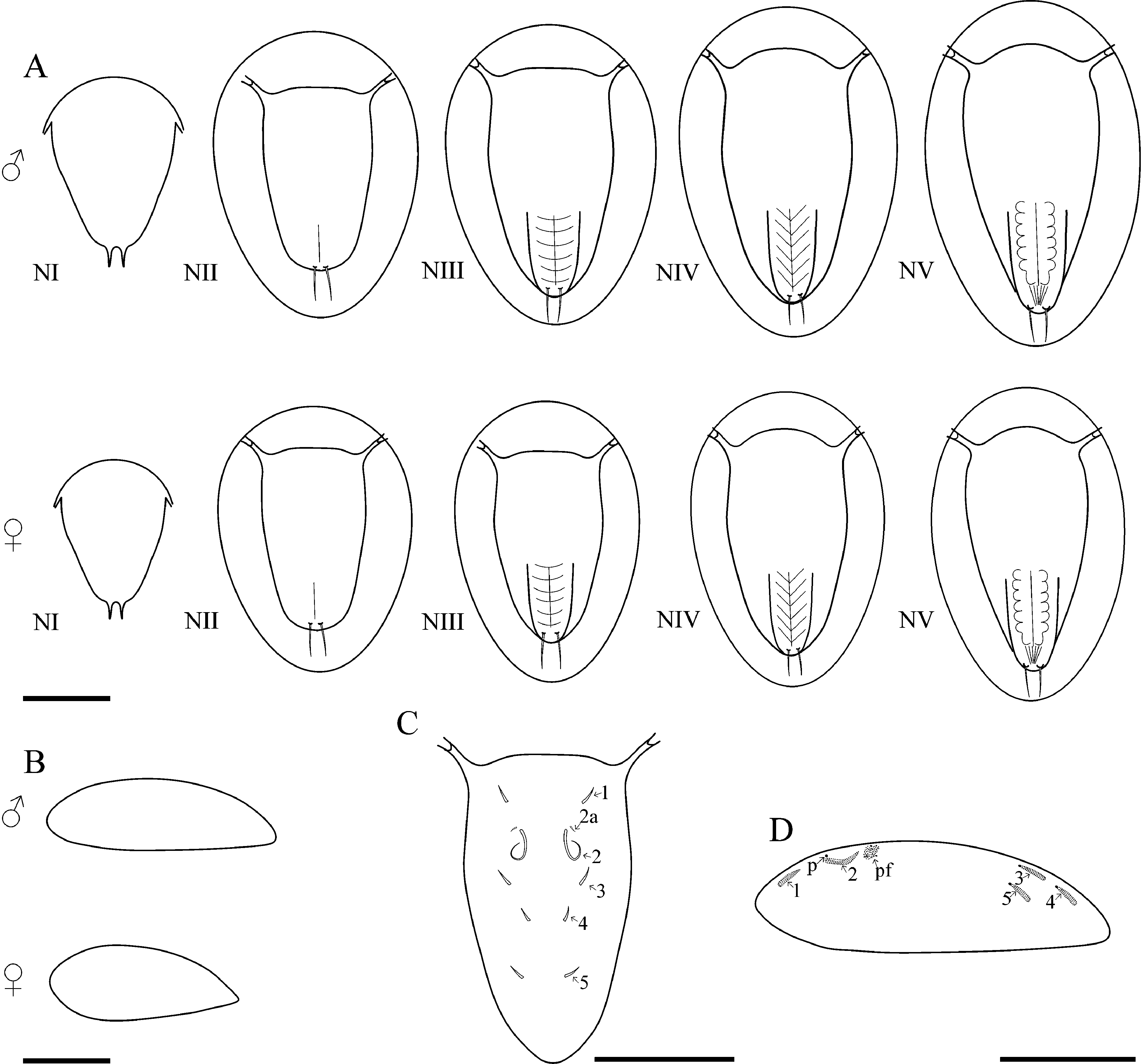
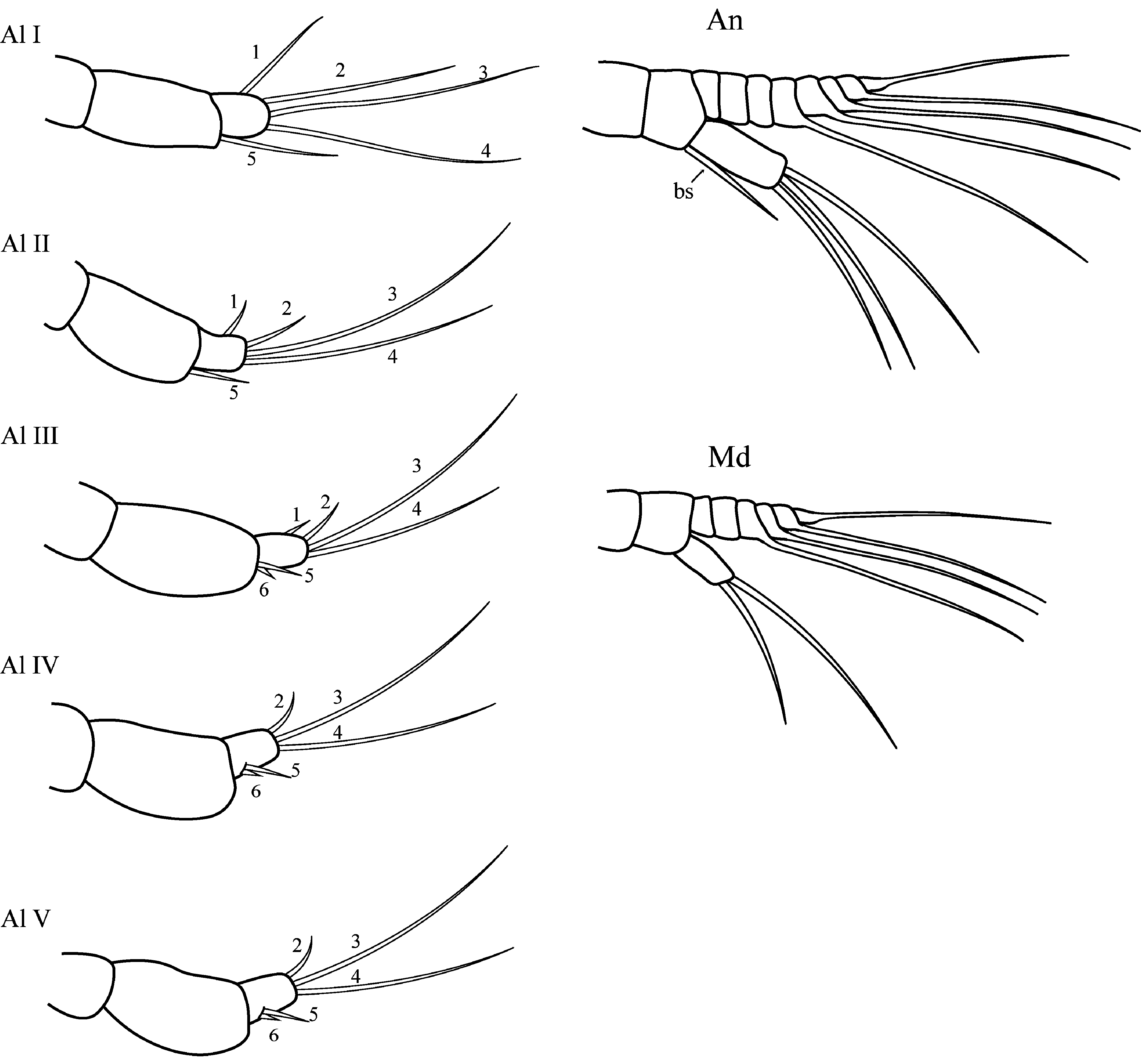
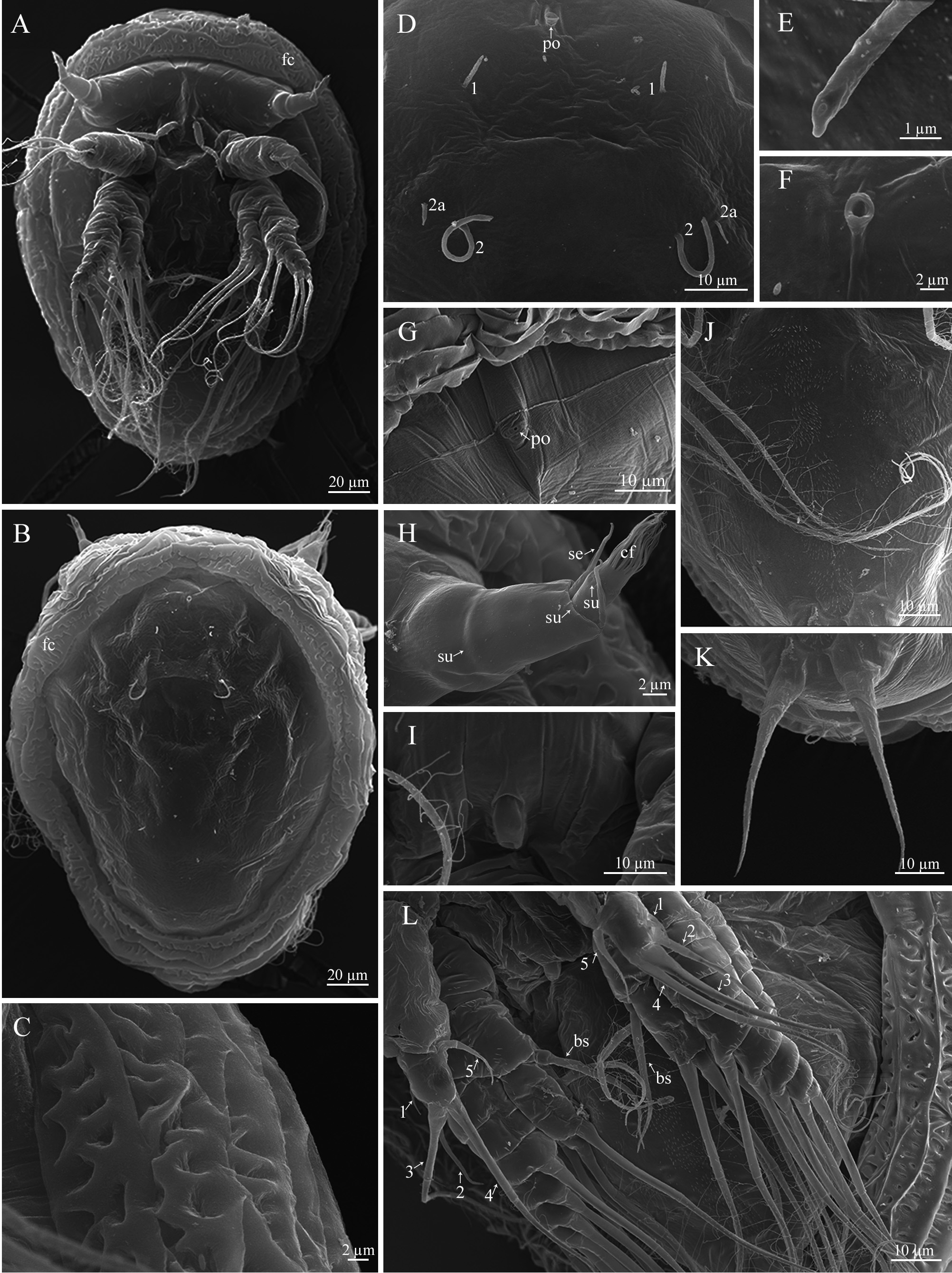
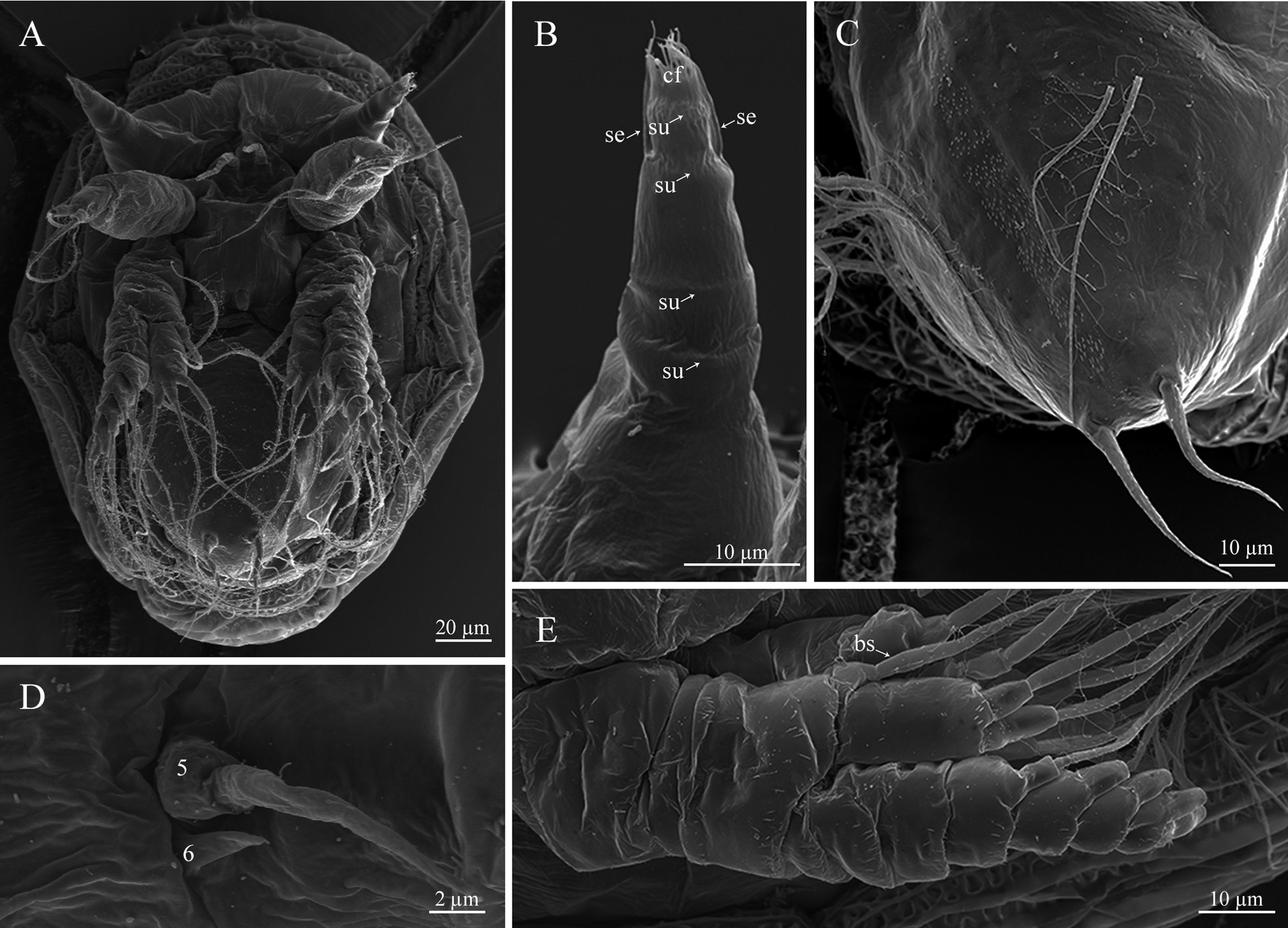
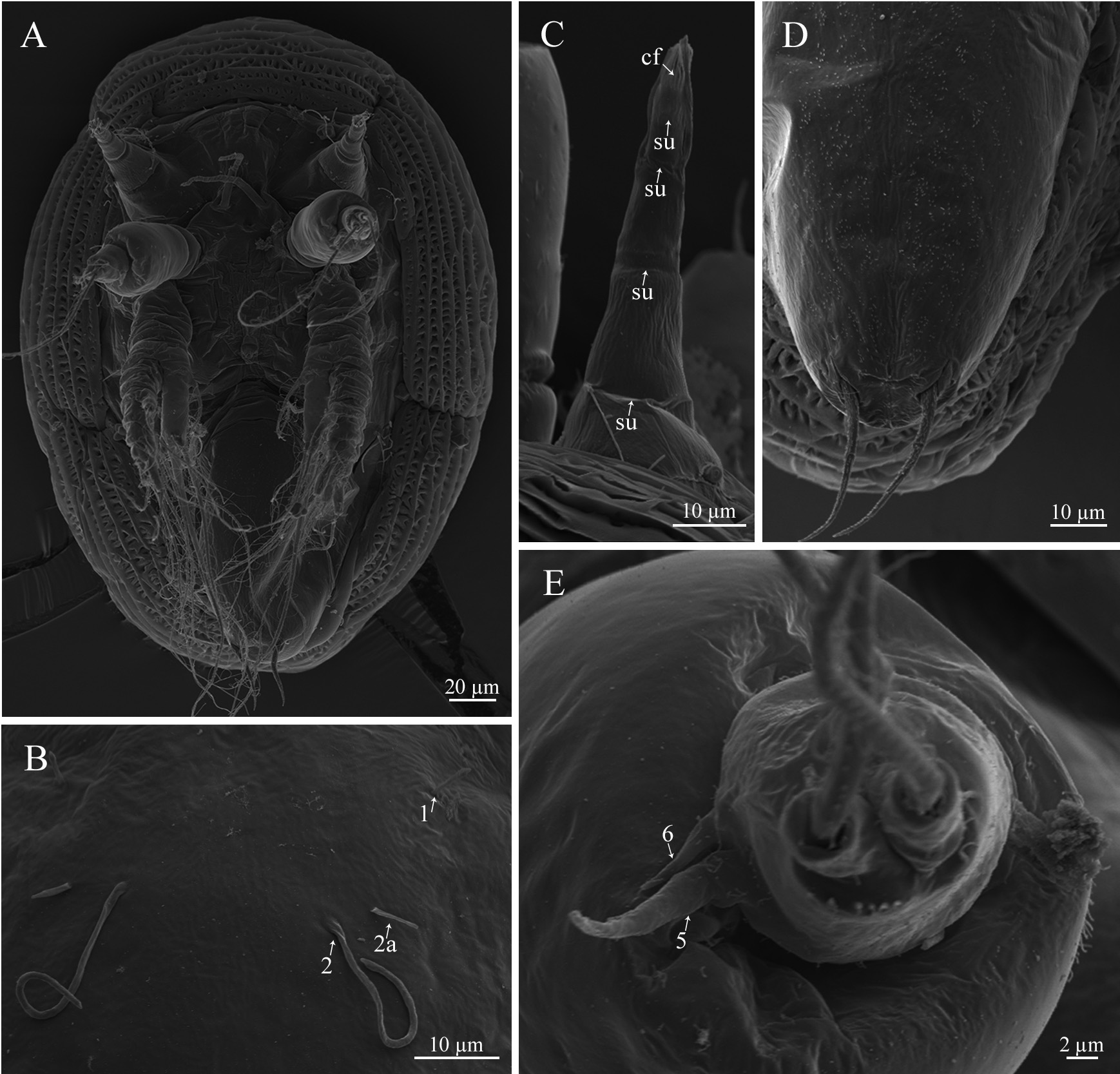
Nauplius I. Nauplius is 253.6± 11.2 µm (♂) and 225.6± 9.6 µm (♀) in length ( Figs. 6A View FIGURE 6 , 7A, B View FIGURE 7 ); larva lacks the pigmented nauplius eye and shows no positive phototaxis. Frontal filaments are present in this and all following naupliar stages. A true flotation collar is absent; however, a narrow transparent border around the larval body is visible in LM. The surface of the dorsal head shield bears two pairs of setae (2 and 2a) with terminal pores, seta 2 is straight or slightly curved ( Fig. 7B, C View FIGURE 7 ); a single pore is slightly visible in the middle of the anterior region ( Fig. 7B View FIGURE 7 ). Frontolateral horns are slightly curved backward, lack terminal fringes, and terminate in an opening of the frontal horn gland; a quite long anterior subterminal seta with a terminal pore is present; one transverse suture is already visible ( Fig. 7D View FIGURE 7 ). Larva has three pairs of appendages; segments lack any denticles ( Fig. 7E View FIGURE 7 ). Uniramous antennule has three segments with five plumose setae; setae 1 and 5 are shorter than 2–4; setae 1–4 arise from the distal antennular segment, seta 5 from the middle one ( Fig. 8 View FIGURE 8 ). The biramous antenna has an 8-segmented exopod (including a small segment carrying the distal seta) with five long plumose setae, and an unsegmented endopod with three long plumose setae; the basis of the antenna has a plumose seta longer than the endopod ( Fig. 8 View FIGURE 8 ). The biramous mandible has a 6-segmented exopod (including a small segment carrying the distal seta) with four long, plumose setae, and an unsegmented endopod with two long, plumose setae ( Fig. 8 View FIGURE 8 ). The mandible is unchanged during the larval development.
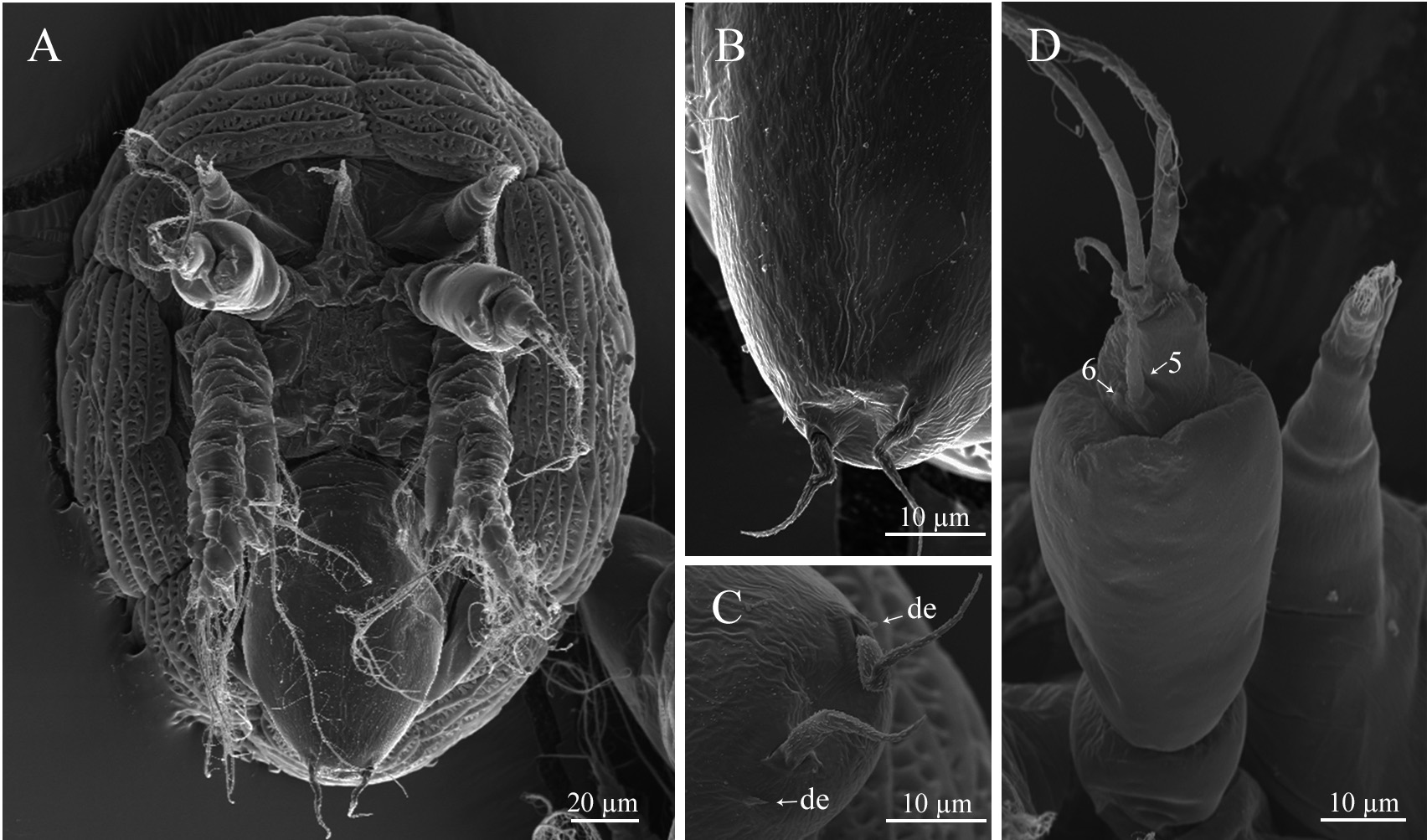
Nauplius II. The larva is 281.5± 11.4 µm (♂) and 255.6± 6.9 µm (♀) in length ( Figs. 6A View FIGURE 6 , 9A, B View FIGURE 9 ). The nauplius is characterized by the presence of a true, conspicuous flotation collar ornamented by a reticulated pattern with longitudinal and transverse ridges ( Fig. 9C View FIGURE 9 ). A pair of pores was found on the lateral sides of the attachment ridge ( Fig. 9G View FIGURE 9 ). The surface of the dorsal head shield has five pairs of setae (1, 2, 2a, 3, 5), setae 4 are not found; seta 2 is the longest, strongly curved, U-shaped; all setae, except 2a, with slightly subterminal pores; seta 2a with a terminal pore ( Fig. 9D, E View FIGURE 9 ). A single pore in the middle of the anterior region is well visible now ( Fig. 9F View FIGURE 9 ). The frontolateral horns are long, from this stage directed anterolaterally, with terminal cuticular fringes and two thick subterminal setae (anterior and posterior); horns are subdivided into three portions by two sutures; the distal portion is also subdivided by an additional, poorly expressed suture ( Fig. 9H View FIGURE 9 ). A conical rudimentary labrum has a small terminal opening ( Fig. 9I View FIGURE 9 ). In LM, the segmentation of the hind body is not visible ( Fig. 6A View FIGURE 6 ). In SEM, the denticles on the hind body are gathered in a median longitudinal strip and sparse lateral groups ( Fig. 9J View FIGURE 9 ). The furcal rami are thin, long, covered with denticles ( Fig. 9K View FIGURE 9 ). The long setae of the appendages are covered with denticles and very long, slender setules; the distal margins of the antennular, antennal and mandibular segments are clearly defined by rows of long denticles ( Fig. 9L View FIGURE 9 ). Antennular setae 1, 2, and 5 are reduced in size ( Fig. 8 View FIGURE 8 ).
Nauplius III. The larva is 285.6± 8.2 µm (♂), 258.8±10.3 (♀) µm in length ( Figs. 6A View FIGURE 6 , 10A View FIGURE 10 ). The dorsal head shield surface has six pairs of setae ( Fig. 6C View FIGURE 6 ). The pattern of dorsal surface setae is unchanged during following naupliar development. The number of sutures on the frontolateral horns is increased, with five portions with four sutures ( Fig. 10B View FIGURE 10 ). The hind body is more elongated. In LM, the segmentation pattern of the hind body looks like a median longitudinal line and weakly pronounced transverse lines ( Fig. 6A View FIGURE 6 ); in SEM, denticles have the same pattern as in nauplius II; furcal rami are inserted into cuticular sockets ( Fig. 10C View FIGURE 10 ). Seta 5 is located at the border between the distal and middle segments; a short seta appears near the base of seta 5 ( Figs. 8 View FIGURE 8 , 10D View FIGURE 10 ); the second antennular segment is more swollen. The long setae of appendages are inserted into cuticular sockets ( Fig. 10E View FIGURE 10 ).
Nauplius IV. The larva is 291.6± 12.6 µm (♂), 266.0±9.6 (♀) µm in length ( Figs. 6A View FIGURE 6 , 11A View FIGURE 11 ). In LM, the segmen- tation of the hind body comprises oblique lines ( Fig. 6A View FIGURE 6 ); in SEM, sparse denticles on the hind body are gathered in weakly pronounced oblique strips, indicating the location of the developing thoracic appendages of the future cypris larva ( Fig. 11B View FIGURE 11 ). A pair of denticles near the base of the rami are slightly pronounced ( Fig. 11C View FIGURE 11 ). The antennule has only four setae, seta 1 is reduced; setae 5 and 6 are already inserted in the distal antennular segment ( Figs. 8 View FIGURE 8 , 11D View FIGURE 11 ).
Nauplius V. The larva is 301.0± 8.9 µm (♂), 269.1±11.5 (♀) µm in length ( Figs. 6A View FIGURE 6 , 12A View FIGURE 12 ). The head shield is more convex, the posterior margin of the head shield is more prominent in lateral view. The dorsal head shield setae 2 are lengthened ( Fig. 12B View FIGURE 12 ). Long frontolateral horns are directed ventrally ( Fig. 12A, C View FIGURE 12 ). The hind body becomes narrower; in LM, the thoracic appendages of the future cypris larva are well visible in the bottom of the hind body ( Fig. 6A View FIGURE 6 ). In SEM, denticles on hind body are gathered in oblique or transverse strips ( Fig. 12D View FIGURE 12 ). The second antennular segment is bulbous; seta 6 is sometimes quite long ( Fig. 12E View FIGURE 12 ).
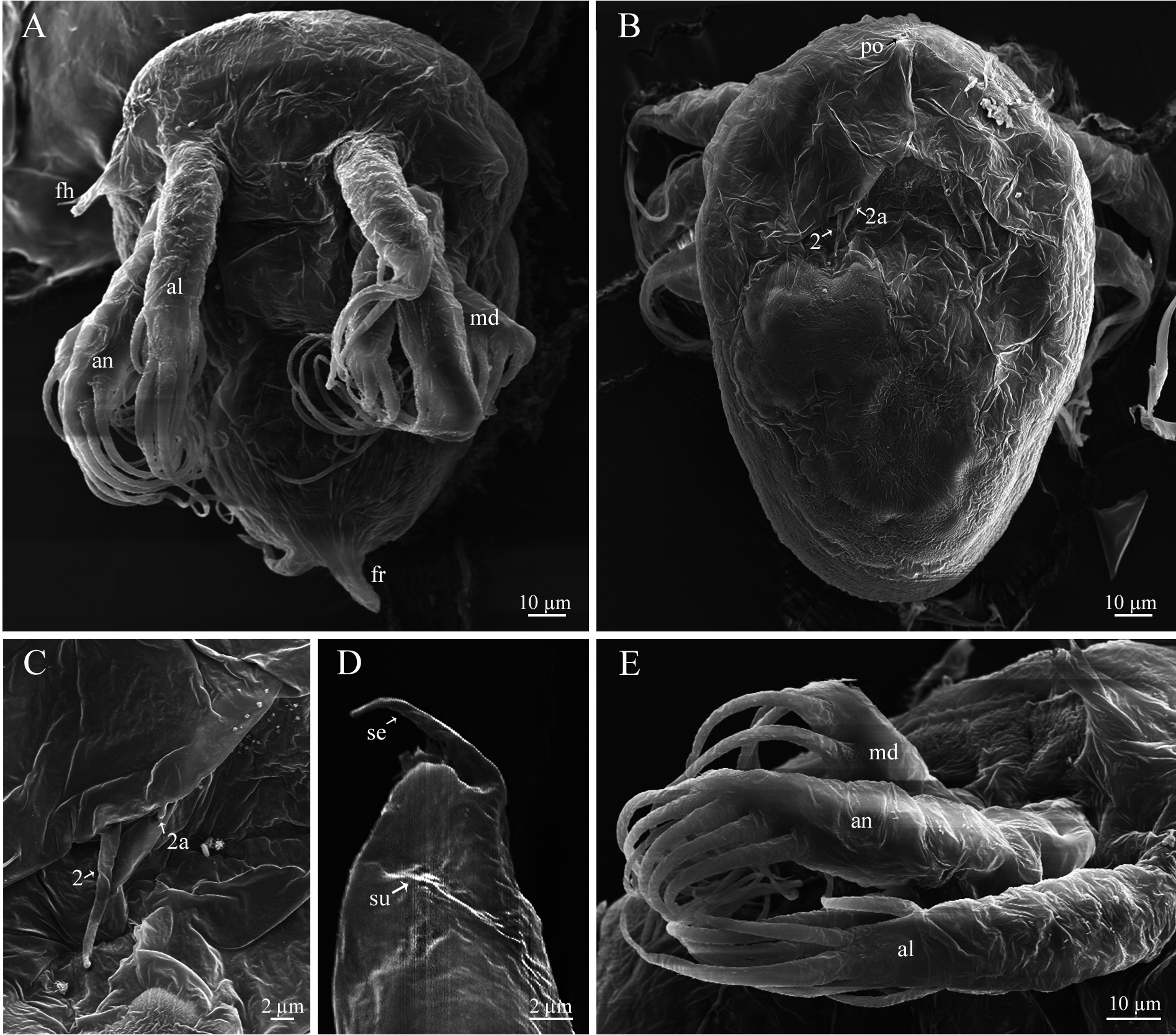
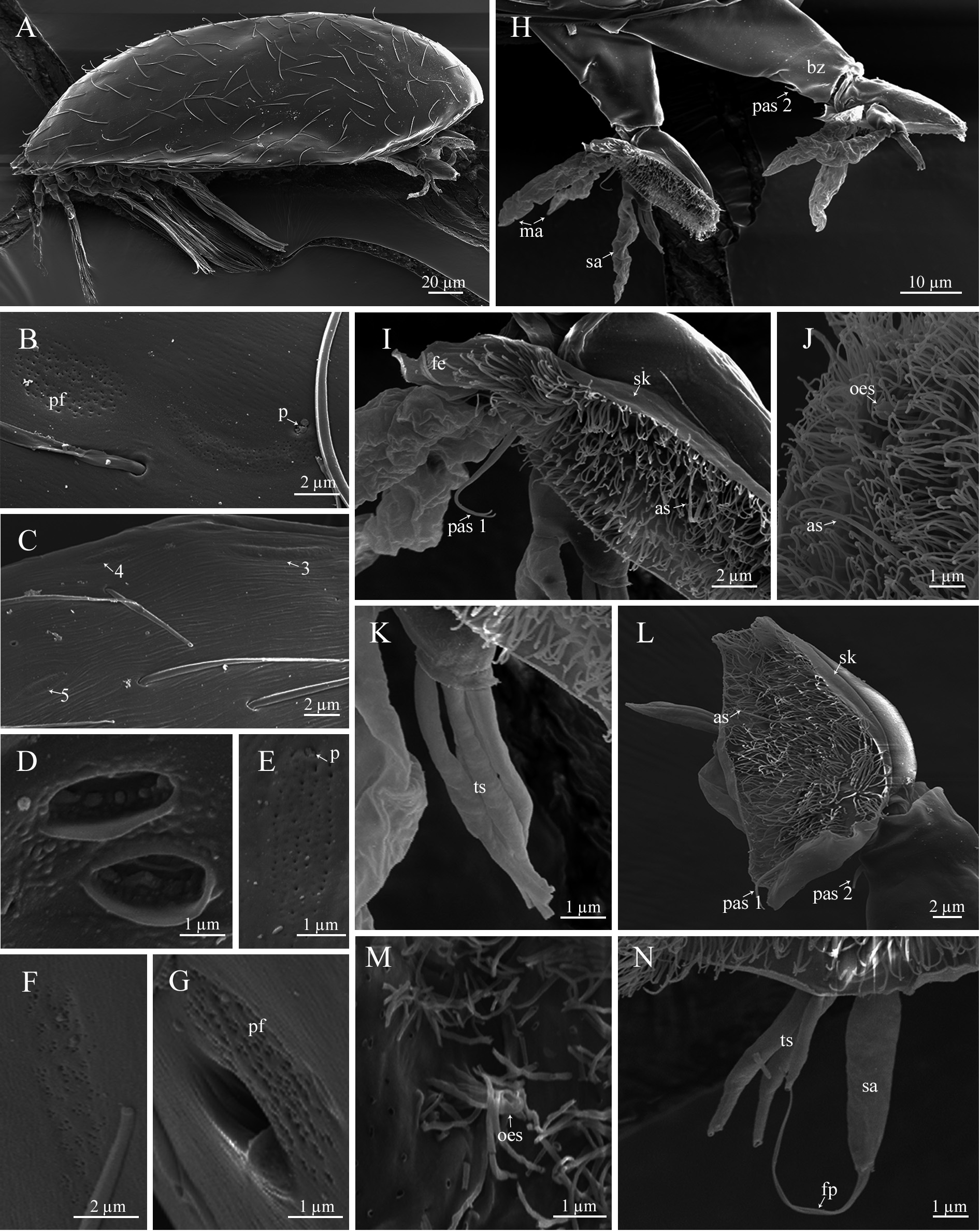
Cypris larva. The larva is 257.0± 12.9 µm (♂), 230.8±9.5 (♀) µm in length ( Figs. 6B View FIGURE 6 , 13A View FIGURE 13 ). The carapace surface is wavy, densely covered with long setae and scattered pores (possible traces of lost setae). Five pairs of lattice organs (two anterior and three posterior) are placed on the carapace surface. LO1–4 are along the midline, LO5 lateral to LO4 ( Fig. 6D View FIGURE 6 ). The second pair (LO2) is crescent-shaped, the convex side is lateral, and the associated porefield is posterior ( Fig. 13B View FIGURE 13 ); the remaining lattice organs (LO1 and LO3–5) are nearly straight ( Fig. 13C View FIGURE 13 ). The frontolateral horn glands open with two separate pores on the anteroventral margins ( Fig. 13D View FIGURE 13 ). Numerous porefields looking like lattice organs are found on the lateral sides of the carapace ( Fig. 13E, F View FIGURE 13 ). A large hole with an adjacent porefield is also found anteriorly along the midline ( Fig. 13G View FIGURE 13 ).
The antennule of the cypris larva consists of four segments. The long second segment ends with a distal breakage zone ( Fig. 13H View FIGURE 13 ). The third segment forms an attachment disc, covered with cuticular villi. The perimeter of the disc is bordered by a cuticular skirt, more pronounced in the female larvae ( Fig. 13I, L View FIGURE 13 ). The attachment disc of the male larva has a flap-like extension at the posterior margin, which is lacking in the female cyprid ( Fig. 13I, L View FIGURE 13 ). An open-ended seta is located in the distal part of the attachment disc of both male and female larvae ( Fig. 13J, M View FIGURE 13 ); a spinous process is absent in both male and female cyprids. The antennule of the male cyprid possesses three sense setae: an axial sense seta in the middle of the attachment disc, a slightly bifurcated postaxial sense seta in the pos- terior (proximal) part, and a short postaxial sense seta in the distal part of the second antennular segment ( Fig. 13H, I, J View FIGURE 13 ). In thefemale cyprid, the axial sense seta is slightly shifted to the distal part of the attachment disc ( Fig. 13L View FIGURE 13 ). A large male aesthetasc is located on the proximal margin of the attachment disc and bifurcated into two separate lobes of unequal length ( Fig. 13H View FIGURE 13 ). In the male cyprid, the short fourth segment has a long, subterminal aesthetasc ( Fig. 13H View FIGURE 13 ) and four nearly equal, open-ended setae ( Fig. 13K View FIGURE 13 ). In the female cyprid, the subterminal aesthetasc is shorter than the subterminal aesthetasc in the male larva and terminates in two thin long filamentous processes; one open-ended seta is shorter than others ( Fig. 13N View FIGURE 13 ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Peltogaster reticulata Shiino, 1943
| Korn, Olga M., Golubinskaya, Darya D. & Sharina, Svetlana N. 2020 |
Peltogaster reticulata
| Yoshida, R. & Naruse, T. 2016: 215 |
| Yoshida, R. & Osawa, M. & Hirose, M. & Hirose, E. 2011: 853 |
Peltogaster reticulatus
| Rybakov, A. V. & Hoeg, J. T. 2002: 98 |